Abstract
A common dermal exposure assessment strategy estimates the systemic uptake of chemical in contact with skin using the fixed fractional absorption approach: the dermal absorbed dose is estimated as the product of exposure and the fraction of applied chemical that is absorbed, assumed constant for a given chemical. Despite the prominence of this approach there is little guidance regarding the evaluation of experiments from which fractional absorption data are measured. An analysis of these experiments is presented herein, and limitations to the fixed fractional absorption approach are discussed. The analysis provides a set of simple algebraic expressions that may be used in the evaluation of finite dose dermal absorption experiments, affording a more data-driven approach to dermal exposure assessment. Case studies are presented that demonstrate the application of these tools to the assessment of dermal absorption data.
Keywords: risk assessment, skin absorption, percent absorption, fractional absorption, dermal load, maximum flux, evaporation
INTRODUCTION
The potential for adverse systemic health effects resulting from dermal contact with a chemical is broadly recognized. In situations where dermal contact may contribute significantly to the total body burden, assessment of the systemic uptake of the chemical following skin contact allows for greater accuracy in the estimation of total absorbed dose, hence, a more comprehensive understanding of the risks of systemic toxicity. Therefore, it is incumbent upon the risk assessor to consider the dermal absorption potential of a chemical. This in turn requires a reasonable estimate of the dermal absorbed dose, that is, the amount of chemical that is systemically absorbed following contact with skin.
A common strategy1 practiced in dermal exposure assessment estimates the systemic uptake of chemical by the dermal route using the fixed fractional absorption approach, in which the dermal-absorbed dose is related to some measure of exposure times the fraction of applied chemical that is absorbed. As an example, in the US EPA Standard Operating Procedures for Residential Pesticide Exposure Assessment,2 the dermal absorbed dose is calculated as:
 |
where D is the absorbed dose (mg/kg/day), E is the exposure (mg/day), BW is body weight (kg), and AF is the fractional absorption factor. This factor—or its equivalent expressed as a percent absorption—is typically an empirical quantity that is assumed to be a fixed value specific to a given chemical regardless of exposure conditions. The fractional absorption factor is commonly determined from finite dose in vitro or in vivo dermal absorption studies. In these experiments, at the end of a specified exposure duration, the dermal-absorbed dose is measured and the fractional absorption factor is calculated as the dermal-absorbed dose divided by the applied load.
The fractional absorption approach has been advocated by regulatory and advisory agencies in North America and Europe. In the United States, the EPA guidance for Superfund remediation3 as well as the Office of Pesticide Programs,2, 4 adopt this approach. Additionally, the US Army,5, 6 the Department of Homeland Security,7 and the National Institute for Occupational Safety and Health8 have incorporated fractional absorption in dermal risk assessment strategies. In Europe, the European Commission's technical guidance for risk assessment9 emphasizes the primacy of fractional absorption for dermal risk assessment. The EC guidance document on dermal absorption10 provides a process for setting dermal absorption percentages using default values or, preferably, values determined experimentally.
There are two main advantages of the fractional absorption approach to estimating dermal absorption. First, the finite dose experiment can represent a good model for realistic environmental and occupational exposure scenarios. In-use conditions, including the use of vehicle, expected dose loading, and exposure duration can be manipulated to mimic realistic exposures. The approach is also appealing in its simplicity. The fractional absorption factor is readily determined from dermal absorption experiments, and this factor is easily slotted into simple algebraic expressions such as Eq. (1) to derive the total dermal-absorbed dose from a given exposure.
However, this apparent simplicity is belied by complicating factors. It has been observed that an inverse relationship often exists between dermal loading and fractional absorption.11, 12, 13 As the loading increases, the fraction of chemical that is absorbed diminishes. Thus, the fractional absorption factor is not a constant for a given chemical, and it is typically highest for low dermal loads that are characteristic of environmental and occupational exposures. For dermal absorption testing of a quality sufficient for regulatory submission, a range of loads are often required to be investigated to span the range of expected exposures.14 However, peer review reports in the open literature typically cover a narrow range or just one load. Experiments at low loads present technical complications for the investigator.11, 15 The uniform application of a thin homogeneous layer of the chemical of interest is challenging and the evaluation of absorbed dose under low loading may stress the limits of chemical detection (The term “low loads” is somewhat ambiguous, but its meaning will become clearer through the information presented in this manuscript.). The OECD Guideline for the Testing of Chemicals16 recommends a load of 1–5 mg/cm2 for solids and up to 10 μl/cm2 for liquids for applications that mimic human exposure, with considerations for using the appropriate vehicle, for example, neat, diluted, or formulated material containing the test substance. This recommendation does not account for differences in dermal absorption rates. These loads may realistically approximate the infinite dose regime for poorly absorbed materials, whereas those that are more readily absorbed may exhibit absorption profiles that are more characteristic of a finite dose application.
Other complicating factors are related to the disposition of the applied load. For example, a volatile chemical will both penetrate the skin and evaporate from the skin's surface. Evaporation will diminish the dermal absorbed amount, but it is not evident how to account in any quantitatively meaningful manner for the competing kinetic processes of absorption and evaporation. The exposure duration, or the time during which the skin remains in contact with the chemical, is clearly a factor in determining the absorbed amount and, hence, the fractional absorption factor. An 8-h exposure might be most relevant for occupational exposures, but low-level environmental exposures, for example, from indoor air contaminants, may persist indefinitely. For the experimental design and evaluation of finite dose dermal absorption data, it is therefore important to consider the relationship between the dermal absorption kinetics of a given compound and the exposure duration, in order to evaluate whether a calculated fractional absorption represents a steady state or if additional absorption would be expected from a longer exposure duration.
Despite the prominence of the fractional absorption approach in dermal exposure assessment, there is little guidance on the proper evaluation of dermal exposure and absorption data derived from experimental observations. The purpose of this paper is to present a systematic approach to the evaluation of finite dose in vitro dermal absorption experiments. Limitations of the fixed fractional absorption approach are presented and analyzed. The order of presentation is not necessarily a function of their importance; rather for a given set of experimental conditions, one or more may represent important considerations. We present a quantitative framework for the analysis of finite dose dermal absorption data that can aid in the evaluation of the potential of a chemical to be dermally absorbed and contribute to the systemic dose. The objective is to provide those tasked with evaluating dermal absorption data with a sensible, data-driven framework for dermal exposure assessment. Another goal is to lay the framework for researchers to use in the future when designing, conducting, and interpreting dermal absorption studies. More detailed theoretical analyses of finite dose absorption kinetics may be found elsewhere.17, 18, 19, 20
LIMITATIONS OF THE FIXED FRACTIONAL ABSORPTION APPROACH FOR ASSESSING SYSTEMIC TOXICITY FROM DERMAL EXPOSURE
Loading Affects Fraction Absorbed
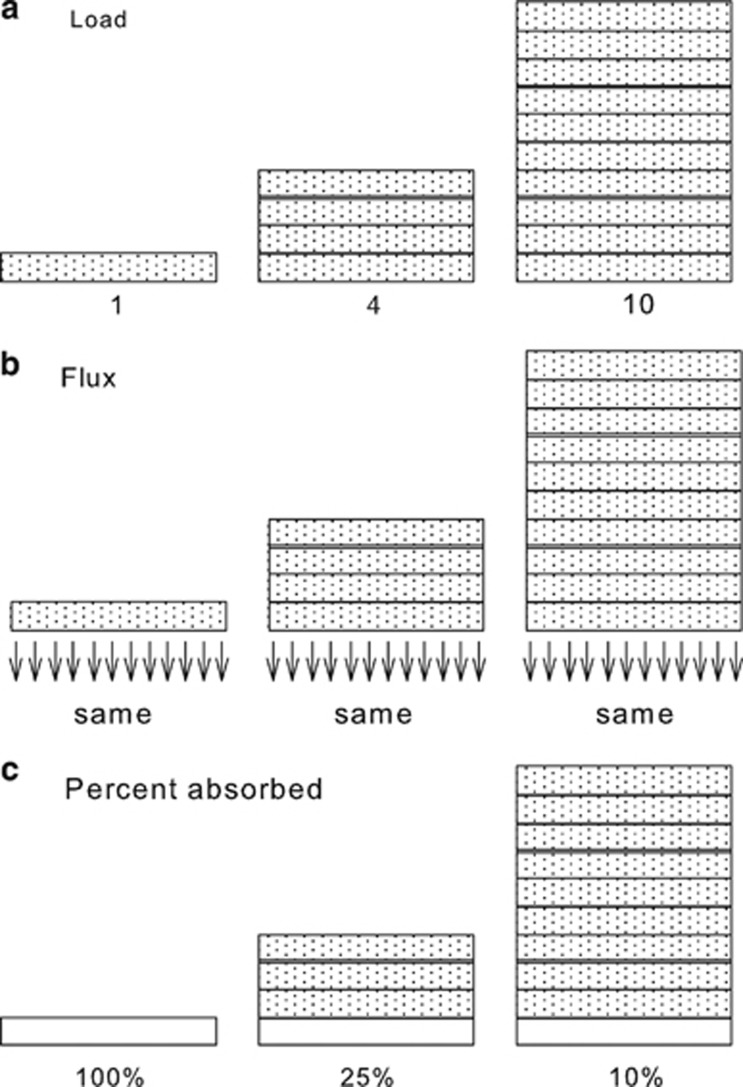
Loading conditions can have enormous effects on fractional or percent absorption.13 Figure 1 presents a simplified case to elucidate the effect of loading on measured percent absorbed. This simple illustration demonstrates the crucial effect of loading on the measured result when absorption is reported solely as the fraction or percent of the applied amount. The example on the right is a case where load depletion is small relative to the applied load. This condition can readily be extrapolated to the case of an infinite load, whereby the percent absorbed must be practically zero. Thus, absorbed amount may range from zero to 100% for the same chemical, depending on the applied load. There are several reasons why 100% absorption may not be achieved in a practical setting. Chemical may bind to skin components or it may be removed from the skin by volatilization, debridement, sweating, or washing. This simple illustration, nevertheless, demonstrates that the use of a specified fixed cutoff value to evaluate the dermal absorption potential of a chemical seems unwarranted.
Figure 1.
Illustration of how applied load affects percent that is absorbed. (a) Three examples of different loads (1, 4, and 10 units) of a given chemical on skin. (b) As long as absorption has not depleted the amount of chemical on the skin too much, flux of the chemical is the same under all conditions. (c) If the experimental duration is such that one load unit penetrates the skin into receptor fluid then on the left, 100% of the load is absorbed; in the middle, the value is 25% and on the right it is 10%.
Given this, is it possible to draw any conclusions regarding the absorption potential of a chemical based on the knowledge of the fraction of applied finite dose that is absorbed? Kissel13 proposed a dimensionless ratio, Nderm, that may prove useful in the evaluation and interpretation of finite dose experiments. The dermal number quantifies the ratio of applied load and absorbable amount:
 |
Experimental load is the mass of chemical applied per unit area of exposed skin, and steady-state flux (Jss) is the steady-state absorption rate (mass/area/time) for the chemical at a specified dilution. Jss can be experimentally determined using infinite doses of chemical at the dilution of interest as donor. In the absence of experimental knowledge of Jss, it may be estimated by using any number of theoretical models that predict the steady-state permeability coefficient (kp,v, length/time) of a compound from a given vehicle (typically, water), along with the knowledge of the concentration (Cv, mass/volume) of the compound in that vehicle (providing the compound or other components in the vehicle do not significantly alter the skin's barrier):
We note that this definition of Nderm (Eq. (2)) differs from that of Kissel13 in that Kissel uses maximum flux in place of steady-state flux. Maximum flux represents the steady-state flux from neat chemical or saturated solution, and its use is appropriate for such donors. Because in practice diluted solutions of chemical are often studied, Nderm as defined here is more generally applicable.
The distinction is made between maximum flux and peak flux. The latter is defined as the highest flux achieved from a given dose. Any in vitro diffusion experiment will exhibit a peak flux, the magnitude of which depends on donor concentration and loading. As loading increases, so too will peak flux until a value is reached, which corresponds to the highest flux obtainable from a given donor. If the donor is a neat chemical or a saturated solution, the peak flux will equal maximum flux (once again, provided the chemical or other components in the vehicle do not significantly alter the skin's barrier).
High values of Nderm signify an experiment that is flux limited; that is, one where there is ample load that is not significantly depleted through the time course of the experiment. Under this condition, % absorption varies inversely with load. In the simplified case described in Figure 1, a load of 10 units resulted in an absorption of 10% of the applied load. Consider a load of 100 units. Under the described conditions, only 1% of the applied load will be absorbed; for a load of 1000 units, 0.1% is absorbed; and so on. This inverse relationship between dermal load and relative dermal absorption has been noted in recent reviews of the literature,11, 12, 13 suggesting that a substantial proportion of finite dose experiments are performed in the high Nderm regime. Generally, high values of Nderm are not representative of the low loads typically encountered in occupational or environmental exposures and the reported % absorption under these conditions is not a useful indicator of the potential of a chemical to be dermally absorbed.
If the entire time course of dermal absorption is provided by the report, then important information can be obtained from experiments with high Nderm values. This regime allows the most reliable estimates of maximum flux and lag time. Because load depletion does not limit flux, it is likely that the experimental flux under high Nderm conditions approaches Jmax of the chemical, if the chemical is applied neat or in a saturated solution. An exception to this rule may occur if a solid chemical is applied without a solvent, particularly, if it is coarsely divided or highly crystalline; in this case, absorption may become dissolution limited.
A low value of Nderm signifies a delivery-limited state, where significant load depletion is expected to occur. This condition is a more proper application of the term “finite dose” and the measured peak flux under this condition will be less than the maximum steady-state flux possible for the specified donor, owing to depletion of the load.
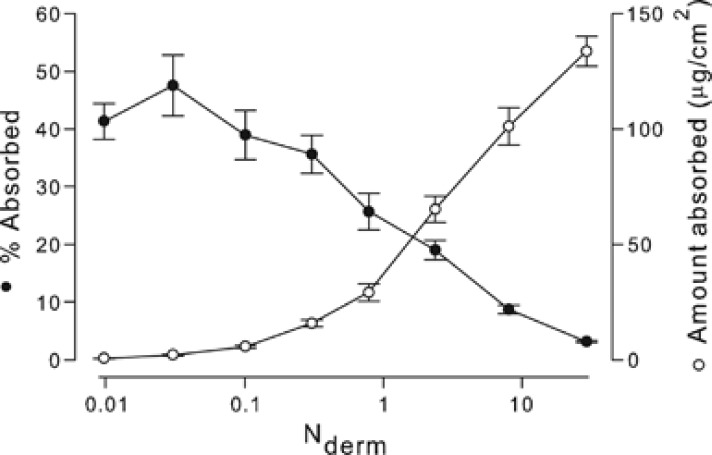
Figure 2 displays experimental data that illustrate how Nderm affects both the percent of applied load and the total amount that are absorbed. Previously published17 in vitro split thickness human cadaver skin absorption data of the model compound vanillylnonanamide, a synthetic capsaicin, have been recast to display percent absorption and total absorbed amount as functions of the parameter Nderm. For an experimental duration of 72 h and maximum flux (measured) of 2 μg/cm2/h, the load equivalent to an Nderm of 1 was 144 μg/cm2. For small loads (Nderm<0.1) of this particular compound, absorption plateaus at ∼40–50% of the applied dose, whereas for larger loads (Nderm>10), absorption is less than 10%. On the other hand, as dose is increased the total amount of the compound that is absorbed also increases, as expected. We emphasize that absorption here refers to the amount of chemical that has passed through the skin and into the receptor compartment, and does not include the amount deposited within the skin. This may contribute to the observation that absorption does not approach 100% at low loads. Another possibility is that the experimental duration was not long enough to observe 100% absorption.
Figure 2.
Percent absorption and total amount absorbed for the chemical vanillylnonanamide. Previously published in vitro human skin absorption data12 has been recast as a function of the dimensionless dermal number Nderm, which quantifies the ratio of applied load and dermal absorption. Data demonstrate that % absorption diminishes with increasing load. Absorption does not include the skin depot amount.
It is difficult to assign a precise cutoff value of Nderm to distinguish between flux-limited and delivery-limited absorption. A complicating issue is the fact that the experimental duration (a variable in the denominator of Eq. (2)) may have been selected without consideration for the kinetics of the absorption process for the particular chemical investigated. For the data displayed in Figure 2, flux limitation does not appear to be reached at the maximum Nderm studied (∼30). There exists a near linear relationship between dermal loading and total absorbed amount for Nderm∼1 and greater, but a plateau in total absorbed amount is not reached.
Case study 1 provides an example from the literature of a failure to consider the effect of loading on fractional absorption and demonstrates how a critical analysis, as advocated herein and by Kissel,13 may be applied in the evaluation of finite dose absorption data.

Evaporation or Sublimation of Volatile Compounds Affects Fraction Absorbed
Volatility of the test material is another variable that requires consideration. Fractional absorption of a volatile compound will depend not only on the experimental load, but also on the rate of evaporation compared with the rate of absorption. A highly volatile compound will evaporate from an unoccluded donor and will not be available for dermal absorption. Although small doses of a volatile compound may well represent a reasonable in-use exposure to splashes of chemical, and as such does yield useful data, this scenario may not provide much information regarding the permeability potential of the compound.
The Nderm parameter described above (Eq. (2)) does not take into consideration the volatility of the chemical. Therefore, in analogy with Nderm, we propose a dimensionless number that quantifies the balance between applied load and depletion through evaporation. The evaporation number is defined as:
 |
Units for load, flux, and duration are the same as those for Nderm. Measured values of evaporation flux are unlikely to be available for most chemicals of interest. In these cases, approximations can be made using empirical or semi-theoretically based models. An example is the US EPA equation for estimating evaporation flux from the surface of chemical spills.24 This equation has also been applied to estimate evaporation from the donor compartment of diffusion cells25, 26, 27 and is presented here in the Appendix.
High values of Nevap signify the flux-limited condition in which there is no significant load depletion through evaporation. Low values of Nevap suggest a delivery-limited condition, whereby significant evaporative losses reduce the observed flux. In contrast with low values of Nderm, the percent of applied dose that is dermally absorbed may be quite low for low Nevap. Owing to substantial evaporation, less chemical is available for dermal absorption.
The dimensionless flux number (Nflux) should prove useful in the evaluation of dermal absorption studies using volatile chemicals. It quantifies the balance between evaporation and absorption:
 |
Both dermal flux and evaporation flux should be evaluated under the same experimental donor conditions (e.g., neat or diluted). If both fluxes represent maximum fluxes, then Nflux is exactly equal to the inverse of the parameter χ described by Kasting and Miller.18 Low values of Nflux indicate a condition in which the measured dermal absorption will be diminished by evaporative losses. For Nflux≪1, the permeant will largely evaporate from the skin surface. Conversely, large values of Nflux are indicative of a compound that will primarily be absorbed. The time scale over which these competing processes occur depends on the membrane lag time, as demonstrated by Kasting and Miller.18 In brief, surface evaporation commences immediately following application of the load to the skin, whereas dermal flux requires some amount of time, related to the membrane lag time, to become established.
Another useful parameter can be used in the evaluation of finite dose absorption data from volatile compounds. One may estimate the time for evaporation of the applied dose to occur using the evaporation time, defined as:
 |
The comparison of tevap with the exposure duration of the applied dose is key. If tevap is much greater than the exposure duration, the experiment is flux limited. That is, there is insignificant load depletion due to evaporation. If tevap is less than the exposure duration, this implies a delivery-limited condition, in which evaporation will limit the load available for dermal absorption, and experimental flux values will be reduced by evaporation.
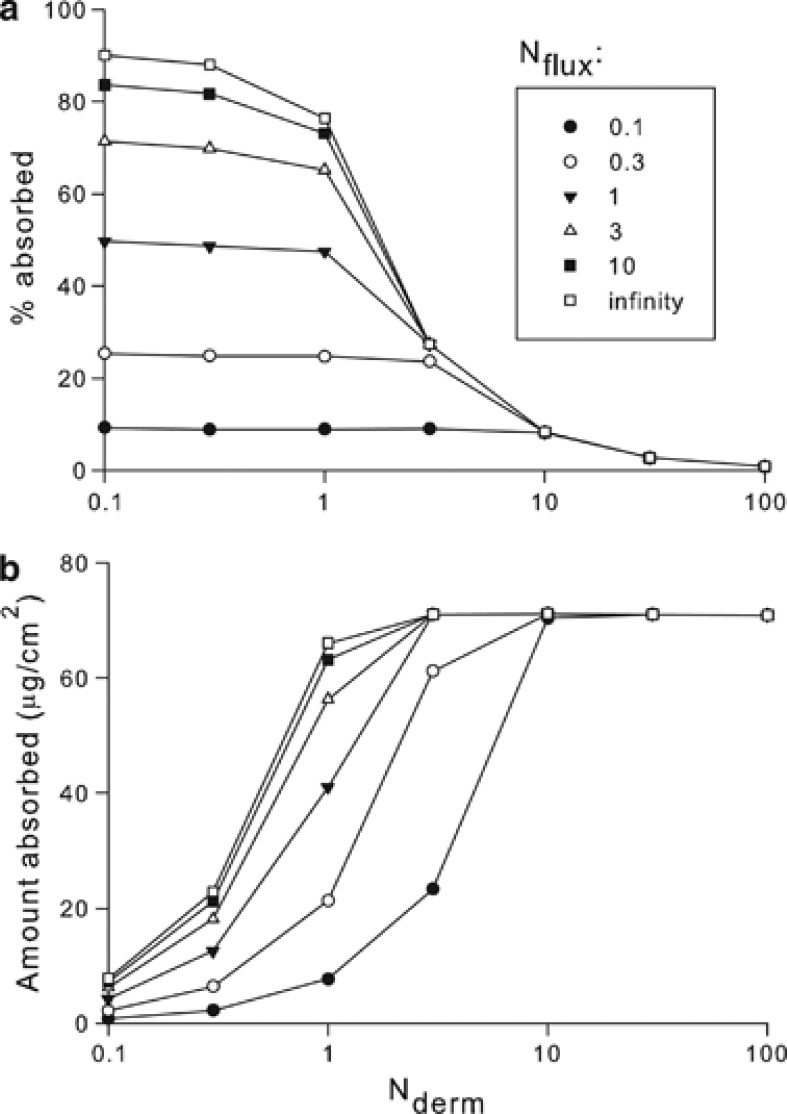
Figure 3 displays model-based predictions of the % of applied dose that is absorbed and the total amount absorbed as functions of Nderm, for a broad range of Nflux values. Calculations were made using the Finite Dose Skin Permeation Calculator,28 which solves for the disposition of an applied surface load and is based on research undertaken by Kasting's group.17, 18 These simulations are based on a hypothetical model compound for which the vapor pressure was arbitrarily varied to achieve the specified values of Nflux. Modeled maximum flux was 10.8 μg/cm2/h and lag time was 1.33 h. The vapor pressure required to achieve an Nflux of 1 was 0.48 Pa at 32 °C. Simulated experimental duration was 8 h.
Figure 3.
Model-based data showing how % absorption (a) and total absorbed amount (b) are modified by volatility of the compound for selected values of Nderm. The dimensionless flux number, Nflux, quantifies the balance between absorptive and evaporative fluxes. Dermal absorption depends both on volatility and load. Symbols represent calculated values; the lines are a guide to the eye.
For Nderm less than about 10, Figure 3 shows that percent absorbed increases with Nflux. Frasch showed29 through theoretical analysis of Kasting's finite dose model that for low loads, the total absorbed fraction of applied dose at infinite time after exposure may be estimated as:
 |
Here, f is the fractional thickness of the desquamating layer of the stratum corneum; a reasonable value for f is 0.1.18 Equation (7) may be used to estimate fractional absorption under low-load conditions.
Not only does % absorbed increase with Nflux, but so does the total amount that is absorbed (Figure 3b). For a non-volatile compound (Nflux→∞), flux limitation appears at Nderm∼1. Total absorbed amount approaches its maximum value for Nderm≥1, indicating that absorption is approaching the regime of infinite loading. Any additional loading beyond this point is excess; it does not contribute to absorption but does diminish the observed percent of load that is absorbed. For a high-volatility compound (Nflux=0.1), this flux-limited regime is not revealed until Nderm∼10 (see Figure 3).
Although the data shown in Figure 3 are model based, the models have been validated with experimental data.17, 26, 30 For any given compound, in vitro absorption data may not be accurately predicted by this family of curves, but we would expect the trends to conform to these modeled data.
Case study 2 presents an application of concepts outlined here to the evaluation of dermal toxicity and absorption data from a volatile compound. This study demonstrates the importance of considering concurrent effects of both loading and evaporation in analyzing the presented data. As suggested by the data in Figure 3, it is important to consider both Nderm and Nevap for volatile compounds. As a general approach, it may be prudent to consider evaporation for cases where Nflux ≤10; that is, where evaporation contributes 10% or more to applied load losses.

Experimental Duration Affects Measured % Absorbed
Another variable that needs to be considered in the evaluation of finite dose experiments is the experimental duration in relationship to the dermal absorption kinetics. The experimental duration is a term in the expressions for both Nderm (Eq. (2)) and Nevap (Eq. (4)), and therefore merits some discussion.
The experimental duration is generally established at the discretion of the investigator and it may be influenced by a number of considerations, including an attempt to mimic a defined occupational exposure duration, concern for tissue deterioration in an in vitro setting, and convenience. For the experimental duration to be a meaningful metric in the equations for Nderm and Nevap, its relationship to the kinetics of the absorption process is important. Consider a case where the finite dose is fully depleted, through dermal absorption, evaporation, or some combination. Any increase in experimental duration beyond this time will result in lower values of Nderm and Nevap, but this decrease is misleading because the dynamic interplay of the diffusion/evaporation process has already abated. One means of evaluating this is to compare the experimental duration with the diffusion lag time (tlag or τ) using the time number:
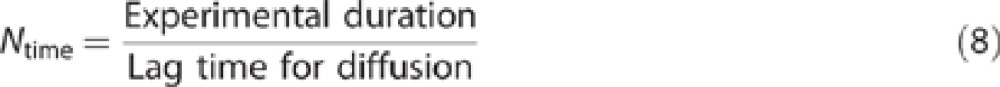 |
The lag time is generally measured from an infinite dose in vitro permeation experiment as the time axis intercept of the asymptote of the absorption curve; typical values range from several minutes to several hours. Lag time measurements for the chemical of interest may be reported in the literature or estimated from the absorption profile, if it has been presented. However, their measurement may be problematic and there could be large variance in the reported quantity. Lag time estimates predicted from physico-chemical descriptors have had limited success.
If the lag time is not reported, an evaluation of whether steady-state absorption has been achieved is possible if the entire time course of dermal absorption is available. If a plateau in the absorption profile has not been reached by the end of the experimental duration, one may infer that additional absorption is to be expected over additional time.
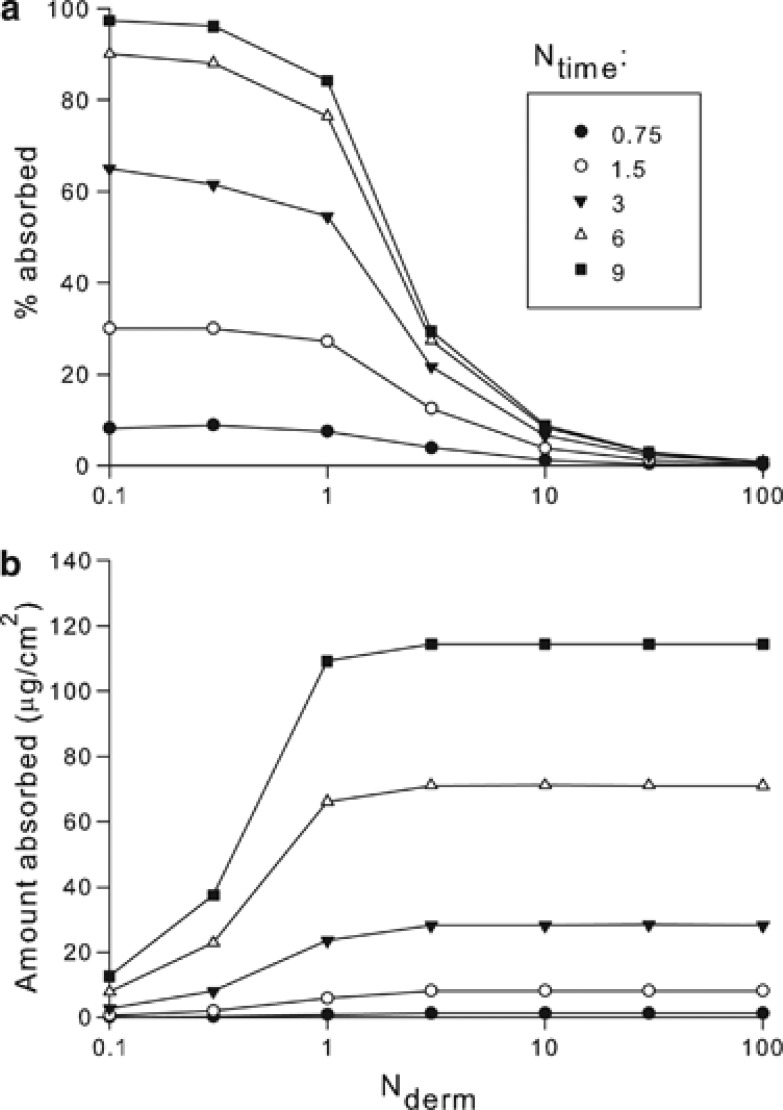
Figure 4 presents model-based data (again, using the Finite Dose Skin Permeation Calculator) for a hypothetical non-volatile compound (vapor pressure=0) that demonstrate the effect of experimental duration on absorbed amount as a function of Nderm. The same model parameters that were used to generate the data for Figure 3 were used here as well, except that experimental duration and applied dose were modified to achieve the specified values of Nderm and Ntime. For small values of Nderm, 100% absorption is possible but very long experimental durations are required. According to the data in Figure 4, experimental durations in excess of about 10 lag times would be required to approach 100% absorption. Depending on the chemical of interest, this may range from hours to days, during which time surface removal of the chemical through sweating, washing, and debridement will reduce absorption. For experimental durations approximately equal to the lag time, one can expect no more than ∼20% of the applied dose to be absorbed. For these short experimental durations, most of the applied dose resides on the skin surface and within the stratum corneum.
Figure 4.
Model-based data showing how % absorption (a) and total absorbed amount (b) are modified by the experimental duration for selected values of Nderm. The dimensionless time number, Ntime, is the ratio of experimental duration to the lag time for diffusion. For a given compound, increasing experimental duration increases the applied load amount that defines a specific Nderm, in accordance with Eq. (2). Symbols represent calculated values; the lines are a guide to the eye.
Note that for a given value of Nderm, the applied load increases with Ntime for a given chemical, in accordance with Eq. (2). It is important to keep in mind the interplay among these different parameters when evaluating finite dose absorption data. Figure 4b shows the total mass absorbed for the hypothetical model chemical. This amount increases substantially with experimental duration. For all values of Ntime, a plateau is reached at Nderm≥1, indicating flux limitation, but the height of the plateau increases for a given compound with Ntime because both total load and also the time over which absorption is measured increase with this parameter.
Absorption Continues After Removal of Load
If a load is applied to the skin's surface and later removed, chemical will continue to penetrate the skin for some time afterward, even with 100% efficiency in skin residue removal. Some chemical remains in the skin after washing. This reservoir has a higher concentration near the surface, which drives transport through the skin. For volatile compounds, evaporation through the skin surface competes with this process so that post-exposure absorption diminishes with increasing volatility. For in vitro exposures designed to mimic a specific scenario, for example, 8 h followed by wash to mimic workplace exposures, it is therefore appropriate to follow permeation beyond the exposure duration to account for absorption from the skin reservoir. This phenomenon has been studied theoretically with some experimental validation.20 The authors propose the following to estimate the total mass absorbed, per unit area of exposed skin, for a transient exposure to a non-volatile permeant:20, 35
with kp,v the permeability coefficient for the permeant in the given vehicle, Cv the concentration in that vehicle, and texp the exposure duration. Steady-state flux may be substituted for the product kp,vCv (Eq. (3)). Equation (9) gives the total absorption that occurs through the duration of the exposure period, plus that which occurs after removal of the compound from the skin surface. The equation is valid if load depletion has not diminished absorption before removal of the compound. Volatility of the applied permeant will lead to evaporative losses through the skin surface upon removal of the compound, reducing the absorbed amount. For highly volatile compounds (say, Nevap≤0.1), the following may be used:20
Equation (9) thus represents the maximum possible absorbed amount and can be used as a conservative estimate, if a reasonable estimate of τ is available.
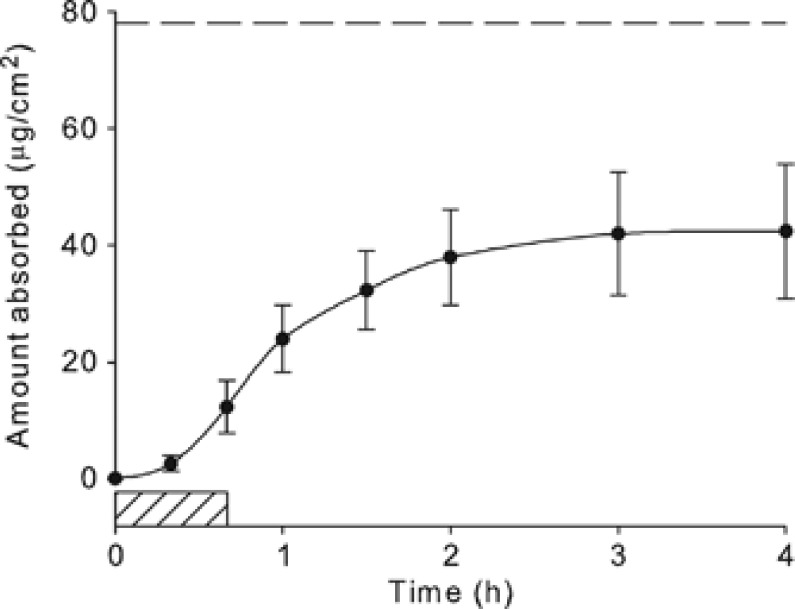
Figure 5 displays the time course of absorption of the model compound, diethyl phthalate,20 presented here to demonstrate that dermal absorption continues after removal of the load. Following a 40 min exposure (represented by the hashed box on the time axis) to dermatomed hairless guinea pig skin, the chemical was removed and the skin was thoroughly rinsed. Absorption continues for almost three more hours, and over three times more chemical was absorbed (42 μg/cm2) than had been at the time the load was removed (13 μg/cm2). The dashed line at 78 μg/cm2 represents the total mass absorbed predicted by Eq. (9) using independently measured kp and τ for this non-volatile compound. Equation (9) overestimates the measured quantity by about a factor of 2 in this case. Binding of the chemical to skin components may explain the discrepancy, and Eq. (9) may thus represent a conservative estimate as there is some question as to whether this bound fraction is potentially bioavailable.
Figure 5.
Time course of in vitro dermal absorption of diethyl phthalate. Following a 40-min exposure (hashed box on time axis), the chemical was removed and skin was thoroughly rinsed. Absorption continues well after the exposure duration. Mass accumulation at 4 h (42 μg/cm2) was over three times more than that at 40 min (13 μg/cm2). Dashed line at 78 μg/cm2 represents the total absorbed amount predicted by Eq. (9).
SUMMARY AND CONCLUSIONS
Finite dose skin permeation experiments serve to model “real world” exposures and thus provide important information that can be used in evaluating the risk associated with dermal exposures. However, care must be taken in analyzing and interpreting results from such experiments. This manuscript has described four limitations to the fixed fractional absorption paradigm. Our analyses of the first two pose strategies for evaluating whether loading effects or evaporation effects might be expected, and if so they alert to the possibility that the reported values of fractional absorption require consideration. The analyses outlined in the case studies are exemplary of strategies to approach the evaluation of finite dose absorption data. These studies suggest an informative strategy for the evaluation of the dermal absorption potential of a chemical based on careful assessment of the dermal number, flux number, evaporation number, time number, and auxiliary equations as presented here. We propose that an evaluation of finite dose dermal absorption data consider the following questions.
Is the Load or Range of Loads Investigated Appropriate?
The fraction or percent of applied dose that is absorbed from finite dose experiments depends on the amount of chemical that is initially loaded onto the skin surface. The dependence is so strong that it is conceivable that the absorbed amount can range from 0 to 100% for a given chemical. Experimental observations derived at large Nderm (Eq. (2)) may under represent the fractional absorption that would be expected at realistic exposures.
Thus, a single, fixed fractional absorption factor may be inadequate to indicate the true extent of systemic absorption. Consideration of the dimensionless dermal number Nderm can guide the risk assessment of finite dose exposure data. Low values of Nderm are likely to be representative of exposures that can be expected in occupational and environmental settings. Therefore, absorption from experiments at low Nderm can provide important information regarding the risk for systemic uptake. High values of Nderm place the experiment in the flux-limited regime, where excess loading results in low percent absorption that is not representative of the absorption potential of the applied chemical. On the other hand, this flux limited, “infinite dose” regime enables the measurement of steady-state flux, permeability, and lag time and in our proposed strategy of evaluation its significance is not overlooked.
Does Evaporation Play a Significant Role?
For volatile chemicals, the complication of the competing interplay between absorption and evaporation must be considered. Evaporation will diminish the applied load such that less permeant is available for absorption. The dimensionless evaporation number Nevap (Eq. (4)) and related tevap (Eq. (6)) may be used to evaluate whether load depletion through evaporation is significant. Additionally, the flux number Nflux (Eq. (5)) quantifies the balance between evaporation and absorption, and fractional or percent absorption may be estimated based on the knowledge of this parameter using Eq. (7). For highly volatile chemicals, the percent of applied dose that is absorbed may not achieve a significant level, even for small applied loads. Assessment of the risk of dermal exposure should then consider the types of exposures that are likely in the occupational or environmental setting. Exposures to unoccluded splashes may not lead to significant systemic uptake, but submersion or occluded exposures may contribute to a greater uptake, and thus are better characterized by considering the maximum flux obtainable for the given chemical.
Is the Experimental Duration Appropriate?
Dermal absorption from a given load of a permeant with a given volatility depends on the kinetics of the absorption and evaporation processes. Therefore, the exposure duration is an essential consideration in the evaluation of finite dose exposures. Exposure duration may be established at the discretion of the investigator or may be prescribed for regulatory submission but in either cases it is a significant variable in the measured absorption. The dimensionless time number Ntime (Eq. (8)) can be used to help evaluate results from finite dose exposures in terms of evaluating the impact of experimental duration.
Has the Study Considered the Absorption that Occurs Following the Exposure time?
We have proposed algebraic expressions to estimate the total absorption of a non-volatile (Eq. (9)) or volatile (Eq. (10)) chemical for a given exposure duration or contact time, as long as depletion of load has not significantly limited absorption over the time course of exposure. These equations may be used in the evaluation of experiments in which the load is removed after a given time by washing. The investigator may continue to measure absorption following removal, or removal may represent the end point of the experiment. In the latter case, Eqs. (9) and (10) address the question of whether significant absorption may be expected after the exposure time. As demonstrated in Figure 5, post exposure absorption can be substantial.
For investigators undertaking finite dose experiments, the preceding analysis suggests strategies for the design of experiments that would enhance the utility of the reported results. The entire absorption profile should be reported, rather than simply any specified end point. The kinetics of the absorption process provide important information, for example, whether a plateau in absorption has been reached or if additional absorption would be expected over additional time. It would also be beneficial if a range of loads were applied to span a broad range of Nderm. This provides additional information for evaluating the absorption potential of the chemical. Although there is an increasing emphasis on exposure periods and donor conditions that reflect anticipated occupational exposures,36 the role of infinite dose exposure data remains important. These provide the most reliable measurements of lag time and steady-state flux, which not only can be used to predict absorption kinetics from arbitrary loads, but also provide means of evaluating and interpreting finite dose absorption data as outlined herein.
The analysis presented in this paper provides a strategy for the evaluation of dermal absorption data that acknowledges the important contribution of finite dose dermal absorption experiments, and addresses the limitations of a dermal risk assessment strategy that relies on the single, fixed fractional absorption paradigm. The application of this strategy should serve to better characterize the dermal absorption of industrial and environmental chemicals.
Glossary
- Absorption
the process of chemical transport from the outer surface of skin and into the receptor compartment in an in vitro experiment, or into the systemic circulation from an in vivo exposure
- Delivery limited
a condition where dermal absorption is limited by the supply of chemical applied to the surface (dose). Peak flux will be diminished from its highest value attainable from a given donor. Compare with flux limited
- Dermal absorbed dose
the total amount of chemical that is absorbed (for in vitro experiments, mass/area; in an in vivo setting, units are consistent with exposure units)
- Donor
the substance applied to the skin surface. It may consist of a solution or mixture containing the chemical of interest or the neat (undiluted) chemical
- Dose
related to the amount of chemical applied to skin, dose is used more loosely than load, and may refer to either an amount (mass/area) of chemical or a specified volume of a concentration (mass/volume) of chemical
- Exposure
the mass of chemical in contact with skin, typically normalized by time (mass/time) and possibly body mass (mass/mass/time) or exposed area (mass/area/time) of the organism
- Exposure duration
the amount of time the chemical is in contact with the skin
- Experimental duration
the amount of time that absorption is measured in an experiment. It may be longer than the exposure duration
- Finite dose
a defined, limited dose
- Flux
the rate of mass accumulation per area of exposed surface (mass/area/time)
- Flux limited
a condition where dermal absorption is limited only by the peak steady-state flux attainable from a given donor. Compare with delivery limited
- Fractional absorption
the fraction of applied dose that is absorbed, calculated as the dermal absorbed dose divided by the applied load. It may equivalently be expressed as percent absorption
- Infinite dose
an unlimited dose; in practice one where the applied dose is minimally depleted through absorption or evaporation
- Lag time
a function of the finite time it takes for a chemical to permeate the skin, it is typically calculated as the time-axis intercept of the asymptote of the steady-state absorption curve (time)
- Load
amount of chemical that is in contact with the skin (mass/area)
- Maximum flux
the highest flux attainable from a given chemical, most reliably measured as the slope of the steady-state absorption curve using an infinite dose of neat chemical
- Peak flux
the highest flux attainable from a given dose
- Steady-state flux
the equilibrium flux that is achieved from an infinite dose
- Systemic uptake
the quantity of chemical that enters the systemic circulation from a given skin exposure (units are consistent with exposure units)
- Vehicle
the solvent or agent mixed with the target chemical in contact with the skin surface
APPENDIX
Estimation of Evaporation Flux
The US Environmental Protection Agency24 suggests the use of the following equation (their Eq. (D-1)) to estimate evaporation from the surface of a pool of neat liquid, at or near the ambient temperature, for risk management of chemical spills. It may also be used to estimate maximum evaporation flux from the donor compartment of a diffusion cell,25 or from the skin surface in an in vivo experiment:
 |
where Jevap is evaporation flux (μg/cm2/h), Pvap is the vapor pressure of the chemical at the ambient temperature (mm Hg), MW is molecular weight, u is wind speed above the liquid surface (m/s), and T is liquid temperature (°C). Typical values of u for indoor air range from 0.1 to 0.5 m/s.
Vapor pressure depends on temperature. If it is known at one temperature (T1), it can be estimated at a different temperature (T2) using a form of the Clausius–Clapeyron equation:37
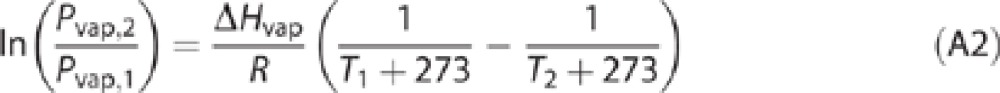 |
where ΔHvap is the molar enthalpy of vaporization (J/mol) and R is the gas constant (8.314 J/mol/K); temperatures T1 and T2 are in °C. If no experimental data on vapor pressure are available, a calculated value may be used, for example, using Episuite.38
In Eq. (A1), Jevap represents the maximum evaporation flux of the neat chemical. For diffusion cell experiments using diluted compound, when a chemical is present at a concentration (Cv) less than the saturation limit in the same vehicle (Sv), the evaporation flux will be less than the maximum flux by an amount that often is proportional to the saturation ratio (SR), defined as:
 |
and the evaporation flux under this subsaturated condition may be estimated:
Equations (A3) and (A4) are appropriate for solutions in which thermodynamic activity is proportional to concentration. Although this approximation is often a good one, significant departures from this behavior are possible.39
The authors declare no conflict of interest.
Footnotes
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Sahmel J, Boeniger M.Appendix II: Dermal exposure assessmentsIn: Bullock WH, Ignacio JS, (eds). A Strategy for Assessing and Managing Occupational Exposures3rd ednAmerican Industrial Hygiene Association: Alexandria; 2006305–333. [Google Scholar]
- Standard Operating Procedures for Residential Exposure Assessments. Office of Pesticide Programs, US Environmental Protection Agency2012 . http://www.epa.gov/pesticides/science/EPA-OPP-HED_Residential%20SOPS_Feb2012.pdf . Accessed on 20 August 2012.
- Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final. Office of Superfund Remediation and Technology Innovation. EPA/540/R/99/005.US Environmental Protection Agency: Washington2004 . http://www.epa.gov/oswer/riskassessment/ragse/pdf/part_e_final_revision_10-03-07.pdf . Accesssed on 20 August 2012.
- Standard Operating Procedures (SOPs) for Residential Exposure Assessments. Office of Prevention, Pesticides, and toxic Substances, US Environmental Protection Agency1997 . http://www.epa.gov/oppfead1/trac/science/trac6a05.pdf . Accessed on 20 August 2012.
- Derivation of Health-based Environmental Screening Levels for Chemical Warfare Agents . A Technical Evaluation. US Army Center for Health Promotion and Preventive Medicine US Army CHPPM: Aberdeen Proving Ground, MD; 1999. [Google Scholar]
- Reference Document 230: Methodology for Determining Chemical exposure Guidelines for Deployed Military Personnel. US Army Public Health Command (Provisional)2010 . http://phc.amedd.army.mil/PHC%20Resource%20Library/RD230%20June%202010%20Revision%20_EntiretyCorrected.pdf . Accessed on 20 August 2012.
- Watson A, Dolislager F, Hall L, Raber E, Hauschild VD, Love AH. Developing health-based pre-planning clearance goals for airport remediation following a chemical terrorist attack: decision criteria for multipathway exposure routes. Hum Ecol Risk Assess. 2011;17:57–121. doi: 10.1080/10807039.2010.534722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current Intelligence Bulletin 61: A Strategy for Assigning New NIOSH Skin Notations. National Institute for Occupational Safety and Health, DHHS Publication 2009-1472009 . http://www.cdc.gov/niosh/docs/2009-147/ . Accessed on 13 December 2011.
- Technical Guidance Document on Risk Assessment, Part I. Europan Commission Joint Research Centre2003 . http://ihcp.jrc.ec.europa.eu/our_activities/public-health/risk_assessment_of_Biocides/doc/tgd/tgdpart1_2ed.pdf . Accessed on 20 August 2012.
- Guidance Document on Dermal Absorption European Commission. Health and Consumer Protection Directorate-General. SANCO/222/2000 rev. 72004 . http://ec.europa.eu/food/plant/protection/evaluation/guidance/wrkdoc20_rev_en.pdf . Accessed on 20 August 2012.
- Reddy MB, Bunge AL.Dermal absorption from pesticide residues: data analysisIn: Kruse J, Verhaar H, de Raat WK, (eds). The Practical Applicability of Toxicokinetic Models in the Risk Assessment of Chemicals Kluwer Academic Press: Dordrecht; 200255–79. [Google Scholar]
- Buist HE, Schaafsma G, van de Sandt JJM. Relative absorption and dermal loading of chemical substances: consequences for risk assessment. Regul Toxicol Pharmacol. 2009;54:221–228. doi: 10.1016/j.yrtph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Kissel JC. The mismeasure of dermal absorption. J Expo Sci Environ Epidemiol. 2011;21:302–309. doi: 10.1038/jes.2010.22. [DOI] [PubMed] [Google Scholar]
- Health Effects Test Guidelines. OPPTS 870.7600 Dermal Penetration. EPA 712-C-98-350. Office of Prevention, Pesticides and Toxic Substances, US Environmental Protection Agency, Washington, DC,1998
- Selzer D, Abdel-Mottaleb MMA, Hahn T, Schaefer UF, Neumann D. Finite and infinite dosing: difficulties in measurements, evaluations and predictions. Adv Drug Deliv Rev. 2013;65:278–294. doi: 10.1016/j.addr.2012.06.010. [DOI] [PubMed] [Google Scholar]
- OECD guideline for the testing of chemicals # 428. Skin absorption: in vitro methods. Paris, Organization for Economic Cooperation and Development2004 . http://www.oecd-ilibrary.org/environment/test-no-428-skin-absorption-in-vitro-method_9789264071087-en . Accessed on 13 December 2011.
- Kasting GB. Kinetics of finite dose absorption through skin 1. vanillylnonanamide. J Pharm Sci. 2001;90:202–212. doi: 10.1002/1520-6017(200102)90:2<202::aid-jps11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kasting GB, Miller MA. Kinetics of finite dose absorption through skin 2: volatile compounds. J Pharm Sci. 2006;95:268–280. doi: 10.1002/jps.20497. [DOI] [PubMed] [Google Scholar]
- Anissimov YG, Roberts MS. Diffusion modeling of percutaneous absorption kinetics: 2. Finite vehicle volume and solvent deposited solids. J Pharm Sci. 2001;90:504–520. doi: 10.1002/1520-6017(200104)90:4<504::aid-jps1008>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Frasch HF, Barbero AM. The transient dermal exposure: theory and experimental examples using skin and silicone membranes. J Pharm Sci. 2008;97:1578–1592. doi: 10.1002/jps.21035. [DOI] [PubMed] [Google Scholar]
- European Union Risk Assessment Report: Diphenyl Ether, Pentabromo derivative (Pentabromodiphenyl ether), CAS No. 32534-81-9; EINECS No. 251-084-2. European Chemicals Bureau, Office for Official Publications of the European Communities, Ispra, Italy,2001
- Wester RC, Bucks DA, Maibach HI, Anderson J. Polychlorinated biphenyls (PCBs): dermal absorption, systemic elimination, and dermal wash efficiency. J Toxicol Environ Health. 1983;12:511–519. doi: 10.1080/15287398309530445. [DOI] [PubMed] [Google Scholar]
- Roper CS, Simpson AG, Madden S, Serex TL, Biesemeier JA. Absorption of [14C]-tetrabromodiphenyl ether (TeBDE) through human and rat skin in vitro. Drug Chem Toxicol. 2006;29:289–301. doi: 10.1080/01480540600652954. [DOI] [PubMed] [Google Scholar]
- Risk Management Program Guidance for Offsite Consequence Analysis. EPA-550-B-99-009.US Environmental Protection Agency: Washington1999 . http://www.epa.gov/region6//6sf/pdffiles/rmp_offsite_guidance.pdf . Accessed 12/13/11.
- N'Dri-Stempfer B, Bunge AL.How much can evaporation of dermally absorbed chemical reduce the systemically absorbed dose Proceedings of the Occupational and Environmental Exposures of Skin to ChemicalsStockholm, Sweden, 12–15 June2005. P81
- Chaudhuri SR, Gajjar RM, Krantz WB, Kasting GB. Percutaneous absorption of volatile solvents following transient liquid exposures II. ethanol. Chem Eng Sci. 2009;64:1665–1672. [Google Scholar]
- Frasch HF, Dotson GS, Barbero AM. In vitro human epidermal penetration of 1-bromopropane. J Toxicol Environ Health A. 2011;74:1249–1260. doi: 10.1080/15287394.2011.595666. [DOI] [PubMed] [Google Scholar]
- NIOSH. Finite Dose Skin Permeation Calculator. National Institute for Occupational Safety and Health2011 . http://www.cdc.gov/niosh/topics/skin/finiteSkinPermCalc.html . Accessed on 13 December 2011.
- Frasch HF. Dermal absorption of finite doses of volatile compounds. J Pharm Sci. 2012;101:2616–2619. doi: 10.1002/jps.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Miller MA, Kasting GB. Absorption and evaporation of N,N-diethyl-m-toluamide from human skin in vitro. Toxicol Appl Pharmacol. 2005;204:81–90. doi: 10.1016/j.taap.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Elf Atochem. Acute dermal toxicity in rats. n-propyl bromide. CIT/Study No. 13113 TAR Paris, Elf Atochem. Available in public docket A-91-42, US Environmental Protection Agency: Washington, DC,1998
- ACGIH 1-Bromopropane: TLV Chemical Substances Documentation7th ednAmerican Conference of Governmental Industrial Hygienists: Cincinnati; 2005 [Google Scholar]
- 1-Bromopropane. Hazardous Substances Data Bank. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~UfReWG:1 . Accessed on 13 December 2011.
- Abboud J-LM, Notario R. Critical compilation of scales of solvent parameters. Part I. Pure, non-hydrogen bond donor solvents. Pure Appl Chem. 1999;71:645–718. [Google Scholar]
- Cleek RL, Bunge AL. A new method for estimating dermal absorption from chemical exposure. 1. General approach. Pharm Res. 1993;10:497–506. doi: 10.1023/a:1018981515480. [DOI] [PubMed] [Google Scholar]
- Guidance Document for the Conduct of Skin Absorption Studies. OECD Environemtnal Health and Safety Publications Series on Testing and Assessment Number 28. Paris, Organization for Economic Co-operation and Development2004 . http://www.oecd-ilibrary.org/environment/guidance-document-for-the-conduct-of-skin-absorption-studies_9789264078796-en . Accessed on 13 December 2011.
- Smith EB.Basic Chemical Thermodynamics5th ednImperial College Press: London; 200445–48. [Google Scholar]
- Estimation Programs Interface Suite for Microsoft Windows, Vers. 4.11. US Environmental Protection Agency: Washington, DC2013
- Bunge AL, Persichetti JM, Payan JP. Explaining skin permeation of 2-butoxyethanol from neat and aqueous solutions. Int J Pharm. 2012;435:50–62. doi: 10.1016/j.ijpharm.2012.01.058. [DOI] [PubMed] [Google Scholar]