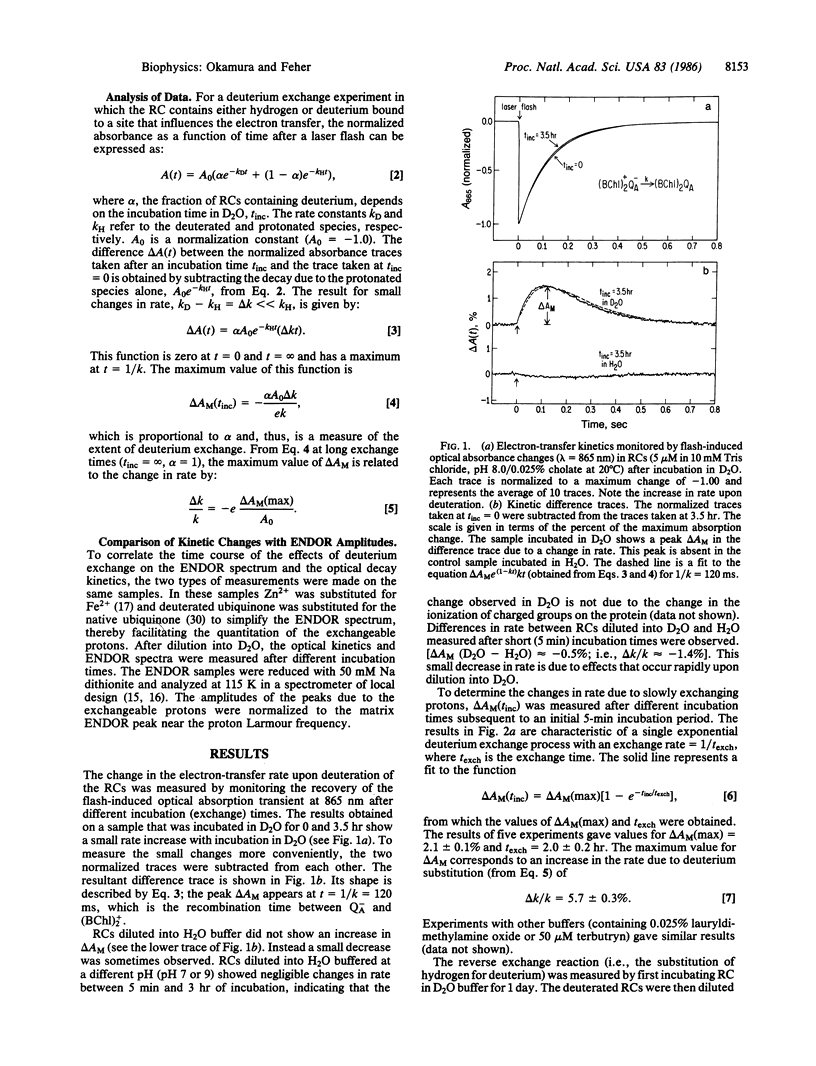
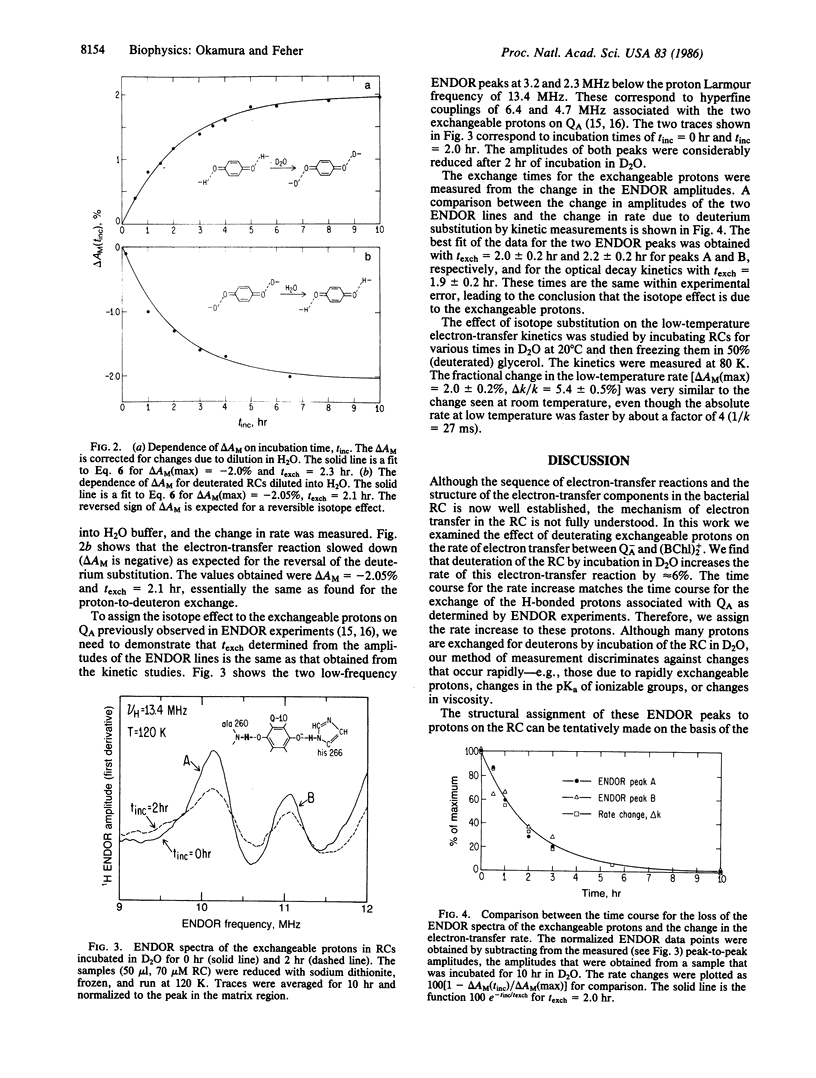
Abstract
Previous ENDOR studies on reaction centers from Rhodopseudomonas sphaeroides have shown the presence of two hydrogen-bonded protons associated with the primary, ubiquinone, acceptor QA. These protons exchange with deuterons from solvent 2H2O. The effect of this deuterium substitution on the charge-recombination kinetics (BChl)2+QA- → (BChl)2QA has been studied with a sensitive kinetic difference technique. The electron-transfer rate was found to increase with deuterium exchange up to a maximum Δk/k of 5.7 ± 0.3%. The change in rate was found to have an exchange time of 2 hr, which matched the disappearance of the ENDOR lines due to the exchangeable protons. These results indicate that these protons play a role in the vibronic coupling associated with electron transfer. A simple model for the isotope effect on electron transfer predicts a maximum rate increase of 20%, which is consistent with the experimental results.
Keywords: bacterial photosynthesis, vibronic coupling, hydrogen bonding, kinetics
Full text
PDF
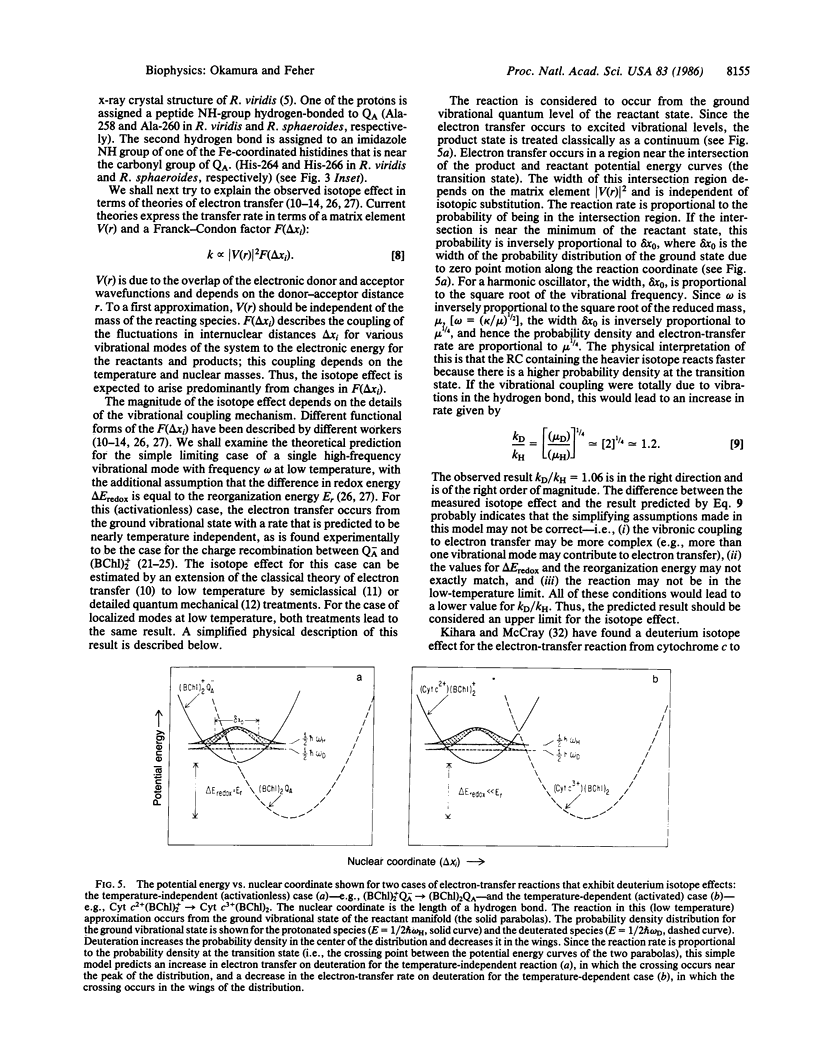

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton R. K., Yau H. F. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. I. Kinetics of the oxidation and reduction of P-870 as affected by external factors. Biophys J. 1972 Jul;12(7):867–881. doi: 10.1016/S0006-3495(72)86130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Feher G., Okamura M. Y. Iron-depleted reaction centers from Rhodopseudomonas sphaeroides R-26.1: characterization and reconstitution with Fe2+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+. Biochemistry. 1986 Apr 22;25(8):2276–2287. doi: 10.1021/bi00356a064. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi E. S., Bolton J. R. Flash photolysis-electron spin resonance study of the effect of o-phenanthroline and temperature on the decay time of the ESR signal B1 in reaction-center preparations and chromatophores of mutant and wild strains of Rhodopseudomonas spheroides and Rhodospirillum rubrum. Biochim Biophys Acta. 1974 Apr 23;347(1):126–133. doi: 10.1016/0005-2728(74)90205-9. [DOI] [PubMed] [Google Scholar]
- Kakitani T., Kakitani H. A possible new mechanism of temperature dependence of electron transfer in photosynthetic systems. Biochim Biophys Acta. 1981 May 13;635(3):498–514. doi: 10.1016/0005-2728(81)90109-2. [DOI] [PubMed] [Google Scholar]
- Kihara T., McCray J. A. Water and cytochrome oxidation-reduction reactions. Biochim Biophys Acta. 1973 Feb 22;292(2):297–309. doi: 10.1016/0005-2728(73)90037-6. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron transfer in reaction centers of Rhodopseudomonas sphaeroides. I. Determination of the charge recombination pathway of D+QAQ(-)B and free energy and kinetic relations between Q(-)AQB and QAQ(-)B. Biochim Biophys Acta. 1984 Jul 27;766(1):126–140. doi: 10.1016/0005-2728(84)90224-x. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Kung M., Hales B. J. Characterization of the phototrap in photosynthetic bacteria. Ann N Y Acad Sci. 1975 Apr 15;244:297–319. doi: 10.1111/j.1749-6632.1975.tb41537.x. [DOI] [PubMed] [Google Scholar]
- Lubitz W., Abresch E. C., Debus R. J., Isaacson R. A., Okamura M. Y., Feher G. Electron nuclear double resonance of semiquinones in reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1985 Aug 7;808(3):464–469. doi: 10.1016/0005-2728(85)90155-0. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarai A. Possible role of protein in photosynthetic electron transfer. Biochim Biophys Acta. 1980 Jan 4;589(1):71–83. doi: 10.1016/0005-2728(80)90133-4. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]