Abstract
An N-butyl analog of benztropine, JHW007 [N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane], binds to dopamine transporters (DAT) but has reduced cocaine-like behavioral effects and antagonizes various effects of cocaine. The present study further examined mechanisms underlying these effects. Cocaine dose-dependently increased locomotion, whereas JHW007 was minimally effective but increased activity 24 hours after injection. JHW007 (3–10 mg/kg) dose-dependently and fully antagonized the locomotor-stimulant effects of cocaine (5–60 mg/kg), whereas N-methyl and N-allyl analogs and the dopamine (DA) uptake inhibitor GBR12909 [1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride] stimulated activity and failed to antagonize effects of cocaine. JHW007 also blocked the locomotor-stimulant effects of the DAT inhibitor GBR12909 but not stimulation produced by the δ-opioid agonist SNC 80 [4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide], which increases activity through nondopaminergic mechanisms. JHW007 blocked locomotor-stimulant effects of cocaine in both DA D2- and CB1-receptor knockout and wild-type mice, indicating a lack of involvement of these targets. Furthermore, JHW007 blocked effects of cocaine on stereotyped rearing but enhanced stereotyped sniffing, suggesting that interference with locomotion by enhanced stereotypies is not responsible for the cocaine-antagonist effects of JHW007. Time-course data indicate that administration of JHW007 antagonized the locomotor-stimulant effects of cocaine within 10 minutes of injection, whereas occupancy at the DAT, as determined in vivo, did not reach a maximum until 4.5 hours after injection. The σ1-receptor antagonist BD 1008 [N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide] blocked the locomotor-stimulant effects of cocaine. Overall, these findings suggest that JHW007 has cocaine-antagonist effects that are deviate from its DAT occupancy and that some other mechanism, possibly σ-receptor antagonist activity, may contribute to the cocaine-antagonist effect of JHW007 and like drugs.
Introduction
Evidence suggests that “atypical” dopamine (DA) uptake inhibitors, compounds that bind to the dopamine transporter (DAT) but lack cocaine-like effects, may prove useful in searching for stimulant-abuse medicines (Tanda et al., 2009a). For example, Desai et al. (2005a) showed that JHW007, a DAT inhibitor and N-butyl analog of benztropine (BZT), antagonized locomotor-stimulant effects of cocaine, without producing stimulant activity. Furthermore, several N-substituted BZT analogs had reduced psychostimulant-like effects and antagonized effects of cocaine and methamphetamine on locomotor activity, self-administration, place conditioning (Katz et al., 2004; Li et al., 2005; Hiranita et al., 2009, 2013; Velazquez-Sanchez et al., 2009; 2010), and DA efflux in the nucleus accumbens (Tanda et al., 2009a,b). Together, the results suggest therapeutic potential and further studies to assess mechanisms for these atypical effects.
Several authors have suggested that drug-abuse medicines would benefit from slow onsets of action—to decrease abuse liability—and long durations of action (Mello and Negus, 1996; Vocci et al., 2005). Studies of in vivo receptor occupancy, microdialysis, and association kinetics show that compared with standard DAT inhibitors (cocaine, WIN35,428), BZT analogs, including JHW007, have slower DAT-association rates with long durations of action (Raje et al., 2003; Desai et al., 2005a,b; Tanda et al., 2009a,b; Kopajtic et al., 2010). Slow onsets of effects may reduce cocaine-like activity (Desai et al., 2005a,b; Tanda et al., 2009a, 2013). Additionally, in contrast to standard DAT inhibitors, BZT analogs may shift DAT conformational equilibrium toward one that is open to the cytosol (Loland et al., 2008), which may be related to distinctive binding kinetics and atypical DAT inhibitory effects.
BZT analogs alone or with cocaine may also induce DAT-mediated stereotypic behaviors that interfere with the expression of cocaine-like stimulant effects. A recent study in rats showed that stereotypies induced by BZT analogs contribute little to their antagonism of cocaine self-administration (Li et al., 2013). The present study assessed the possibility that stereotypies induced by JHW007 compete with cocaine-induced locomotion, producing an apparent antagonism of cocaine.
Kopajtic et al. (2010) compared the binding profile of [3H]JHW007 to that of the cocaine analog [3H]WIN 35428. In contrast to the cocaine analog, the saturation isotherm for [3H]JHW007 binding in striatum from rat and mouse was better fit to a two-site than a one-site model. Conversely, the binding in membranes from N2A neuroblastoma cells transfected with human DAT was best fit with a one-site model. Further, multiple-site binding of JHW007 was found in striatum from DAT knockout (KO) mice (Kopajtic et al., 2010). BZT analogs have affinity for other targets which may contribute to multiple-site binding and atypical DAT effects. The most prominent of these off-target sites include muscarinic M1 and histamine H1 receptors, sites for which the parent compound, BZT, has affinity. These sites, however, appear to have no substantive influence on the behavioral effects of BZT analogs (Tanda et al., 2009a).
To assess the contribution of off-target sites to JHW007 effects, sites were selected based on previous receptor screens (Katz et al., 2004; Li et al., 2011), which indicated affinities of several BZT analogs for DA D2 and sigma receptors (σRs). Additionally, a potential role of cannabinoid (CB1) receptors was proposed by Navarro et al. (2009). Screening of cocaine analogs through functional assays identified RTI-371 as a positive allosteric modulator of CB1 receptors. Because this compound had effects like JHW007, both were studied for effects on the efficacy of the CB1 agonist CP55,940 in stimulating calcium mobilization. Both compounds increased the efficacy of the CB1 agonist but were inactive as modulators of the efficacy of the mu-opioid agonist DAMGO. As a result, the authors proposed that atypical effects of RTI-371 and JHW007 were due at least in part to positive modulation of CB1 receptors.
The present studies compared JHW007 with other BZT analogs and a selective DA uptake inhibitor (GBR12909) to assess whether the antagonism by JHW007 was unique. To assess the role of the DAT, the antagonism by JHW007 of stimulation via DAT actions and nondopaminergic mechanisms was examined. Because the cocaine-antagonist effects of JHW007 may involve its slow onset (Desai et al., 2005a; Tanda et al., 2009b), its interaction with cocaine over time was compared with its in vivo DAT occupancy. In other experiments, the contributions of DA D2, CB1, and sigma-receptor mechanisms to the effectiveness of JHW007 in blocking locomotor-stimulant effects of cocaine were assessed using genetic and pharmacological approaches. Finally, interactions of cocaine with BZT analogs on stereotypic behaviors assessed the contribution of stereotypic behaviors to the cocaine-antagonist effects of JHW007.
Materials and Methods
Animals.
Male Swiss Webster mice (Taconic Farms, Germantown, NY) weighing 25–40 g at the time of testing were used. In addition, for some studies, DA D2 receptor and CB1 receptor KO and wild-type (WT) male mice were used. One of the CB1 WT subjects was female. All mutant mice had a C57BL/6J genetic background and were bred within the National Institute on Drug Abuse Intramural Research Program. The DA D2R mutants were descendants of mating pairs provided by the Department of Physiology and Pharmacology, Oregon Health and Science University, and the CB1 mutants from Dr. Andreas Zimmer of the National Institute of Mental Health (Bethesda, MD).
Subjects were housed in groups of four in plastic cages with pine sawdust bedding. Food and water were continuously available, except during testing. All subjects were kept in a colony room maintained at 21 ± 2°C under a 12-hour light/dark cycle (lights on 7:00 AM). All experiments were conducted during the light phase of the light/dark cycle between 8:00 AM and 3:00 PM. Subjects used in this study were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Animal care procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP).
In Vivo Displacement of [125I]RTI-121.
Each subject received an intravenous injection of 2 μCi of [125I]RTI-121 and was sacrificed by cervical dislocation 2 hours later. Displacement of [125I]RTI-121 by JHW007 was assessed with intraperitoneal injection of JHW007 (3–17 mg/kg), or vehicle was administered at various doses and times relative to sacrifice according to methods of Scheffel et al. (1989). Displacement was examined at 8, 16, 24, and 48 hours after 3.0, 10.0, and 17.0 mg/kg JHW007. Each point was determined in sets of four mice. Whole brains were rapidly removed and striatum and cerebellum were dissected on ice. Each dissected brain region was placed into a separate plastic vial (Rohren Tubes, 55 × 12 mm), weighed, and tissue radioactivity measured using an automated gamma counter (Micromedic Systems, 10/600 PLUS; MP Biomedicals, LLC, Santa Ana, CA).
For the analysis of in vivo binding data, regional radioactivity levels were divided by weight (gram) of the tissue (CPM/tissue weight). Specific binding was calculated as CPM/tissue weight in striatum divided by cerebellum minus 1 (S/C – 1), which is based on the observation that dopaminergic transporter sites are highly concentrated in the striatum and relatively absent in the cerebellum (Scheffel et al., 1991). These values were expressed as a percentage of specific binding after vehicle injection. Data were analyzed using two-way analysis of variance (ANOVA), and a post-hoc Tukey’s test was used to determine significance of effects for individual doses at different time periods. Data for the effects of JHW007 from 5 minutes to 4.5 hours after injection (n = 5–13/time point) have been included from a previous report (Desai et al., 2005a), a full depiction of the time course of JHW007 binding in vivo.
Locomotor Activity.
For the assessment of horizontal locomotor activity (ambulation), mice were transferred from the housing room to the laboratory in their housing cages and allowed to acclimate for approximately 30 minutes. The subjects were then individually transferred to clear acrylic experimental chambers (40 cm3) equipped with light sensitive detectors, spaced 2.5 cm apart along two perpendicular adjoining walls (Omnitech Electronics, Columbus, OH). Infrared light sources were mounted on the opposing walls and directed at the detectors. One horizontal activity count was recorded with each interruption of a single light beam.
For studies of drugs in combination with cocaine, JHW007 (3 or 10 mg/kg), AHN2-005 (3 or 10 mg/kg), AHN1-055 (3 or 10 mg/kg), GBR 12909 (10 or 30 mg/kg), or vehicle were injected immediately before subjects were placed in the experimental chamber and saline or cocaine (5–60 mg/kg) was administered 4.5 (JHW007), 3.5 (AHN2-005), 2.5 (AHN1-055), or 2 (GBR 12909) hours later; the times for the N-substituted BZT analogs and GBR 12909 are based on maximum in vivo DAT occupancy (Desai et al., 2005a,b). The antagonism of locomotor-stimulant effects of GBR 12909 (3–56 mg/kg) and SNC 80 (1–30 mg/kg) were examined by injecting JHW007 (3 or 10 mg/kg) or vehicle 4.5 hours before administration of each of these drugs. In experiments assessing the effects of 10 mg/kg JHW007 in combination with cocaine over time, JHW007 or vehicle was injected immediately before subjects were placed in the experimental chamber, and cocaine (5–60 mg/kg, or saline) was administered 10 minutes, 1, 2, 4.5, 8, or 24 hours later.
The effects of JHW007 (10 mg/kg) or vehicle pretreatment was also studied according to two additional procedures. With one procedure, the subjects were transferred to the laboratory as described above in their housing cages (HC), allowed to acclimate for 30 minutes, injected, and returned to their housing cage, which was now in the laboratory. At 2 or 24 hours later, the subjects were injected with different doses of cocaine (5–60 mg/kg) or vehicle and placed in the experimental chamber for the recording of locomotor activity. With a second procedure, the subjects were injected in their home cages in the housing room (HR) and transferred to the laboratory 23.5 hours later. They were allowed to acclimate for 30 minutes, then injected with different doses (5–60 mg/kg) of cocaine (i.e., at 24 hours) or vehicle and placed in the experimental chamber for the recording of locomotor activity.
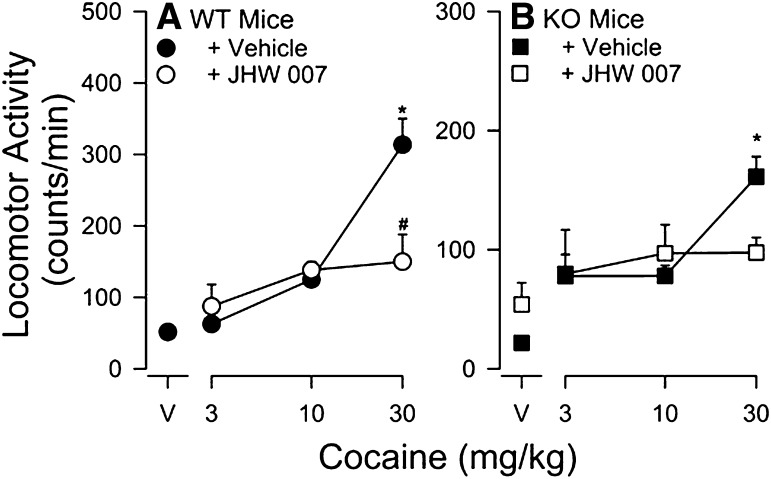
Finally, the effects of pretreatment (4.5 hours) with JHW007 (10 mg/kg) before administration of cocaine (3–30 mg/kg) in DA D2-receptor KO and WT mice and prior to injections of 20 mg/kg cocaine or vehicle in cannabinoid CB1-receptor KO and WT mice was also examined. In all studies, activity was monitored for a period of 90 minutes after the second injection. Total activity count data was collected every 10 minutes. Mice were used only once, and each treatment condition was studied in at least six to eight mice.
Data are shown as horizontal locomotor activity counts per minute over a 90-minute period following the second injection. All results are presented as group means (±S.E.M.). Data were analyzed using a two-way analysis of variance (ANOVA) with pretreatment drug (i.e., JHW007) and test drug (i.e., cocaine) as factors. Data from the DA D2 and CB1 receptor WT and KO mice were analyzed using a three-way ANOVA with pretreatment drug dose, cocaine dose, and genotype as factors. Post-hoc Tukey’s tests were used to determine significance of effects of individual doses or pretreatments with vehicle. Data were considered to be significant when P < 0.05. Data for the antagonism of cocaine’s effects by JHW007 (10 mg/kg) and AHN2-005 (10 mg/kg) are those from a preliminary report (Desai et al., 2005a) and are included in this report for comparison with the other doses and other compounds.
Stereotypy Measures.
The effects of cocaine (5–60 mg/kg) alone and in combination with JHW007 (3 or 10 mg/kg), AHN2-005 (3 or 10 mg/kg), AHN1-055 (3 or 10 mg/kg), and GBR 12909 (10 or 30 mg/kg) on the frequencies of rearing, sniffing, and grooming were measured. Behavioral observations began 10 minutes after administration of cocaine or saline using a multiple-subject, time-sampling procedure (Desai and Terry, 2003). In brief, behaviors were scored in groups of eight subjects. Within a group each subject was observed over nine periods of 1-minute each, starting at an interval of 10 minutes after injections. Subjects in the group were observed in turn during the ensuing 8 minutes. After a 2-minute rest period the observations began again until each subject was observed nine times. In each 1-minute sampling period, behavior was checked as either present or absent every 5 seconds (yielding a maximum of 12 positives per 1-minute observation period). Hence, for each mouse the maximum number of occurrences of any behavior was 108 for the 90-minute observation period. The behaviors scored included rearing (body in a vertical or near-vertical plane with front paws off the floor), sniffing (multiple rhythmic movements of the snout directed at the chamber floor, walls, or air), and grooming (strokes of the forepaws along face, scratching with hind limbs, anogenital licking, and tail nibbling), as adapted from Desai and Terry (2003). No other forms of grossly observable behaviors were observed with any substantial frequency at any of the doses tested. Furthermore, no significant interaction effects between combinations of cocaine and BZT analogs or GBR 12909 were observed on grooming behavior and consequently these data are not shown.
Drugs.
Drugs used in the present studies were: (–)-cocaine HCl (Sigma-Aldrich, St. Louis, MO), GBR 12909 [1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine diHCl; Sigma-Aldrich], BD 1008 di-HBr (Tocris Bioscience, Ballwin, MO), SNC 80 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide; Tocris Cookson Inc., Ellisville, MO], and several N-substituted analogs of BZT (Agoston et al., 1997). Cocaine was prepared fresh daily in 0.9% NaCl. GBR 12909 and all of the BZT analogs examined were dissolved in sterile water with sonication and heating. SNC 80 was dissolved in 8% 1 M HCl solution (Jutkiewicz et al., 2004). All drugs were administered by the intraperitoneal route on a milligram per kilogram body weight basis in a volume of 1 ml/0.1 kg. “Drugs alone” nominally refers to two injections: the reference drug with an appropriate vehicle control injection at the appropriate time before the second injection.
The radioligand used for in vivo displacement studies was [125I]RTI-121 (specific activity 2200 Ci/mmol; PerkinElmer Life and Analytical Sciences, Boston, MA; Lever et al., 1996), which has a high selectivity and a high affinity for the DAT over other monoamine transporters. Furthermore, nonmetabolized radioligand represents 85% of the signal observed (Lever et al., 1996), and the radioligand has been used to assess occupancy at the DAT by ligands that interact both rapidly and slowly with the DAT (e.g., Desai et al., 2005a; Scheffel et al., 1992; Lever et al., 1996).
Results
In Vivo Displacement of [125I]RTI-121.
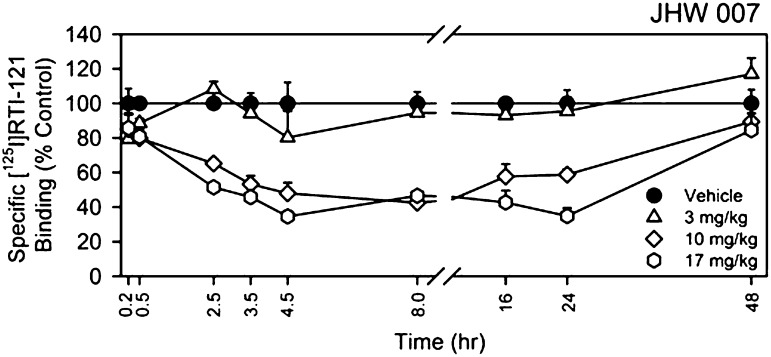
JHW007 displaced [125I]RTI-121 in a dose- and time-dependent manner (Fig. 1). The ANOVA indicated that dose (F3,224 = 78.9, P < 0.05) and time (F8,224 = 9.60, P < 0.05) and their interaction (F24,224 = 3.73, P < 0.05) were significant. At 3.0 mg/kg, there was displacement of approximately 20% of bound [125I]RTI-121 at 5 minutes and 4.5 hours, and no significant displacement was observed at other time points. At the higher doses, there was increasing displacement up to 4.5 hours after injection with comparable levels at 8 hours after injection (P values < 0.05). The displacement at 16 and 24 hours after injection was less than that at 8 hours after 10.0 mg/kg or unchanged from that at 17.0 mg/kg. Both of these higher doses showed little displacement at 48 hours after injection (P values > 0.05).
Fig. 1.
Time course of effects of JHW007 in vivo on specific [125I]RTI-121 binding in mice. Ordinates: specific [125I]RTI-121 binding expressed as a percentage of vehicle control; Abscissae: time after JHW007 injection. Data for the effects of JHW007 from 5 minutes to 4.5 hours after injection (n = 5–13/time point) have been included from a previous report (Desai et al., 2005a) using methods identical to those from experiments newly conducted for this report. Data for the effects of JHW007 from 8 to 48 hours represent effects determined in four mice at each time point, with error bars representing 1 S.E.M.
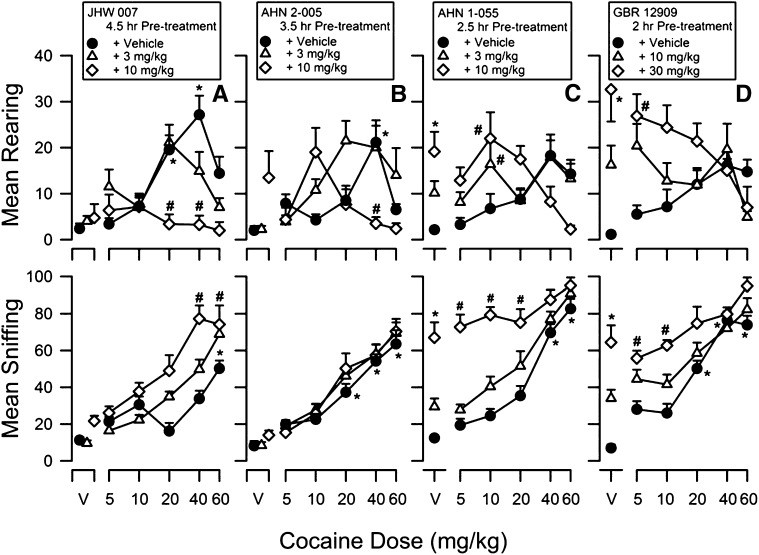
Effects of Cocaine Alone and in Combination with N-Substituted BZT Analogs or GBR 12909 on Locomotor Activity.
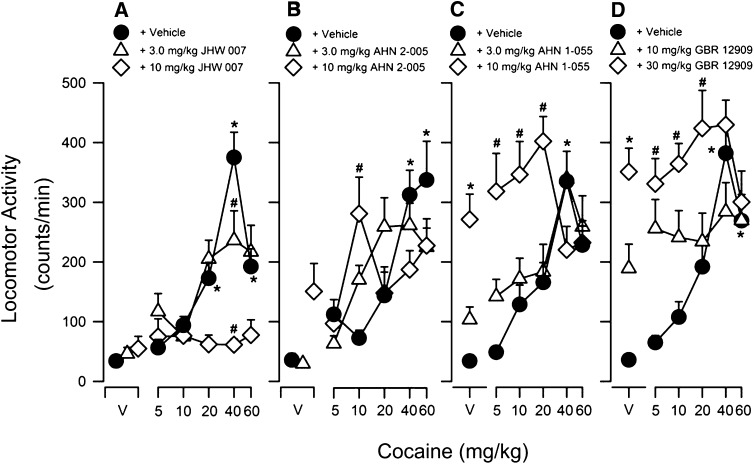
Cocaine alone produced a significant dose-dependent (5.0–60 mg/kg) increase in locomotor activity (Fig. 2A, filled symbols). These effects were typically comparable in all experiments: Doses from 5–40 mg/kg cocaine produced increasing effects up to the maximum stimulation of locomotor activity, which occurred at 40 mg/kg, whereas a higher dose of cocaine (60 mg/kg) increased locomotor activity less than the lower dose. The exception was a maximum stimulation of locomotor activity produced by 60 mg/kg cocaine when administered at 3.5 hours after vehicle injection (Fig. 2B, filled symbols). A two-way ANOVA indicated significant effects of cocaine dose (F5,124–6 values ≥ 6.20, P values < 0.05). Post-hoc analyses typically indicated that 20–60 mg/kg cocaine significantly stimulated activity compared with control vehicle values (P values < 0.05; Fig. 2, A–D; filled symbols).
Fig. 2.
Effects of pretreatment with JHW007 (A), AHN2-005 (B), AHN1-055 (C), or GBR 12909 (D) on cocaine-induced increases in locomotor activity in mice. Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), or cocaine dose in milligrams per kilogram. JHW007, AHN2-005, AHN1-055, or GBR 12909 were administered 4.5, 3.5, 2.5, or 2 hours before administration of different doses of cocaine (5.0–60 mg/kg). Each point represents the average (+S.E.M.) rate in counts/minute determined in six to eight mice for a time period of 90 minutes. Note that in contrast to the other N-substituted BZT analogs and GBR 12909, JHW007 dose-dependently and fully antagonized the locomotor-stimulant effects of cocaine. *P < 0.05 versus vehicle + vehicle; #P < 0.05 versus vehicle + cocaine.
JHW007 alone at doses of 3.0 and 10 mg/kg did not substantially increase locomotor activity compared with control values (Fig. 2A, open symbols above “V”). Post-hoc tests indicated that the effects of 3.0 and 10 mg/kg JHW007 alone were not significantly greater than those of two vehicle injections (P values > 0.05). Pretreatment with JHW007 (3.0 and 10 mg/kg) dose-dependently antagonized the locomotor-stimulant effects of cocaine (Fig. 2A, compare filled and open symbols). A two-way ANOVA revealed a significant main effect of JHW007 (F2,124 = 19.0, P < 0.05) and a significant interaction between JHW007 and cocaine (F10,124 = 6.37, P < 0.05). Post-hoc analyses showed that JHW007 (3.0 and 10 mg/kg) administered 4.5 hours before cocaine dose-dependently antagonized the increase in activity produced by 40 mg/kg cocaine (P values < 0.05).
Administration of 10 mg/kg AHN2-005 alone produced a nonsignificant suggestion of an increase in locomotor activity (Fig. 2B, open diamond above “V”); post-hoc tests indicated that the effects of 3.0 and 10 mg/kg AHN2-005 alone were not significantly greater than those of two vehicle injections (P values > 0.05). Pretreatment with AHN2-005 (3.0 or 10 mg/kg) produced a trend toward increased locomotor-stimulant effects of low doses of cocaine (Fig. 2B, compare filled and open symbols); however, a two-way ANOVA did not indicate a significant main effect of AHN2-005 (F2,126 = 0.337, P < 0.05). A significant interaction between AHN2-005 and cocaine was obtained (F10,126 = 4.08, P < 0.05). Post-hoc analysis showed that the effects of 10 mg/kg AHN2-005 in combination with 10 mg/kg cocaine were greater than those of vehicle in combination with 10 mg/kg cocaine (P < 0.05).
Injections of AHN1-055 (3.0 or 10 mg/kg) and GBR 12909 (10 or 30 mg/kg) alone significantly stimulated locomotor activity in a dose-dependent manner (Fig. 2, C and D, respectively, open symbols above “V”). Post-hoc comparisons showed significant increases produced by the highest doses of AHN1-055 (10 mg/kg) and GBR 12909 (30 mg/kg) compared with two vehicle injections (P values < 0.05). Pretreatment with AHN1-055 (3.0 and 10 mg/kg) and GBR 12909 (10 or 30 mg/kg) shifted the cocaine dose-effect curve upward and to the left (Fig. 2, C and D, open symbols) in a manner corresponding with the effects of these doses alone. Two-way ANOVA indicated a significant main effect of AHN1-055 and GBR 12909 (F2,125 values ≥ 19.5, P values < 0.05), as well as a significant interaction between cocaine and AHN1-055 or GBR 12909 (F10,125 values ≥ 2.85, P values < 0.05). Post-hoc comparisons revealed that the highest doses of AHN1-055 (10 mg/kg) and GBR 12909 (30 mg/kg) significantly increased the effects of low doses of cocaine (5–20 mg/kg) on locomotor activity (P values < 0.05); however, the effects of AHN1-055 or GBR 12909 combined with cocaine were not significantly greater than those of either of the two drugs alone (P values > 0.05; Fig. 2, C and D, respectively).
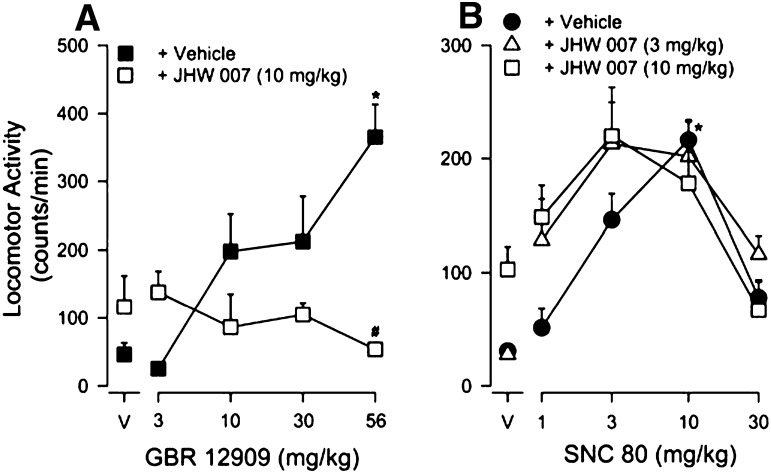
Effects of JHW007 in Combination with GBR 12909.
Consistent with Fig. 2 and previous studies, GBR 12909 alone (3.0–56 mg/kg) produced a dose-related increase in locomotor activity (Fig. 3A, filled squares), with the maximal effect obtained at the highest dose (56 mg/kg) tested. Post-hoc analyses showed that 56 mg/kg GBR 12909 significantly increased locomotor activity compared with vehicle (P < 0.05). The change in locomotor activity produced by JHW007 (10 mg/kg) alone 4.5 hours before testing vehicle failed to reach statistical significance (P < 0.05; Fig. 3A, compare symbols above “V”). In addition, pretreatment with JHW007 attenuated the locomotor-stimulant effects of GBR 12909 (Fig. 3A, compare filled and open symbols). A two-way ANOVA indicated a main effect of GBR 12909 (F4,53 = 3.16, P < 0.05), JHW007 (F1,53 = 6.52, P < 0.05), as well as a significant interaction between the two drugs (F4,53 = 7.19, P < 0.05). Post-hoc comparisons indicated a significant decrease in the effect of 56 mg/kg GBR 12909 produced by JHW007 (P < 0.05).
Fig. 3.
Effects of pretreatment with JHW007 on increases in locomotor activity in mice produced by GBR 12909 (A) or SNC 80 (B). Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), GBR 12909, or SNC 80 dose in milligrams per kilograms. JHW007 (10 mg/kg) was administered 4.5 hours before administration of different doses of GBR 12909 (3.0–56 mg/kg) or SNC 80 (1.0–30 mg/kg). Each point represents the average (+ S.E.M.) rate in counts/minute determined in six to eight mice for a time period of 90 minutes. Note that JHW007 fully blocked the locomotor-stimulant effects of GBR 12909 and failed to alter the increases in locomotor activity produced by the δ-opioid agonist SNC 80. *P < 0.05 versus vehicle + vehicle; #P < 0.05 versus vehicle + GBR 12909.
Effects of JHW007 in Combination with SNC 80.
SNC 80 (1.0–30 mg/kg) dose-dependently increased locomotor activity (Fig. 3B, filled circles). Maximal stimulation of locomotor activity was produced by 10 mg/kg SNC 80, with post-hoc analyses indicating significance compared with vehicle (P < 0.05). Effects of JHW007 alone were not statistically significant by post-hoc test (P < 0.05; compare symbols above “V”). Both tested doses of JHW007 (3.0 and 10 mg/kg) produced a shift to the left in the SNC 80 dose-effect curve (Fig. 3B, compare filled and open symbols). A two-way ANOVA indicated a significant main effect of SNC 80 (F4,105 = 18.2, P < 0.05) and JHW007 (F2,105 = 3.17, P < 0.05), without a significant interaction between the two drugs (F8,105 = 1.80, P < 0.05).
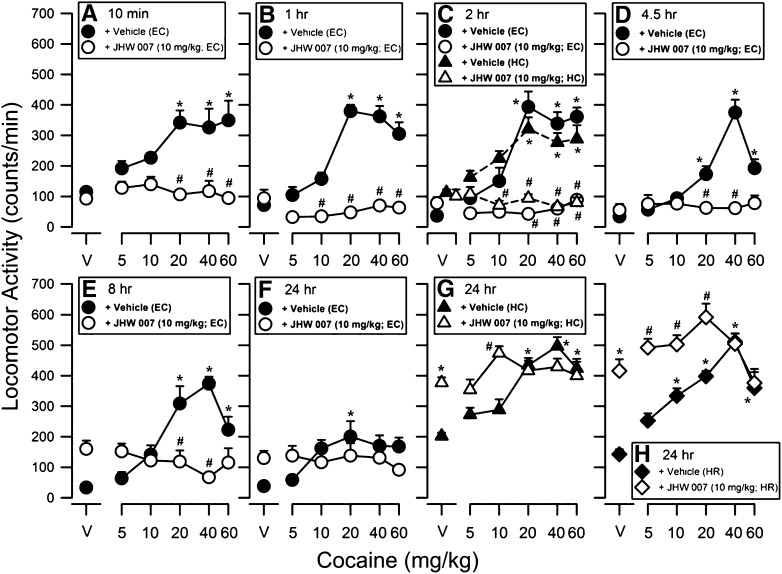
Time Course of Effects of Combinations of Cocaine and JHW007.
The dose-related increases in locomotor activity induced by cocaine alone were generally comparable up to and including 8 hours after vehicle injection and consequently were independent of how long the subjects had been in the chamber before cocaine injection (Fig. 4, A–E, filled circles). Two-way ANOVA revealed a significant main effect of cocaine dose at each of these pretreatment time points (all F5,79–83 values ≥ 3.96, with P values < 0.05). JHW007 (10 mg/kg) at times between 10 minutes and 4.5 hours before cocaine, produced little change in locomotor activity compared with vehicle levels (Fig. 4; compare points above “V”), which was confirmed by post-hoc tests at all time points up to 24 hours.
Fig. 4.
Time course of the effects of pretreatment with JHW007 in combination with cocaine on locomotor activity in mice. Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), or cocaine dose in milligrams per kilograms. JHW007 was administered at 10 minutes, 1, 2, 4.5, 8, and 24 hours before administration of different doses of cocaine (5.0–60 mg/kg). Each point represents the average (+ S.E.M.) rate in counts/minute determined in six to eight mice for a time period of 90 minutes. The combination effects of JHW007 and cocaine at 24 hours were determined under three different experimental conditions where vehicle or 10 mg/kg JHW007 was injected either in the experimental chamber (EC), home cage in the laboratory (HC), or in the housing room (HR) in the animal facility. Note that at time points of 10 minutes to 8 hours, JHW007 alone failed to significantly alter locomotor behavior, whereas JHW007 alone administered after 8 hours produced cocaine-like increases in locomotor activity, particularly under the HC and HR conditions at 24 hours. Administration of 10 mg/kg JHW007 at pretreatment times of 10 minutes to 8 hours fully antagonized the locomotor-stimulant effects of cocaine. At 24 hours after JHW007 administration, the locomotor-stimulant effects of cocaine was potentiated, i.e., the cocaine dose-effect was shifted upward and to the left under both HC and HR conditions. *P < 0.05 versus vehicle + vehicle; #P < 0.05 versus vehicle + cocaine.
When 10 mg/kg JHW007 was injected between 10 minutes and 8 hours before cocaine there was an insurmountable antagonism of the locomotor-stimulant effects of cocaine (Fig. 4, A–E; compare filled to open circles). A two-way ANOVA revealed a significant main effect of JHW007 (10 mg/kg) at pretreatment times of 10 minutes to 8 hours (F1,79–83 values ≥ 13.4, P values < 0.05), as well as a significant interaction between cocaine and JHW007 at all time points (F5,79–83 values ≥ 4.12, P values < 0.05). Post-hoc analyses generally indicated that an injection of 10 mg/kg JHW007 10 minutes to 8 hours before cocaine fully antagonized the locomotor-stimulant effects produced by 20–60 mg/kg cocaine (P values < 0.05).
At 24 hours after vehicle injection, the maximal stimulation of locomotor activity produced by cocaine alone, after subjects had been in the experimental chamber (EC) for those 24 hours, was substantially less than that obtained at the other times, and the main effect of cocaine only approached significance (F5,84 = 2.09, P = 0.075; Fig. 4F, filled circles). However, post-hoc tests indicated that the effects of the 20 mg/kg dose of cocaine were significantly greater than the effects of vehicle (P value < 0.05). Furthermore, there was no significant main effect of JHW007 (F1,84 = 0.230, P > 0.05), although there was a significant interaction between cocaine and JHW007 (F5,84 = 2.80, P < 0.05).
With subjects injected with vehicle in their home cages with those cages kept in the laboratory room in which the testing chambers were housed (Fig. 4, C and G, HC), the effects of cocaine (filled symbols) were similar to those obtained at the other vehicle pretreatment times (F5,83 = 10.5, P < 0.05). Under these conditions JHW007 (10 mg/kg) injected 2 hours, but not 24 hours, before testing blocked the effects of cocaine. At 24 hours JHW007 enhanced the effects of lower cocaine doses, whether subjects were kept in their home cages in the laboratory after JHW007 injection (Fig. 4G) or kept in the housing room in the animal facility (Fig. 4H) between JHW007 injection and locomotor testing. A two-way ANOVA confirmed a significant main effect of JHW007 (F1,83 values ≥ 10.9, P values < 0.05), cocaine (F5,83 values ≥ 10.5, P values < 0.05), as well as a significant interaction between the two (F5,83 values ≥ 6.12, P values < 0.05) with the 24-hour pretreatment. The differences between results with cocaine depending on where the subjects were kept between JHW007 injection and testing (compare Fig. 4, F–G and H) is likely due to the interaction between cocaine injection and the transfer of the subject to a novel environment.
Effects of Cocaine Alone and in Combination JHW007 in DA D2R WT and KO Mice.
Cocaine produced a dose-related increase in locomotor activity in both the DA D2R WT and KO mice (Fig. 5, filled circles and squares, respectively) with greatest effects at the highest dose tested (30 mg/kg). A three-way ANOVA indicated a significant main effect of cocaine (F3,58 = 23.1, P < 0.05). Post-hoc analyses confirmed that 30 mg/kg cocaine significantly increased locomotor activity in both genotypes (P < 0.05). The stimulation in locomotor activity produced by cocaine was less in DA D2R KO than in DA D2R WT mice (Fig. 5, compare filled circles to filled squares), as indicated by a significant post-hoc difference between genotypes in the effects of 30 mg/kg cocaine (P < 0.05; compare filled circles and squares).
Fig. 5.
Effects of pretreatment with JHW007 on cocaine-induced increases in locomotor activity in DA D2R WT and KO mice. Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), or cocaine dose in milligrams per kilograms. JHW007 was administered 4.5 hours before administration of different doses of cocaine (3–30 mg/kg). Each point represents the average (+S.E.M.) rate in counts/minute determined in four to six mice for a time period of 90 minutes. Note that JHW007 attenuated the stimulation of locomotor activity produced by the highest dose of cocaine in both the DA D2 WT and KO mice. *P < 0.05 versus vehicle + vehicle; #P < 0.05 versus vehicle + cocaine.
JHW007 alone (10 mg/kg) failed to significantly stimulate locomotor activity above vehicle levels in the DA D2R KO mice (P < 0.05); due to lack of availability of DA D2R WT mice, the effects of JHW007 (10 mg/kg) alone were not evaluated in this genotype. A three-way ANOVA indicated a nonsignificant effect of JHW007 (F1,58 = 2.23, P < 0.05), an interaction of JHW007 and genotype (F1,58 = 1.10, P < 0.05), and a three-way interaction (F2,58 = 2.01, P < 0.05). Although the main effect of JHW007 was not significant, post-hoc comparisons indicated that none of the effects of cocaine in combination with JHW007 were significantly different from vehicle in either WT or KO mice.
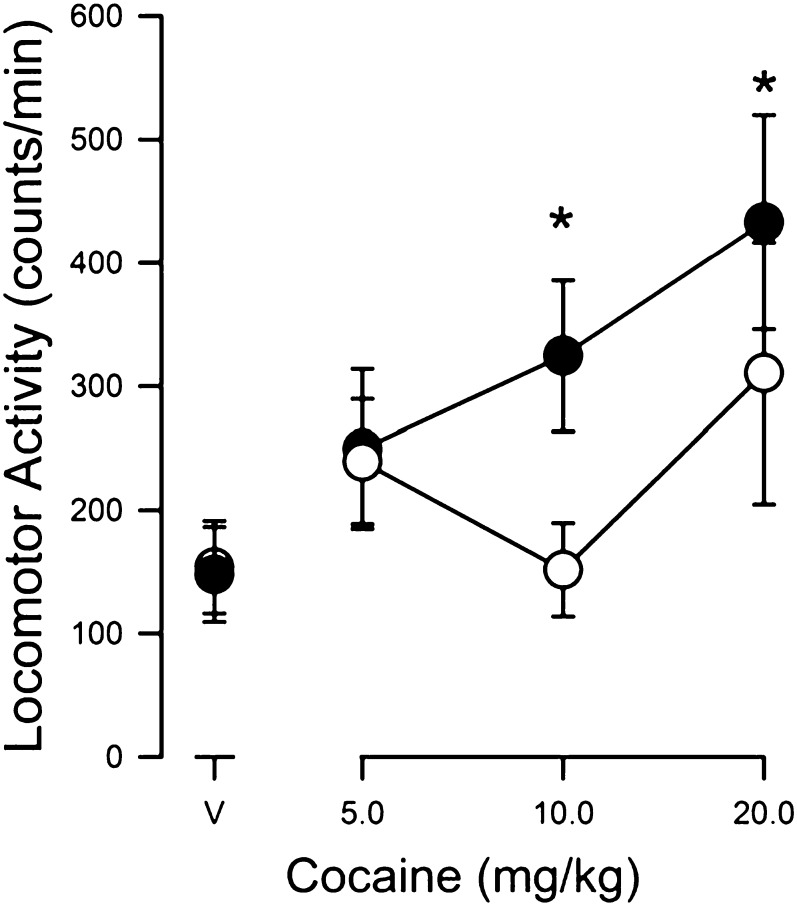
Effects of Cocaine Alone and in Combination with BD 1008.
Cocaine, preceded by a saline vehicle injection, produced dose-related increases in locomotor activity (Fig. 6). When subjects were injected with 10 mg/kg BD 1008 before cocaine, the stimulation of locomotor activity was significantly decreased (Fig. 6, compare filled and open symbols). That dose of BD 1008 had no effects on locomotor activity when administered alone (points above “V”). A two-way ANOVA indicated that the effects of BD 1008 were significant (F1,36 = 9.16, P = 0.005), whereas neither the effects of cocaine nor the interaction were significant. Post-hoc comparisons indicated a significant effect at 10 (t = 2.74; P = 0.009) and 20 (t = 2.461; P = 0.01) mg/kg BD 1008.
Fig. 6.
Effects of pretreatment with BD 1008 on cocaine-induced increases in locomotor activity in Swiss-Webster mice. Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), or cocaine dose in mg/kg. BD 1008 (10 mg/kg) was administered immediately before administration of cocaine (5–20 mg/kg). Each point represents the average (+ S.E.M.) rate in counts/minute determined in six mice for a time period of 60 minutes. Note that BD 1008 attenuated the dose-dependent stimulation of locomotor activity produced by cocaine.
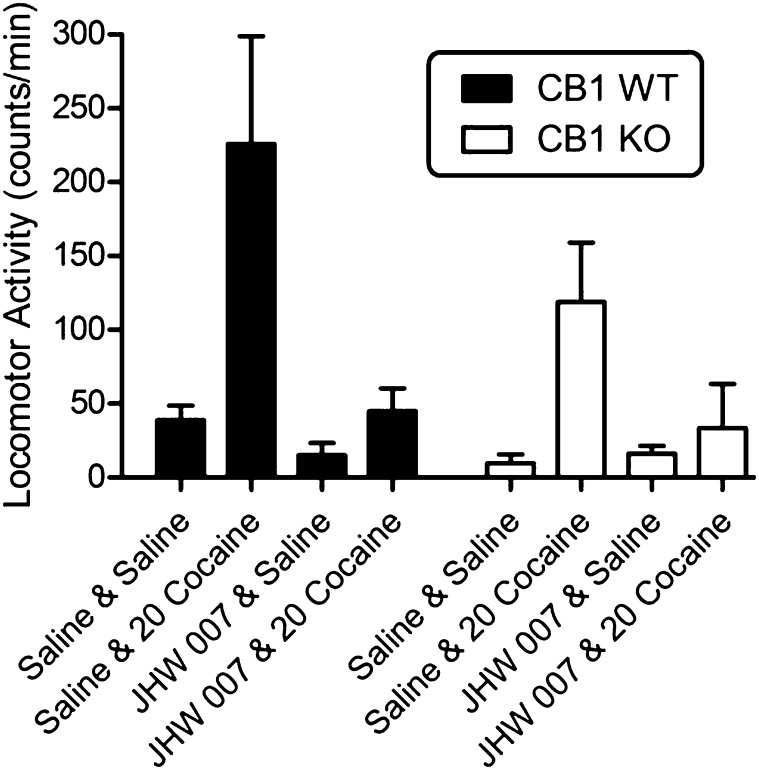
Effects of Cocaine Alone and in Combination JHW007 in Cannabinoid CB1R WT and KO Mice.
Cocaine (20 mg/kg) produced a significant increase in locomotor activity in both the CB1R WT and KO mice (Fig. 7). A two-way ANOVA indicated a significant main effect of treatment (F3,44 = 11.5, P < 0.001) without effects of genotype (F1,44 = 2.67, P = 0.109) or their interaction (F3,44 = 1.24, P = 0.306). Post-hoc analyses confirmed that 20 mg/kg cocaine significantly increased locomotor activity (P < 0.001) and that cocaine with JHW007 did not (P = 0.789).
Fig. 7.
Effects of pretreatment with JHW007 on cocaine-induced increases in locomotor activity in cannabinoid CB1R receptor WT and KO mice. Ordinates: horizontal locomotor activity counts/minute. Abscissae: treatment condition, vehicle (V), or cocaine dose in milligrams per kilograms. JHW007 (20 mg/kg) was administered 4.5 hours before administration of 20 mg/kg doses of cocaine. Each point represents the average (+S.E.M.) rate in counts/minute determined in four to six mice for a time period of 90 minutes. Note that JHW007 attenuated the stimulation of locomotor activity produced by cocaine in both the cannabinoid CB1R receptor WT and KO mice.
Effects of Cocaine Alone and in Combination with N-Substituted BZT Analogs and GBR 12909 on Stereotypic Behavior.
Cocaine (5.0–60 mg/kg) alone typically produced significant dose-dependent increases in the frequencies of rearing and sniffing behaviors (Fig. 8, filled symbols). The maximum increase in rearing was obtained following administration of 40 mg/kg; a higher dose of cocaine (60 mg/kg) produced effects that were less than those produced by 40 mg/kg cocaine. A two-way ANOVA indicated significant effects of cocaine dose for rearing when vehicle was injected 3.5 and 4.5 hours (F5,124–6 values ≥ 3.86, P values < 0.05), and for sniffing at all times (F5,124–6 values ≥ 40.6, P values < 0.05).
Fig. 8.
Effects of pretreatment with JHW007 (A), AHN2-005 (B), AHN1-055 (C), or GBR 12909 (D) on the effects of cocaine on rearing (top panels) and sniffing (bottom panels) in mice. Ordinates: mean frequency of rearing (top) and sniffing (bottom) after drug administration. Abscissae: treatment condition, vehicle (V), or cocaine dose in milligrams per kilograms. JHW007, AHN2-005, AHN1-055, or GBR 12909 were administered 4.5, 3.5, 2.5, or 2 hours before administration of different doses of cocaine (5–60 mg/kg). Each point represents the average frequency (+S.E.M.) determined in six to eight mice over the 90-minute observation period. Note that in contrast to the other N-substituted BZT analogs and GBR 12909, JHW007 dose-dependently antagonized the increases in rearing behavior produced by cocaine. Furthermore, similar to AHN1-055 and GBR 12909, JHW007 also produced a leftward shift in the cocaine dose-effect curve; AHN2-005 had no effect on sniffing. *P < 0.05 versus vehicle + vehicle; #P < 0.05 versus vehicle + cocaine.
The increases in rearing produced by 5.0–60 mg/kg cocaine were dose-dependently antagonized by JHW007 (3.0 and 10 mg/kg) injected 4.5 hours before cocaine (Fig. 8A, top panel; compare diamonds to circles). For sniffing, pretreatment with 3.0 or 10 mg/kg JHW007 shifted the cocaine dose-effect curve to the left in a dose-related manner (Fig. 8A, bottom panel; compare diamonds to circles). For both rearing and sniffing, a two-way ANOVA confirmed a significant main effect of JHW007 dose (F2,124 values ≥ 12.1, P values < 0.05), as well as a significant interaction between JHW007 and cocaine (F10,124 values ≥ 3.45, P values < 0.05). Post-hoc analyses confirmed that combinations of 10 mg/kg JHW007 and cocaine (20 or 40 mg/kg) were significantly different than those of cocaine alone (P values < 0.05) for both rearing and sniffing.
Pretreatment with AHN2-005 produced a leftward shift in the cocaine dose-effect curve (Fig. 8B, top panel; compare triangles to circles); however, a two-way ANOVA did not indicate a significant main effect of AHN2-005 (F2,126 = 2.32, P < 0.05), though a significant interaction between AHN2-005 and cocaine was obtained (F10,126 = 4.61, P < 0.05). In contrast, the effects of cocaine on sniffing were not altered by either dose of AHN2-005 (Fig. 8B, bottom panel; compare triangles to circles). A two-way ANOVA confirmed nonsignificant effects of AHN2-005 (F2,126 = 1.63, P < 0.05), as well as a nonsignificant interaction between AHN2-005 and cocaine (F10,126 = 0.436, P < 0.05).
AHN1-055 (3.0 or 10 mg/kg; 2.5 hours before testing) and GBR 12909 (10 or 30 mg/kg; 2 hours before testing) alone significantly increased the frequencies of rearing and sniffing behavior in a dose-dependent manner (Fig. 8, C–D, top and bottom panels, respectively; compare symbols above “V”). Post-hoc comparisons indicated that the effects of the highest doses of AHN1-055 and GBR 12909 were significantly greater than those obtained with vehicle injections (P values < 0.05). Pretreatment with either drug increased the effects of the low doses of cocaine (Fig. 8, C–D, top and bottom panel, respectively; compare open to filled symbols) to an extent corresponding with the effects of the individual doses of the drugs when administered alone. Two-way ANOVAs indicated significant main effects of AHN1-055 and GBR 12909 for rearing and sniffing (F2,125 values ≥ 5.49, P values < 0.05), as well as a significant interaction between cocaine and AHN1-055 or GBR 12909 (F10,125 values ≥ 2.81, P values < 0.05).
Discussion
Consistent with a previous report (Desai et al., 2005a), JHW007 dose-dependently antagonized the locomotor-stimulant effects of cocaine at a time (4.5 hours) that produced maximum observed in vivo DAT occupancy (Desai et al., 2005a). These results are also consistent with more recent studies that have shown antagonism of cocaine and methamphetamine by JHW007 and other BZT analogs in behavioral (e.g., locomotor activity, self-administration behavior, place conditioning) and neurochemical (e.g., DA efflux in the nucleus accumbens) procedures (Katz et al., 2004; Li et al., 2005; Hiranita et al., 2009, 2013; Velazquez-Sanchez et al., 2009, 2010; Tanda et al., 2009a,b).
In contrast to the cocaine-antagonist effects of JHW007, the N-methyl (AHN2-005) and N-allyl (AHN1-055) analogs of BZT did not antagonize cocaine-induced increases in locomotor activity. Instead, these drugs increased the effects of cocaine up to levels that were comparable with their own stimulant activity. Similar results were obtained in the present study with combinations of cocaine and the selective DA uptake inhibitor GBR 12909. It is interesting to contrast the present studies on locomotor activity in mice with those from studies of cocaine and methamphetamine self-administration in rats (Hiranita et al., 2009, 2013). In the latter studies, JHW007 as well as AHN2-005 and AHN1-055 blocked cocaine or methamphetamine self-administration. The effects of AHN1-055, however, were different from the other BZT analogs in that this compound had effects that resembled both stimulants and the atypical BZT analogs (see also Katz et al., 1999). As in other pharmacological systems, the end response and the characteristics of the drug can combine to determine the degree of antagonism obtained.
The effects of JHW007 on behaviors other than locomotor activity do not appear to contribute to its cocaine-antagonist effects. JHW007 alone failed to induce stereotyped rearing and sniffing, whereas pretreatment with JHW007 dose-dependently antagonized effects of cocaine on rearing and enhanced the effects of low doses of cocaine on sniffing. The possibility that drug-induced stereotypies may have resulted in a decrease in locomotor activity may deserve further consideration; although it should be noted that both AHN1-055 and GBR 12909 enhanced the effects of cocaine on stereotyped behavior but failed to antagonize effects of cocaine on locomotion and rearing, making an enhancement of cocaine-induced stereotypies an unlikely contributor to the antagonism of locomotor-stimulant effects of cocaine by JHW007. That interpretation is consistent with a recent study that demonstrated that stereotypic behaviors induced by JHW007 and other BZT analogs contribute little if anything to the antagonism of cocaine self-administration in rats (Li et al., 2013).
Interestingly, evaluation of the time course of the cocaine-antagonist effects in the present study indicates that the locomotor-stimulant effects were antagonized when JHW007 was administered 10 minutes to 8 hours before cocaine. The antagonism shortly after JHW007 injection was unexpected, as maximal binding of JHW007 in vivo required up to 4.5 hours. In contrast, at 10 minutes after injection, when there was presently a clear and profound antagonism of the effects of cocaine, the 10 mg/kg dose of JHW007 displaced only ∼15–20% of the radiolabel. Thus, levels of in vivo occupancy at the DAT do not provide a full explanation for the behavioral outcomes observed with JHW007 in the present study.
These findings suggest that the antagonism of cocaine observed in the present study may involve actions of JHW007 at sites other than the DAT. Several previous reports have provided results from broad screens of receptor binding and have indicated several sites at which all of the BZT analogs tested had affinities at least in the micromolar range. These sites include histamine H1, muscarinic M1, DA D2, and σRs. As mentioned above, previous studies of the contributions of M1 and H1 receptor antagonism indicate that these effects contribute little to the unique behavioral effects of the BZT analogs (Katz et al., 1999; Campbell et al., 2005; Tanda et al., 2008).
The present study examined the potential contribution of DA D2 receptors by examining antagonism of cocaine in both DA D2R WT and KO mice. While the stimulation of locomotor activity in the DA D2R KO mice was less than that seen in WT mice, as has been previously shown (e.g., Chausmer et al., 2002), treatment with JHW007 was effective in reducing the effects of cocaine in both lines of mice. That antagonism obtained in both lines of mice suggests that DA D2Rs are not critically involved in the antagonist effects of JHW007.
The involvement of allosteric modulation of CB1 receptors was assessed using a similar approach. As with the DA D2R KO mice, the stimulation of locomotor activity in the CB1 receptor KO mice was less than that obtained in their WT littermates. However, an antagonism of the locomotor stimulation produced by cocaine was obtained with JHW007 in both CB1 receptor KO and WT mice. That the antagonism was obtained in both lines of mice suggests that allosteric modulation of CB1 receptors is not necessary for the antagonist effects of JHW007.
More recent studies have suggested a role of σRs in the effects of BZT analogs. For example Katz et al. (2004) and Li et al. (2011) found that several BZT analogs, including JHW007, had high affinity (∼2 nM for JHW007) for σRs. Additionally, previous studies have indicated that σR antagonists block the locomotor-stimulant effects of cocaine (e.g., Menkel et al., 1991; see reviews by Matsumoto, 2009; Katz et al., 2011). In the present study, the σR antagonist BD 1008 decreased cocaine-induced locomotor stimulation. Whether JHW007 has σR agonist or antagonist activity has not as yet been determined, but certainly activity at this site deserves further assessment.
JHW007 pretreatment, antagonized the increases in locomotor activity produced by the selective DA uptake inhibitor GBR 12909 but failed to modify the stimulation of locomotor activity induced by SNC 80, an δ-opioid agonist that increases behavior through nondopaminergic mechanisms (Jutkiewicz et al., 2004). These findings suggest that the antagonism of the locomotor-stimulant effects produced by JHW007 was not simply a nonspecific dampening of any behavioral stimulation but rather was an action upon stimulation evoked by dopaminergic action, and possibly specific to that involving the DAT (see Hiranita et al., 2013).
A previous study (Kopajtic et al., 2010) found the association of [3H]WIN 35428 best fit to a one-phase model, whereas the association of [3H]JHW007 was best fit to a two-phase model. Kopajtic et al. (2010) suggested that the rapid association phase may contribute to the cocaine-antagonist effects of JHW007 consistent with the present findings of antagonism soon after injection. The present findings with those of Kopajtic et al. (2010) suggest that JHW007 may be acting at more than one site, each with different rates of occupancy. Initial actions of JHW007 at one site may interfere with the effects of cocaine. The later appearing increases in locomotor activity seen with JHW007 may result from activity at the second site (with a slower association rate) and a loss of antagonist effects at 24 hours after administration, despite considerable binding at this time. Whether the rapid-association phase of JHW007 binding reflects DAT activity or off-target activity remains to be determined. It is possible that the rapid association is to a site other than the DAT, which would relate “atypical” DAT inhibition and cocaine-antagonist effects to relative rates of association at the DAT and the relevant other site(s). Different relative rates of occupancy of individual BZT analogs would then contribute to the degree of antagonism obtained, with different end responses differentially sensitive to the differential occupancy rates.
Previous studies have shown antagonism of several effects of cocaine by JHW007, including locomotor stimulation (Desai et al., 2005a), increases in nucleus accumbens DA concentrations, and cocaine self-administration. The present results further substantiate that JHW007 antagonizes the locomotor-stimulant effects of cocaine and indicate that those antagonist effects are obtained from virtually immediately to up to 8 hours after injection. The antagonism was not completely related to the occupancy of the DAT by JHW007. Together with previous results using in vivo receptor binding assays, the data suggest that an off-target site may contribute to the antagonism of the effects of cocaine, and that this antagonism is not likely due to actions at CB1 or DA D2Rs, but one possible target for further studies is σRs. Nonetheless, the results provide further support for the view that compounds with characteristics similar to those of JHW007 show promise for development as medications for the treatment of stimulant abuse.
Acknowledgments
The authors thank Patty Ballerstadt and Maryann Carrigan for administrative support. They also also thank A. H. Newman and J. J. Cao for synthesizing the BZT analogs used in this study and J. H. Woods for advice on parts of the study. D.K.G. and C.R.L., respectively, provided DA D2R and cannabinoid CB1R wild-type and mutant mice.
Abbreviations
- AHN1-055
3α-[bis(4′-fluorophenyl)methoxy]-tropane
- AHN2-005
N-allyl-3α-[bis(4′-fluorophenyl)methoxy]-tropane
- ANOVA
analysis of variance
- BZT
benztropine
- BD 1008
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- CB
cannabinoid
- CP55,940
(–)-5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclo-hexyl]-phenol
- DA
dopamine
- DAMGO
(2S)-2-[[2-[[(2R)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]propanoyl]amino]acetyl]-methylamino]-N-(2-hydroxyethyl)-3-phenylpropanamide
- DAT
dopamine transporter
- GBR 12909
1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride
- HC
home cage
- HR
housing room
- JHW007
N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane
- KO
knockout
- RTI-121
propan-2-yl (1R,2S,3S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- σR
σ receptor
- SNC 80
4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide
- WIN 35,428
2β-carbomethoxy-3β-(4-fluorophenyl)tropane
- WT
wild type
Authorship Contributions
Participated in research design: Desai, Katz.
Conducted experiments: Desai, Katz.
Performed data analysis: Desai, Katz.
Wrote or contributed to the writing of the manuscript: Desai, Katz.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse].
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. (1997) Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40:4329–4339 [DOI] [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Newman AH, Katz JL. (2005) Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. J Pharmacol Exp Ther 315:631–640 [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. (2002) Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 163:54–61 [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P. (2003) Evidence of cross-tolerance between behavioural effects of nicotine and cocaine in mice. Psychopharmacology (Berl) 166:111–119 [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. (2005a) Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25:1889–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. (2005b) Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-2-[bis-(4-fluorophenyl)methoxy]ethyl-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther 315:397–404 [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL. (2013) Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther doi: 10.1124/jpet.113.208264 [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. (2004) Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther 309:173–181 [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. (1999) Novel 3α-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288:302–315 [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. (2004) Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309:650–660 [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. (2011) A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 4:880–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopajtic TA, Liu Y, Surratt CK, Donovan DM, Newman AH, Katz JL. (2010) Dopamine transporter-dependent and -independent striatal binding of the benztropine analog JHW 007, a cocaine antagonist with low abuse liability. J Pharmacol Exp Ther 335:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Scheffel U, Stathis M, Seltzman HH, Wyrick CD, Abraham P, Parham K, Thomas BF, Boja JW, Kuhar MJ, et al. (1996) Synthesis and in vivo studies of a selective ligand for the dopamine transporter: 3 β-(4-[125I]iodophenyl) tropan-2 β-carboxylic acid isopropyl ester ([125I]RTI-121). Nucl Med Biol 23:277–284 [DOI] [PubMed] [Google Scholar]
- Li SM, Newman AH, Katz JL. (2005) Place conditioning and locomotor effects of N-substituted, 4′,4″-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313:1223–1230 [DOI] [PubMed] [Google Scholar]
- Li SM, Kopajtic TA, O’Callaghan MJ, Agoston GE, Cao J, Newman AH, Katz JL. (2011) N-substituted benztropine analogs: selective dopamine transporter ligands with a fast onset of action and minimal cocaine-like behavioral effects. J Pharmacol Exp Ther 336:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hiranita T, Hayashi S, Newman AH, Katz JL. (2013) The stereotypy-inducing effects of N-substituted benztropine analogs alone and in combination with cocaine do not account for their blockade of cocaine self-administration. Psychopharmacology (Berl) 225:733–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. (2009) Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol 2:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. (1991) Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol 201:251–252 [DOI] [PubMed] [Google Scholar]
- Navarro HA, Howard JL, Pollard GT, Carroll FI. (2009) Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje S, Cao J, Newman AH, Gao H, Eddington ND. (2003) Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther 307:801–808 [DOI] [PubMed] [Google Scholar]
- Scheffel U, Boja JW, Kuhar MJ. (1989) Cocaine receptors: in vivo labeling with 3H-(-)cocaine, 3H-WIN 35,065-2, and 3H-WIN 35,428. Synapse 4:390–392 [DOI] [PubMed] [Google Scholar]
- Scheffel U, Pögün S, Stathis M, Boja JW, Kuhar MJ. (1991) In vivo labeling of cocaine binding sites on dopamine transporters with [3H]WIN 35,428. J Pharmacol Exp Ther 257:954–958 [PubMed] [Google Scholar]
- Scheffel U, Dannals RF, Wong DF, Yokoi F, Carroll FI, Kuhar MJ. (1992) Dopamine transporter imaging with novel, selective cocaine analogs. Neuroreport 3:969–972 [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. (2008) Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem 106:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. (2009a) Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57:253–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. (2009b) Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther 330:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Li SM, Mereu M, Thomas AM, Ebbs AL, Chun LE, Tronci V, Green JL, Zou MF, Kopajtic TA, et al. (2013) Relations between stimulation of mesolimbic dopamine and place conditioning in rats produced by cocaine or drugs that are tolerant to dopamine transporter conformational change. Psychopharmacology (Berl) 229:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) The dopamine uptake inhibitor 3 alpha-[bis(4′-fluorophenyl)metoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology 34:2497–2507 [DOI] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Murga J, Cardá M, Canales JJ. (2010) The high affinity dopamine uptake inhibitor, JHW 007, blocks cocaine-induced reward, locomotor stimulation and sensitization. Eur Neuropsychopharmacol 20:501–508 [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. (2005) Medication development for addictive disorders: the state of the science. Am J Psychiatry 162:1432–1440 [DOI] [PubMed] [Google Scholar]