Abstract
Atypical dopamine-uptake inhibitors have low abuse potential and may serve as leads for development of cocaine-abuse treatments. Among them, the benztropine (BZT) derivatives, N-butyl (JHW007), N-allyl (AHN2-005), and N-methyl (AHN1-055) analogs of 3α-[bis(4′-fluorophenyl)methoxy]-tropane dose-dependently decreased cocaine self-administration without effects on food-maintained responding. Our study examined selectivity by assessing their effects on self-administration of other drugs. As with cocaine, each BZT analog (1.0–10.0 mg/kg i.p.) dose-dependently decreased maximal self-administration of d-methamphetamine (0.01–0.32 mg/kg/infusion) but was inactive against heroin (1.0–32.0 µg/kg/infusion) and ketamine (0.032–1.0 mg/kg/infusion) self-administration. Further, standard dopamine indirect-agonists [WIN35,428 ((−)-3β-(4-fluorophenyl)-tropan-2-β-carboxylic acid methyl ester tartrate), d-amphetamine (0.1–1.0 mg/kg i.p., each)] dose-dependently left-shifted self-administration dose-effect curves for d-methamphetamine, heroin, and ketamine. Noncompetitive NMDA-glutamate receptor/channel antagonists [(+)-MK-801 (0.01–0.1 mg/kg i.p.), memantine (1.0–10.0 mg/kg i.p.)] also left-shifted dose-effect curves for d-methamphetamine and ketamine (but not heroin) self-administration. The µ-agonists [dl-methadone and morphine (1.0–10.0 mg/kg i.p., each)] dose-dependently decreased maximal self-administration of µ-agonists (heroin, remifentanil) but not d-methamphetamine or ketamine self-administration. The µ-agonist-induced decreases were similar to the effects of BZT analogs on stimulant self-administration and effects of food prefeeding on responding maintained by food reinforcement. Radioligand-binding and behavioral studies suggested that inhibition of dopamine transporters and σ receptors were critical for blocking stimulant self-administration by BZT-analogs. Thus, the present results suggest that the effects of BZT analogs on stimulant self-administration are similar to effects of µ-agonists on µ-agonist self-administration and food prefeeding on food-reinforced responding, which implicates behavioral mechanisms for these effects and further supports development of atypical dopamine uptake inhibitors as medications for stimulant abuse.
Introduction
The dopamine transporter (DAT) is critically involved in reinforcing effects of psychostimulants such as cocaine and d-amphetamine (Ritz et al., 1987; Ritz and Kuhar, 1989). Consequently, studies have assessed drugs acting at this site as potential “substitution treatments” for stimulant abuse, paralleling the use of methadone for heroin abuse (Dole and Nyswander, 1965, 1967). Both preclinical and clinical studies have offered some empirical support for the effectiveness of amphetamine in the treatment of cocaine abuse (Grabowski et al., 2004a). For example, Negus and Mello (2003) found that d-amphetamine dose-dependently decreased cocaine self-administration with minimal effects on food-reinforced responding in rhesus monkeys. Similar effects were reported for the uptake inhibitor GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine] (Glowa et al., 1995). Further, clinical support for this approach to treatment of stimulant abuse was provided by reports that d-amphetamine combined with methadone to treat concurrent cocaine and opioid abuse decreased cocaine use in a randomized, double-blind trial (Grabowski et al., 2004b; Mooney et al., 2009).
One likely problem with use of currently available standard dopamine (DA)-uptake inhibitors as treatments for cocaine abuse rests with their own abuse liability and potential to enhance effects of abused drugs. For example, several laboratory studies have demonstrated that standard DA indirect agonists enhance the self-administration of cocaine exhibited as leftward shifts in the self-administration dose-effect curve (Schenk, 2002; Barrett et al., 2004; Hiranita et al., 2011). When the self-administration is compared with responding maintained by another reinforcer such as food, under some conditions this enhancement can look like a selective decrease in self-administration without effects on food-maintained responding (Hiranita et al., 2011).
Several DA-uptake inhibitors have been identified as “atypical,” as these drugs bind to the DAT but produce substantially blunted psychostimulant-like effects. For example, benztropine (BZT) analogs produce less locomotor stimulation compared with cocaine and do not fully substitute in rodents trained to discriminate cocaine (e.g., Katz et al., 1999, 2004). Additionally, these compounds do not produce place conditioning (Li et al., 2005) and are either minimally self-administered or not self-administered at rates above those obtained with vehicle (Ferragud et al., 2009; Hiranita et al., 2009; Li et al., 2013). Further, the N-substituted BZT analog (n-butyl)-3α-[bis-(4′-fluorophenyl)methoxy]-tropane (JHW007) decreases locomotor stimulation produced by cocaine (Desai et al., 2005), and it as well as others [N-allyl-3α-[bis(4′-fluorophenyl)methoxy]-tropane (AHN2-005); 3α-[bis(4′-fluorophenyl)methoxy]-tropane (AHN1-055)] dose-dependently decrease cocaine self-administration at doses lacking effects on comparable food-reinforced responding (Hiranita et al., 2009; Li et al., 2013). Moreover, JHW007 reduces the effects of cocaine on DA levels in the nucleus accumbens shell, an area critical for reinforcing effects of drugs (Tanda et al., 2009a).
A recent study suggested that N-substituted BZT analogs may also block abuse-related effects of DA releasers. In that study (Velázquez-Sánchez et al., 2013), JHW007 blocked locomotor stimulation produced by a dose of d-amphetamine in mice. Thus, our study assessed whether N-substituted BZT analogs blocked the self-administration of d-methamphetamine. Initial studies indicated that several N-substituted BZT analogs produced an insurmountable antagonism, whereas standard DA-uptake inhibitors produced leftward shifts in the d-methamphetamine dose-effect curve.
The differences between BZT analogs and standard DA-uptake inhibitors with respect to their interactions with cocaine self-administration (Hiranita et al., 2009; Li et al., 2013) prompted the present comparisons of effects of drug pretreatments on self-administration of drugs from within the pharmacologic class (i.e., among drugs having the same primary biologic target) and across classes to place outcomes within a pharmacologic context. Whether the previously reported insurmountable antagonism was specific to cocaine self-administration was assessed by examining the effects of BZT analogs on self-administration of µ-opioid agonists (heroin, remifentanil) and the noncompetitive N-methyl-d-aspartate (NMDA) receptor/channel antagonist ketamine (in lieu of the less selective phencyclidine). Those effects were compared with those of standard DA indirect agonists. To minimize the likelihood that effects were idiosyncratic to particular test drugs, we made comparisons with several drugs from within pharmacologic classes. Whether insurmountable antagonism of self-administration was more commonly produced by drugs from within the class prompted comparisons with the effects of µ-opioid agonists and NMDA antagonists pretreatments on self-administration of drugs within and across classes.
Because previous studies had suggested that dual inhibition of the DAT and σ receptors (σRs) can block cocaine self-administration (Hiranita et al., 2011), our study assessed the sensitivity of d-methamphetamine self-administration to pretreatments with combinations of the DA-uptake inhibitor WIN35,428 [(−)-3β-(4-fluorophenyl)-tropan-2-β-carboxylic acid methyl ester tartrate] and the σR antagonist BD1008 [N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide], and affinities at these sites were determined. Finally, to place the findings within a behavioral context, the effects of satiation with the reinforcer were assessed by examining the effects of prefeeding on food-reinforced behavior in another group of subjects.
Materials and Methods
Self-Administration Procedures.
Twenty-four male Sprague-Dawley rats (Taconic Farms, Germantown, NY), weighing approximately 300 g at the start of the study, served as subjects. The subjects were acclimated to a temperature- and humidity-controlled vivarium for at least 1 week with a 12-hour light/dark cycle (lights on at 07:00 hours) during which food (Scored Bacon Lover Treats; Bio-Serv, Frenchtown, NJ) and tap water were available at all times. After acclimation, body weights were maintained at approximately 320 g by adjusting daily food rations. Water continued to be available at all times in the home cages. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by Association for Assessment and Accreditation of Laboratory Animal Care International.
Experimental sessions were conducted daily with subjects placed in operant-conditioning chambers (modified ENV-203; Med Associates, St. Albans, VT) that measured 25.5 × 32.1 × 25.0 cm and were enclosed within sound-attenuating cubicles equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of a lever with a force approximating 0.20 N defined a response, and always activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of 45-mg food pellets via a pellet dispenser (Model ENV-203-20; Med Associates) was mounted on the midline of the front wall between the two levers and 2.0 cm above the floor. A syringe infusion pump (Model 22; Harvard Apparatus, Holliston, MA) placed above each chamber delivered injections of specified volumes from a 10-ml syringe. The syringe was connected by Tygon tubing to a single-channel fluid swivel (375 Series Single Channel Swivels; Instech Laboratories, Plymouth Meeting, PA) that was mounted on a balance arm above the chamber. Tygon tubing from the swivel to the subject’s catheter was protected by a surrounding metal spring and completed the connection to the subject.
Subjects were surgically prepared under anesthesia (ketamine 60.0 mg/kg i.p., and xylazine 12.0 mg/kg i.p.) in the right external jugular vein with a chronic indwelling catheter that exited at the midscapular region of the animal’s back. Catheters were infused daily with 0.1 ml of a sterile saline solution containing heparin (30.0 IU/ml) and penicillin G potassium (250,000 IU/ml) to minimize the likelihood of infection and the formation of clots or fibroids. All animals were allowed to recover from surgery for approximately 7 days before drug self-administration studies were initiated.
Experimental sessions initially lasted for 120 minutes. Sessions started with the illumination of the LEDs above each lever. Each downward deflection of the right lever turned off the LEDs, produced an audible click, and activated the infusion pump for 10 seconds (fixed ratio or FR 1-response schedule) followed by a 20-second time-out period during which the LEDs were off and responding had no scheduled consequences. Drug injections were d-methamphetamine (0.1 mg/kg/injection, n = 6), (−)-heroin (0.01 mg/kg/injection, n = 6), or (±)-ketamine (0.32 mg/kg/injection, n = 6). After the time-out, the LEDs were illuminated, and responding again had the scheduled consequences. Responses on the left lever were recorded but had no scheduled consequences. These conditions remained in effect over the course of 14 sessions. Subjects were returned to their home cages in the vivarium after each session.
The self-administration procedure was then modified to facilitate pharmacologic assessments. Subjects were further trained to self-administer d-methamphetamine (0.1 mg/kg/injection), (−)-heroin (0.01 mg/kg/injection), or (±)-ketamine (0.32 mg/kg/injection) under an FR 5 schedule of reinforcement (each fifth response produced an injection with stimulus changes as previously described) until self-administration was consistent from one session to the next, as evidenced by the absence of visually apparent shifts in dose-effect curves. The session was then divided into five 20-minute components, each preceded by a 2-minute time-out period. This arrangement allowed the assessment of a range of self-administered doses in a single session (Hiranita et al., 2009). By adjusting infusion volumes and durations, the drug dose per injection was incremented in the sequential components as follows: no injection (also referred to as extinction, or EXT, because responses had no scheduled consequences), 0.01, 0.03, 0.10, and 0.32 mg/kg/injection for d-methamphetamine, EXT, 0.001, 0.003, 0.01, and 0.032 mg/kg/injection for (−)-heroin, and EXT, 0.03, 0.10, 0.32, or 1.0 mg/kg/injection for (±)-ketamine. The infusion volumes (and durations) were, respectively, 0 μl (0 seconds), 5.6 μl (0.32 seconds), 18.0 μl (1.0 second), 56.0 μl (3.2 seconds), and 180 μl (10.0 seconds) based on a body weight of 0.32 kg. A sample injection of the drug at the corresponding dose occurred independently of responding at the end of the time-out period that preceded each component, except the first during which there were no injections.
Training continued until responding was maintained with less than 20% variation in response rates across three consecutive sessions. Once performances were stable, various compounds were substituted for each of the drugs to assess their self-administration. The compounds substituted in half-log dose (in mg/kg/injection) increments were: d-methamphetamine (0.01–0.32), (−)-heroin (0.001–0.032), (±)-ketamine (0.032–1.0), d-amphetamine (0.01–0.32), (−)-cocaine (0.032–1.0), WIN35,428 (0.0032–0.1), remifentanil (0.0001–0.0032), dl-methadone (0.01–0.32), (−)-morphine (0.01–0.32), (+)-MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine)] (0.00032–0.01), memantine (0.032–1.0), JHW007 (0.032–1.0), AHN2-005 (0.032–1.0), AHN1-055 (0.032–1.0), and saline. Due to high rates of remifentanil self-administration (Panlilio and Schindler, 2000; Hutchinson et al., 2012), infusion durations of remifentanil were reduced to 0, 0.24, 0.75, 2.4, and 7.5 seconds to avoid excessive fluid intake and emptying of the syringe.
Effects of presession intraperitoneal injections of the atypical indirect-acting DA agonists (JHW007, AHN2-005, AHN1-055) were compared with those of standard DA indirect-agonists (WIN35,428, d-amphetamine). The specificity of the interactions between these indirect DA agonists and self-administered d-methamphetamine was assessed by comparing their effects on the self-administration of µ-opioid receptor agonists (heroin, remifentanil) and a noncompetitive NMDA receptor/channel antagonist (ketamine). To put these findings in a pharmacologic context, pretreatments from within and across pharmacologic class were compared on self-administration of heroin, remifentanil, and ketamine. Additionally, the effects of pretreatments with the σR antagonist BD1008 were studied when administered alone or in combination with WIN35,428. The effects of presession treatments on respective drug self-administration were separated by a minimum of 72 hours. The effects on pretreatment were studied with a mixed order of drugs and doses.
To put the results in a behavioral context, responding was maintained by different amounts of food in another group of subjects using a procedure similar to the present drug self-administration schedule (Hiranita et al., 2011). Six experimentally naïve subjects were trained with food reinforcement (20-mg grain-based food pellets; Bio-Serv) to press the right lever under an FR 5-response schedule of reinforcement. Each fifth response produced a food pellet, which was followed by a 20-second time-out period, during which responses had no scheduled consequences (other than feedback clicks). Sessions ended after delivery of 30 food pellets or 20 minutes. After obtaining 30 food pellets within three consecutive sessions, the subjects were further trained under a five-component procedure similar to that used for drug self-administration. All conditions were identical to those used with drug self-administration except that food pellet presentations replaced the injections. Subjects were fed their daily food ration (∼35 g of 1-g chocolate-flavored pellet; Bio-Serv) 150 minutes before sessions so that their response rates approximated those maintained by drug injections. Once performances were stable (as defined previously), the effects of presession feeding conditions were varied by giving the subjects unlimited amounts of food for time periods from 10 minutes to 22 hours (continuous food) before the ∼2-hour sessions. These studies were designed to provide an indication of effects of satiation under this schedule with which drug pretreatments could be compared.
Response rates were determined by dividing responses by appropriate elapsed times (excluding time-outs, and 0.032–10.0 seconds for each drug injection, or 0.1 seconds for each food presentation). The statistical significance of various effects was assessed by appropriate one-way or two-way repeated-measures analyses of variance (ANOVA) as indicated below. A post-hoc Bonferroni t test was used for all pairwise comparisons. For all analyses, P < 0.05 was considered statistically significant.
Radioligand Binding.
For DAT assays, brains from male Sprague-Dawley rats weighing 200–225 g (Bioreclamation, Westbury, NY) were removed, the striata dissected, and the tissue quickly frozen. Membranes were prepared by homogenizing tissues in 20 volumes (w/v) of ice-cold modified sucrose phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkman Polytron (setting 6 for 20 seconds; Kinematica AG, Lucerne, Switzerland) and centrifuged at 50,000g for 10 minutes at 4°C. The resulting pellet was resuspended in buffer, recentrifuged, and resuspended in buffer to a concentration of 10 mg/ml. Experiments were conducted in assay tubes containing 0.5 ml sucrose phosphate buffer for 120 minutes on ice. Each tube contained 0.5nM [3H]WIN35,428 (specific activity 76 Ci/mmol) (PerkinElmer Life and Analytical Sciences, Waltham, MA) and 1.0 mg of striatal tissue (original wet weight [OWW]). Nonspecific binding was determined using 0.1 mM cocaine HCl (Sigma-Aldrich, St. Louis, MO).
For σR binding, frozen whole guinea-pig brains (minus cerebellum) were thawed on ice, weighed, and homogenized (with a glass and Teflon homogenizer) in 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 (10 ml/g tissue). Guinea-pig brains were used because of the relatively higher density of σRs in that tissue compared with rat brains (Tam, 1983). The homogenate was centrifuged at 1000g for 10 minutes at 4°C. The supernatant was collected into a clean centrifuge tube, and the remaining pellet was resuspended by vortex in 10-ml buffer (10 mM Tris HCl, pH 8.0) and centrifuged again at 50,000g for 15 minutes at 4°C. The resulting pellet was resuspended in 50 mM Tris-HCl, pH 8.0 buffer to 80 mg/ml OWW. Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0. For σ1R binding, each tube contained 3 nM [3H](+)-pentazocine (PerkinElmer Life and Analytical Sciences) and 8.0 mg tissue, OWW. Nonspecific binding was determined using 10 μM haloperidol. For σ2R binding, each tube contained 3 nM [3H]1,3-di-o-tolylguanidine (DTG) (PerkinElmer Life and Analytical Sciences), 200 nM (+)-pentazocine, and 8.0 mg tissue, OWW. Nonspecific binding was determined using 100 μM haloperidol. The reaction was started with the addition of tissue, and the tubes were incubated for 120 minutes at room temperature.
Incubations for all binding assays were terminated by rapid filtration through Whatman GF/B filters (Whatman/GE Healthcare, Maidstone, Kent, United Kingdom), presoaked in polyethylenimine, using a Brandel R48 filtering manifold (Brandel, Gaitherburg, MD). The filters were washed twice with 5-ml ice cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter at 50% efficiency (Beckman Coulter, Brea, CA).
All assays were typically conducted in at least three independent experiments, each performed in triplicate. From the displacement data, IC50 values were computed using a nonlinear, least-squares regression analysis (GraphPad Prism; GraphPad Software Inc., San Diego, CA). Affinities (Ki values) were calculated using the Cheng-Prusoff equation (Cheng and Prusoff, 1973).
Drugs.
The drugs used in the present study and their salt and enantiomeric forms were as follows: d-methamphetamine HCl (Sigma-Aldrich), (−)-heroin HCl (RTI International, Research Triangle Park, NC), (±)-ketamine HCl (Fort Dodge Animal Health, Fort Dodge, IA), d-amphetamine hemi sulfate (Sigma-Aldrich), (−)-cocaine HCl (Merck, Whitehouse Station, NJ), WIN35,428 (National Institute on Drug Abuse, Drug Supply Program), remifentanil HCl (Ultiva; Hospira, Inc. Lake Forest, IL), dl-methadone HCl (Sigma-Aldrich), (−)-morphine sulfate salt pentahydrate (Sigma-Aldrich), (+)-MK-801 hydrogen maleate (Sigma-Aldrich), memantine HCl (Sigma-Aldrich), BD1008 di-HBr (Tocris Cookson, Ellisville, MO), and haloperidol (Sigma-Aldrich). The N-substituted BZT analogs JHW007 HCl, AHN2-005 oxalate, and AHN1-055 HCl were synthesized in the Medicinal Chemistry Section of National Institute on Drug Abuse Intramural Research Program according to procedures previously published elsewhere (Newman et al., 1995; Agoston et al., 1997). Saline (0.9% sodium chloride, USP; Hospira) was used as the vehicle for all compounds with heat and sonication, as necessary.
Self-administered drugs were delivered intravenously whereas those delivered as pretreatments were injected into the peritoneal cavity. Pretreatment compounds were administered at the following times before sessions: 5 minutes, d-amphetamine, WIN35,428, BD1008; 30 minutes, dl-methadone, (−)-morphine, (+)-MK-801, memantine; 90 minutes, AHN1-055; 120 minutes, AHN2-005; and 150 minutes, JHW007. Times were selected based on the published literature or preliminary data.
Results
Radioligand Binding.
Among N-substituted BZT analogs tested, JHW007 was the most potent in displacing the binding of the radioligands [3H](+)-pentazocine and [3H](+)-DTG from σ1 and σ2 receptors, respectively (Table 1). AHN2-005 was the second most potent in displacing both [3H](+)-pentazocine and [3H](+)-DTG to σ1 and σ2 receptors, respectively (Table 1). Though AHN1-055 was least potent in displacing binding of both [3H](+)-pentazocine and [3H](+)-DTG to σ1 and σ2 receptors, respectively, it was the most potent in displacing binding of [3H]WIN35,428 to the DAT among the DA uptake inhibitors tested (Table 1). The standard DA indirect-agonists (cocaine, WIN35,428, d-methamphetamine) had µM affinity for σ1 receptors and, with the exception of WIN35,428, had lower affinity for σ2 receptors. Neither heroin nor ketamine had appreciable affinity for σ1 or σ2 receptors.
TABLE 1.
Inhibition of binding of radioligands labeling DAT, σ1, and σ2 receptors
Values are Ki values, except as indicated, for displacement of the listed radioligands. Values in parentheses are 95% confidence limits, except as indicated. Values listed from previous studies were obtained in this laboratory with conditions identical to the ones presently employed.
| Compound | DAT Ki Value [3H]WIN35,428 | σ1R Ki Value [3H](+)-Pentazocine | σ2R Ki Value (High Affinity)a [3H]DTG |
|---|---|---|---|
| nM | |||
| Cocaineb | 76.6 (72.6–80.5) | 5190 (3800–7060) | 19,300 (16,000–23,300) |
| WIN35,428 | 5.24 (4.92–5.57)b,c | 5700 (4060–8020) | 4160 (3120–5550) |
| d-Methamphetamined | NT | 4390 (3740–5160) | 15,900 (11,800–21,500) |
| JHW007 | 12.0 (11.2–12.8)e | 2.40 (2.07–2.80) | 12.0 (10.0–14.4) |
| AHN2-005 | 8.82 (8.13–9.56) | 15.5 (13.2–18.3) | 28.5 (23.6–34.4) |
| AHN1-055 | 4.09 (3.67–4.56) | 119 (98.9–142) | 78.7 (50.6–122) |
| Heroin | NT | ND | 68,600 (27,100–173,000) |
| Ketamine | NT | 227,000 (53,400–963,000) | 36,900 (20,100–67,900) |
ND, no displacement at concentrations up to 10 mM; NT, not tested.
The value for displacement of DTG is a high-affinity site if the data modeled better for two than one site. The high-affinity site is similar to the site previously identified as the σ2 site.
All values are from Garces-Ramirez et al. (2011).
Value is a Kd value obtained by homologous competition experiments.
Values from Hiranita et al. (2013).
Values from Kopajtic et al. (2010).
Self-Administration.
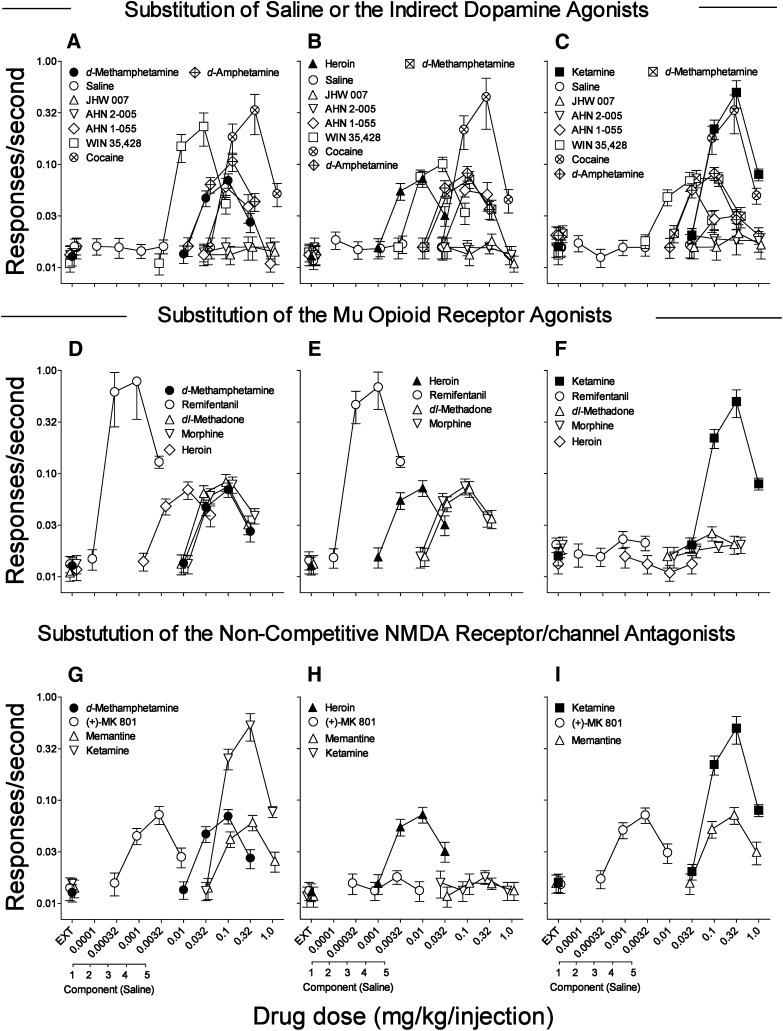
Dose-effect curves for d-methamphetamine, heroin, and ketamine self-administration were biphasic, with dose-related increases in rates of responding up to maximal at doses of 0.1, 0.01, and 0.32 mg/kg/injection which maintained rates of 0.070, 0.073, and 0.499 responses per second (Fig. 1, A–C, filled symbols; Table 2). In contrast, rates of responding obtained when saline was available were uniformly low, regardless of the drug with which self-administration was trained (Fig. 1, A–C, ○). Maximal rates of responding maintained by each of the training drugs were significantly greater than those for saline (Table 3). Further, the dose-effect curves for the training drugs over the course of our study were not appreciably changed from the first to last three replications, though there were statistically significant but small changes at some doses with each of the drugs (Table 4).
Fig. 1.
Substitution of various compounds in rats trained to self-administer either d-methamphetamine, heroin, or ketamine. Ordinates: responses per second, log scale; abscissae: drug dose (mg/kg/injection), log scale, or sequential component of the session. Each point represents the mean ± S.E.M. of six subjects. Each dose-effect curve of d-methamphetamine (0.01–0.32 mg/kg/injection), heroin (0.001–0.032 mg/kg/injection), and ketamine (0.032–1.0 mg/kg/injection) was shown as an average of 45, 53, and 45 assessments, respectively). Substitutions include: saline injections, JHW007, AHN2-005, AHN1-055 (0.032–1.0 mg/kg/injection, each), WIN35,428 (0.0032–0.1 mg/kg/injection), cocaine (0.032–1.0 mg/kg/injection), d-amphetamine (0.01–0.32 mg/kg/injection), and d-methamphetamine (panels B and C). (A) Dose-effects of substitutions of indirect DA agonists in subjects trained to self-administer d-methamphetamine. Effects were determined once except saline and d-amphetamine (shown as averages of two assessments, each). (B) Dose-effects of substitutions of indirect DA agonists in subjects trained to self-administer heroin. Each dose-effect curve was determined only once except saline (shown as an average of two assessments). (C) Dose-effects of substitutions of indirect DA agonists in subjects trained to self-administer ketamine. Each dose-effect curve was determined only once except saline (shown as an average of two assessments). (D) Dose-effects of substitutions of µ-opioid agonists in subjects trained to self-administer d-methamphetamine. Each dose-effect curve of the µ-opioid agonists tested was determined only once. (E) Dose-effects of substitutions of µ-opioid agonists in subjects trained to self-administer heroin. Each dose-effect curve of the µ-opioid agonists tested was determined only once except remifentanil (shown as an average of two assessments). (F) Dose-effects of substitutions of µ-opioid agonists in subjects trained to self-administer ketamine. Each dose-effect curve of the µ-opioid receptor agonist was determined once. (G) Dose-effects of substitutions of NMDA antagonists in subjects trained to self-administer d-methamphetamine. Each dose-effect curve of the NMDA antagonists was determined once. (H) Dose-effects of substitutions of NMDA antagonists in subjects trained to self-administer heroin. Each dose-effect curve of the noncompetitive NMDA receptor/channel antagonists tested was determined once. (I) Dose-effects of substitutions of NMDA antagonists in subjects trained to self-administer ketamine. Each dose-effect curve of the NMDA antagonists was determined once except (+)-MK-801 (shown as an average of two assessments).
TABLE 2.
Maximal responses rates (in responses/seconds) if maintained above saline values
| Substitution Compound | Self-Administered Drug |
||
|---|---|---|---|
| d-Methamphetamine | (−)-Heroin | (±)-Ketamine | |
| Saline | 0.0161 ± 0.0029 @ the second component | 0.0185 ± 0.0036 @ the second component | 0.0173 ± 0.0031 @ the second component |
| JHW007 | X | X | X |
| AHN2-005 | X | X | X |
| AHN1-055 | 0.061 ± 0.013 @ 0.1 mg/kg/inj | 0.056 ± 0.008 @ 0.1 mg/kg/inj | 0.032 ± 0.005 @ 0.32 mg/kg/inj |
| d-Methamphetamine | 0.070 ± 0.011 @ 0.1 mg/kg/inj | 0.072 ± 0.013 @ 0.1 mg/kg/inj | 0.072 ± 0.011 @ 0.1 mg/kg/inj |
| d-Amphetamine | 0.107 ± 0.021 @ 0.1 mg/kg/inj | 0.082 ± 0.013 @ 0.1 mg/kg/inj | 0.082 ± 0.012 @ 0.1 mg/kg/inj |
| WIN35,428 | 0.232 ± 0.083 @ 0.032 mg/kg/inj | 0.101 ± 0.016 @ 0.032 mg/kg/inj | 0.069 ± 0.014 @ 0.032 mg/kg/inj |
| (−)-Cocaine | 0.337 ± 0.142 @ 0.32 mg/kg/inj | 0.451 ± 0.232 @ 0.32 mg/kg/inj | 0.335 ± 0.137 @ 0.32 mg/kg/inj |
| (±)-Methadone | 0.083 ± 0.016 @ 0.1 mg/kg/inj | 0.071 ± 0.012 @ 0.1 mg/kg/inj | X |
| (−)-Morphine | 0.078 ± 0.014 @ 0.1 mg/kg/inj | 0.075 ± 0.013 @ 0.1 mg/kg/inj | X |
| (−)-Heroin | 0.070 ± 0.013 @ 0.01 mg/kg/inj | 0.073 ± 0.013 @ 0.01 mg/kg/inj | X |
| Remifentanil | 0.785 ± 0.449 @ 0.001 mg/kg/inj | 0.701 ± 0.369 @ 0.001 mg/kg/inj | X |
| (+)-MK 801 | 0.072 ± 0.014 @ 0.0032 mg/kg/inj | X | 0.072 ± 0.012 @ 0.0032 mg/kg/inj |
| (±)-Ketamine | 0.533 ± 0.159 @ 0.32 mg/kg/inj | X | 0.499 ± 0.149 @ 0.32 mg/kg/inj |
| Memantine | 0.061 ± 0.010 @ 0.32 mg/kg/inj | X | 0.072 ± 0.013 @ 0.32 mg/kg/inj |
X, did not substitute with rates above those maintained by saline.
TABLE 3.
Statistical analyses of dose-effect curves for training drugs or substitution with various compounds compared with saline availability as shown in the Fig. 1.
| Treatment | Drug | Dose | Interaction | Post Hoc Test |
|---|---|---|---|---|
| Subjects trained with d-methamphetamine | ||||
| d-Methamphetamine vs. saline substitution | F1,20 = 34.1; P = 0.002 | F4,20 = 31.6; P < 0.001 | F4,20 = 32.2; P < 0.001 | 0.032 mg/kg/inj, t = 6.22, P < 0.001 |
| 0.10 mg/kg/inj, t = 11.0, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 2.27, P = 0.034 | ||||
| WIN35,428 vs. saline substitution | F1,20 = 8.08; P = 0.036 | F4,20 = 7.49; P < 0.001 | F4,20 = 7.67; P < 0.001 | 0.01 mg/kg/inj, t = 3.26, P = 0.004 |
| 0.032 mg/kg/inj, t = 5.31, P < 0.001 | ||||
| Cocaine vs. saline substitution | F1,20 = 6.36; P = 0.053 | F4,20 = 5.27; P = 0.005 | F4,20 = 5.35; P = 0.004 | 0.1 mg/kg/inj, t = 2.48, P = 0.022 |
| 0.32 mg/kg/inj, t = 4.72, P < 0.001 | ||||
| d-Amphetamine vs. saline substitution | F 1,20 = 26.4; P = 0.004 | F4,20 = 22.0; P < 0.001 | F4,20 = 22.1; P < 0.001 | 0.032 mg/kg/inj, t = 4.88, P < 0.001 |
| 0.10 mg/kg/inj, t = 9.39, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 2.80, P = 0.012 | ||||
| JHW007 vs. saline substitution | F 1,20 = 3.40; P = 0.125 | F4,20 = 0.0539; P = 0.994 | F4,20 = 5.35; P = 0.712 | NS |
| AHN2-005 vs. saline substitution | F 1,20 = 0.606; P = 0.472 | F4,20 = 0.174; P = 0.949 | F4,20 = 0.863; P = 0.503 | NS |
| AHN1-055 vs. saline substitution | F 1,20 = 11.9; P = 0.018 | F4,20 = 11.3; P < 0.001 | F4,20 = 12.1; P < 0.001 | 0.1 mg/kg/inj, t = 6.77, P < 0.001 |
| 0.32 mg/kg/inj, t = 3.68, P = 0.001 | ||||
| Remifentanil vs. saline substitution | F 1,20 = 3.49; P = 0.121 | F4,20 = 2.84; P = 0.051 | F4,20 = 2.87; P = 0.050 | 0.00032 mg/kg/inj, t = 2.42, P = 0.025 |
| 0.001 mg/kg/inj, t = 3.09, P = 0.006 | ||||
| dl-Methadone vs. saline substitution | F 1,20 = 25.1; P = 0.004 | F4,20 = 23.1; P < 0.001 | F4,20 = 25.0; P < 0.001 | 0.032 mg/kg/inj, t = 6.38, P < 0.001 |
| 0.10 mg/kg/inj, t = 8.91, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 2.11, P = 0.048 | ||||
| Morphine vs. saline substitution | F 1,20 = 40.0; P = 0.001 | F4,20 = 21.9; P < 0.001 | F4,20 = 24.0; P < 0.001 | 0.032 mg/kg/inj, t = 6.74, P < 0.001 |
| 0.10 mg/kg/inj, t = 9.53, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 3.44, P = 0.002 | ||||
| Heroin vs. saline substitution | F 1,20 = 25.3; P = 0.004 | F4,20 = 19.9; P < 0.001 | F4,20 = 19.8; P < 0.001 | 0.0032 mg/kg/inj, t = 4.99, P < 0.001 |
| 0.01 mg/kg/inj, t = 8.47, P < 0.001 | ||||
| 0.032 mg/kg/inj, t = 3.61, P = 0.002 | ||||
| (+)-MK-801 vs. saline substitution | F 1,20 = 24.1; P = 0.004 | F4,20 = 18.9; P < 0.001 | F4,20 = 19.5; P < 0.001 | 0.001 mg/kg/inj, t = 4.59, P < 0.001 |
| 0.0032 mg/kg/inj, t = 9.04, P < 0.001 | ||||
| Memantine vs. saline substitution | F 1,20 = 24.2; P = 0.004 | F4,20 = 31.1; P < 0.001 | F4,20 = 26.1; P < 0.001 | 0.1 mg/kg/inj, t = 5.46, P < 0.001 |
| 0.32 mg/kg/inj, t = 9.60, P < 0.001 | ||||
| Ketamine vs. saline substitution | F 1,20 = 14.2; P = 0.013 | F4,20 = 10.7; P < 0.001 | F4,20 = 10.7; P < 0.001 | 0.1 mg/kg/inj, t = 3.21, P = 0.004 |
| 0.32 mg/kg/inj, t = 6.96, P < 0.001 | ||||
| Subjects trained with heroin | ||||
| Heroin vs. saline substitution | F1,20 = 26.6; P = 0.004 | F4,20 = 25.3; P < 0.001 | F4,20 = 28.5; P < 0.001 | 0.0032 mg/kg/inj, t = 6.57, P < 0.001 |
| 0.010 mg/kg/inj, t = 9.39, P < 0.001 | ||||
| 0.032 mg/kg/inj, t = 2.47, P = 0.025 | ||||
| d-Methamphetamine vs. saline substitution | F 1,20 = 20.5; P = 0.006 | F4,20 = 23.2; P < 0.001 | F4,20 = 22.0; P < 0.001 | 0.032 mg/kg/inj, t = 5.37, P < 0.001 |
| 0.1 mg/kg/inj, t = 8.25, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 2.91, P = 0.011 | ||||
| WIN35,428 vs. saline substitution | F 1,20 = 27.2; P = 0.003 | F4,20 = 28.8; P < 0.001 | F4,20 = 29.4; P < 0.001 | 0.01 mg/kg/inj, t = 6.80, P < 0.001 |
| 0.032 mg/kg/inj, t = 9.56, P < 0.001 | ||||
| Cocaine vs. saline substitution | F1,20 = 4.48; P = 0.088 | F4,20 = 3.64; P = 0.022 | F4,20 = 3.64; P = 0.022 | 0.32 mg/kg/inj, t = 4.01, P < 0.001 |
| d-Amphetamine vs. saline substitution | F1,20 = 32.2; P = 0.002 | F4,20 = 32.7; P < 0.001 | F4,20 = 34.5; P < 0.001 | 0.032 mg/kg/inj, t = 6.88, P < 0.001 |
| 0.1 mg/kg/inj, t = 10.5, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 3.08, P = 0.007 | ||||
| JHW007 vs. saline substitution | F1,20 = 0.253; P = 0.636 | F4,20 = 15.3; P < 0.001 | F4,20 = 4.38; P = 0.011 | NS |
| AHN2-005 vs. saline substitution | F1,20 = 1.57; P = 0.266 | F4,20 = 4.36; P = 0.011 | F4,20 = 1.78; P = 0.173 | 1.0 mg/kg/injection, t = 2.20, P = 0.038 |
| AHN1-055 vs. saline substitution | F1,20 = 12.7; P = 0.016 | F4,20 = 10.7; P < 0.001 | F4,20 = 11.5; P < 0.001 | 0.1 mg/kg/inj, t = 5.80, P < 0.001 |
| 0.32 mg/kg/inj, t = 5.02, P < 0.001 | ||||
| Remifentanil vs. saline substitution | F1,20 = 7.95; P = 0.037 | F4,20 = 6.15; P = 0.002 | F4,20 = 6.13; P = 0.002 | 0.00032 mg/kg/inj, t = 3.22, P = 0.004 |
| 0.001 mg/kg/inj, t = 4.81, P < 0.001 | ||||
| dl-Methadone vs. saline substitution | F1,20 = 36.8; P = 0.002 | F4,20 = 23.0; P < 0.001 | F4,20 = 25.1; P < 0.001 | 0.032 mg/kg/inj, t = 6.38, P < 0.001 |
| 0.1 mg/kg/inj, t = 9.82, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 3.46, P = 0.003 | ||||
| Morphine vs. saline substitution | F1,20 = 33.9; P = 0.002 | F4,20 = 24.2; P < 0.001 | F4,20 = 21.8; P < 0.001 | 0.032 mg/kg/inj, t = 6.10, P < 0.001 |
| 0.1 mg/kg/inj, t = 9.28, P < 0.001 | ||||
| 0.32 mg/kg/inj, t = 3.01, P = 0.007 | ||||
| (+)-MK-801 vs. saline substitution | F1,20 = 0.560; P = 0.488 | F4,20 = 5.82; P = 0.003 | F4,20 = 3.65; P = 0.022 | 0.01 mg/kg/inj, t = 2.22, P = 0.037 |
| Memantine vs. saline substitution | F1,20 = 3.16; P = 0.135 | F4,20 = 6.36; P = 0.002 | F4,20 = 1.88; P = 0.154 | 0.032 mg/kg/inj, t = 2.85, P = 0.009 |
| Ketamine vs. saline substitution | F1,20 = 1.07; P = 0.348 | F4,20 = 4.70; P = 0.008 | F4,20 = 1.06; P = 0.403 | NS |
| Subjects trained with ketamine | ||||
| Ketamine vs. saline substitution | F1,20 = 12.9; P = 0.016 | F4,20 = 10.8; P < 0.001 | F4,20 = 10.3; P < 0.001 | 0.1 mg/kg/inj, t = 2.92, P = 0.008 |
| 0.32 mg/kg/inj, t = 6.84, P < 0.001 | ||||
| d-Methamphetamine vs. saline substitution | F1,20 = 14.9; P = 0.012 | F4,20 = 26.5; P < 0.001 | F4,20 = 13.6; P < 0.001 | 0.032 mg/kg/inj, t = 5.95, P < 0.001 |
| 0.1 mg/kg/inj, t = 5.56, P < 0.001 | ||||
| WIN35,428 vs. saline substitution | F1,20 = 9.14; P = 0.029 | F4,20 = 22.8; P < 0.001 | F4,20 = 9.36; P < 0.001 | 0.01 mg/kg/inj, t = 3.45, P = 0.004 |
| 0.032 mg/kg/inj, t = 5.52, P < 0.001 | ||||
| Cocaine vs. saline substitution | F1,20 = 6.49; P = 0.051 | F4,20 = 5.90; P = 0.003 | F4,20 = 5.35; P = 0.004 | 0.1 mg/kg/inj, t = 2.45, P = 0.023 |
| 0.32 mg/kg/inj, t = 4.75, P < 0.001 | ||||
| d-Amphetamine vs. saline substitution | F1,20 = 17.9; P = 0.008 | F4,20 = 40.9; P < 0.001 | F4,20 = 16.1; P < 0.001 | 0.032 mg/kg/inj, t = 4.75, P < 0.001 |
| 0.1 mg/kg/inj, t = 7.59, P < 0.001 | ||||
| JHW007 vs. saline substitution | F1,20 = 0.644; P = 0.459 | F4,20 = 4.47; P = 0.010 | F4,20 = 0.414; P = 0.797 | NS |
| AHN2-005 vs. saline substitution | F1,20 = 0.609; P = 0.470 | F4,20 = 1.02; P = 0.421 | F4,20 = 2.00; P = 0.133 | NS |
| AHN1-055 vs. saline substitution | F1,20 = 3.66; P = 0.114 | F4,20 = 9.07; P < 0.001 | F4,20 = 0.932; P = 0.465 | NS |
| Remifentanil vs. saline substitution | F1,20 = 1.24; P = 0.317 | F4,20 = 3.49; P = 0.026 | F4,20 = 1.57; P = 0.221 | NS |
| dl-Methadone vs. saline substitution | F1,20 = 2.16; P = 0.201 | F4,20 = 7.49; P < 0.001 | F4,20 = 0.419; P = 0.793 | NS |
| Morphine vs. saline substitution | F1,20 = 1.34; P = 0.300 | F4,20 = 1.89; P = 0.151 | F4,20 = 1.32; P = 0.295 | NS |
| Heroin vs. saline substitution | F1,20 = 0.746; P = 0.427 | F4,20 = 0.904; P = 0.480 | F4,20 = 1.64; P = 0.203 | NS |
| (+)-MK-801 vs. saline substitution | F1,20 = 10.9; P = 0.021 | F4,20 = 30.1; P < 0.001 | F4,20 = 15.9; P < 0.001 | 0.001 mg/kg/inj, t = 4.15, P = 0.001 |
| 0.0032 mg/kg/inj, t = 6.39, P < 0.001 | ||||
| 0.01 mg/kg/inj, t = 2.37, P = 0.037 | ||||
| Memantine vs. saline substitution | F1,20 = 9.98; P = 0.025 | F4,20 = 27.5; P < 0.001 | F4,20 = 11.5; P < 0.001 | 0.1 mg/kg/inj, t = 3.88, P = 0.002 |
| 0.32 mg/kg/inj, t = 5.75, P < 0.001 |
NS, not statistically significant; inj, injection.
TABLE 4.
Statistical analyses of shifts in dose-effect curves of training drugs between the first and the last three assessments
| Treatment | First vs. Last Assessments | Dose | Interaction | Post Hoc Test |
|---|---|---|---|---|
| d-Methamphetamine | F1,20 = 12.5; P = 0.017 | F4,20 = 34.5; P < 0.001 | F4,20 = 21.1; P < 0.001 | 0.032 mg/kg/inj, t = 6.63, P < 0.001 (decrease of 0.013 responses/s) |
| 0.10 mg/kg/inj, t = 5.59, P < 0.001 (decrease of 0.011 responses/s) | ||||
| Heroin | F1,20 = 13.6; P = 0.014 | F4,20 = 26.8; P < 0.001 | F4,20 = 4.49; P = 0.009 | 10 µg/kg/inj, t = 3.28, P = 0.004 (increase of 0.006 responses/s) |
| 32 µg/kg/inj, t = 5.01, P < 0.001 (increase of 0.009 responses/s) | ||||
| Ketamine | F1,20 = 8.99; P = 0.030 | F4,20 = 11.8; P < 0.001 | F4,20 = 5.66; P = 0.003 | 0.32 mg/kg/inj, t = 5.37, P < 0.001 (increase of 0.075 responses/s) |
Substitution for the Drugs Used to Train Self-Administration.
When substituted, the standard DA indirect agonists WIN35,428, cocaine, and d-amphetamine maintained rates of responding greater than those with vehicle (Table 3), and with biphasic dose-effect curves (Fig. 1, A–C, □, ⊗, and  , respectively) similar to those of the training drugs. Maximal response rates maintained by the standard DA indirect agonists were greater than those maintained by d-methamphetamine and heroin, but not greater than those maintained by ketamine (Table 2). Substitution of d-methamphetamine also maintained rates of responding greater than vehicle in subjects trained with heroin and ketamine (Fig. 1, B and C,
, respectively) similar to those of the training drugs. Maximal response rates maintained by the standard DA indirect agonists were greater than those maintained by d-methamphetamine and heroin, but not greater than those maintained by ketamine (Table 2). Substitution of d-methamphetamine also maintained rates of responding greater than vehicle in subjects trained with heroin and ketamine (Fig. 1, B and C,  ; Table 3).
; Table 3).
In contrast to the effects of the standard DA indirect agonists, neither JHW007 nor AHN2-005 maintained rates of responding that were significantly different from those obtained with saline in subjects trained with d-methamphetamine, heroin, or ketamine across the entire range of doses tested (Fig. 1, A–C, △, ▽; Table 3). The other BZT analog tested, AHN1-055, maintained rates of responding that were a bitonic function of dose and statistically significantly greater than those obtained with saline in subjects trained with d-methamphetamine and heroin (Table 2), though at lower maximal rates than those maintained by cocaine, WIN35,428, and d-amphetamine (Fig. 1, A and B, ◊; Table 2). A bitonic dose-effect curve for substitution of AHN1-055 was also obtained in subjects trained with ketamine (Fig. 1C, ◊), but the maximal response rates were lower than those obtained in subjects trained with the other self-administered drugs (Table 2) and were not significantly greater than those obtained with saline (Table 3).
The pharmacologic specificity of the self-administration of the three training drugs was further tested with substitution tests using drugs from within and across pharmacologic class. The μ opioid-receptor agonists (remifentanil ○, dl-methadone △, morphine ▽, and heroin ◊, ▴) substituted (Fig. 1, D and E) for both d-methamphetamine and heroin. Remifentanil was approximately 10-fold more potent and maintained maximal response rates that were approximately 10-fold greater than those maintained by heroin (Table 2). Additionally, dl-methadone and morphine maintained rates of responding comparable to those maintained with heroin (Table 2) across the same range of doses (0.01–0.32 mg/kg/injection), and were each approximately 10-fold less potent than heroin (Fig. 1, D and E). The maximal effects of these compounds were significantly greater than those obtained with saline for each of the μ opioid-receptor agonists substituted for either heroin or d-methamphetamine (Table 3). None of the μ-opioid-receptor agonists maintained rates of responding greater than those obtained with saline in subjects trained with ketamine (Fig. 1F; Table 3).
For subjects trained with ketamine, both (+)-MK-801 (Fig. 1I, ○) and memantine (Fig. 1I, △) dose-dependently substituted and maintained rates of responding significantly greater than those obtained with saline (Table 3). Ketamine maintained rates of responding that were approximately 7-fold greater than those of the other noncompetitive NMDA receptor/channel antagonists (Table 2), and (+)-MK-801 was approximately 100-fold more potent than ketamine and memantine. These compounds as well as ketamine maintained response rates that were significantly greater than that obtained with vehicle in subjects trained with d-methamphetamine (Fig. 1G, ○, △, ▽; Table 3). Additionally, the pattern of substitution obtained with the NMDA antagonists, the potency relations and the differences in maximal response rates were similar in subjects trained with ketamine and d-methamphetamine (Fig. 1G). In contrast, none of the noncompetitive NMDA antagonists maintained rates of responding significantly greater than those obtained with saline in subjects trained with heroin (Fig. 1H; Table 3).
Effects of Pretreatments on Self-Administration.
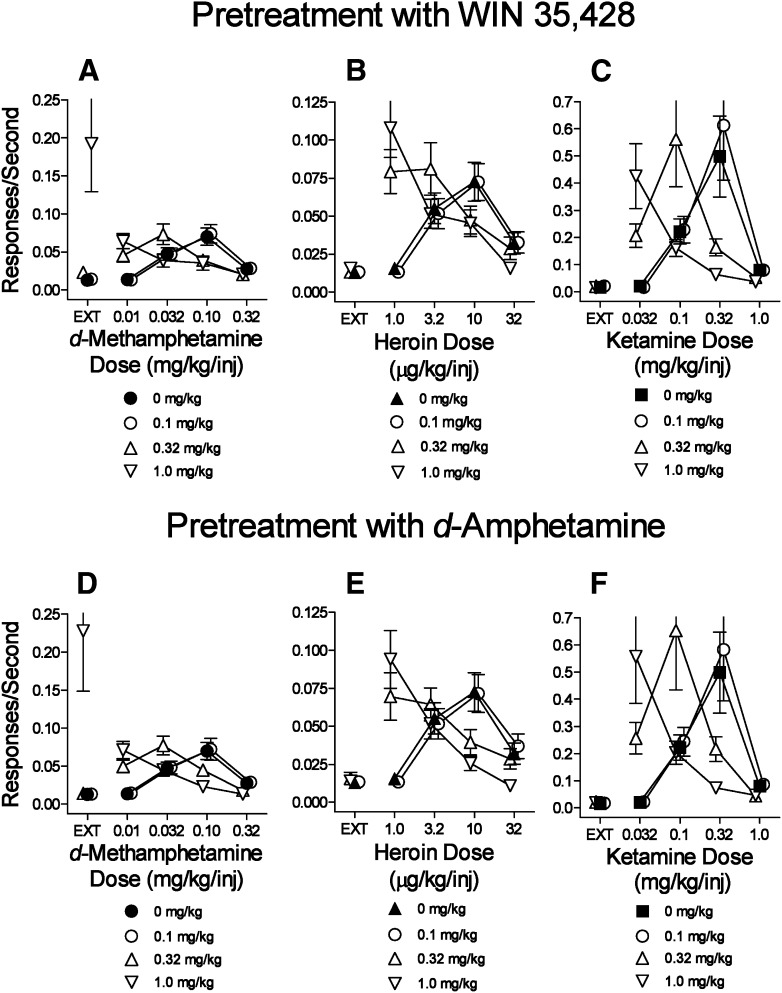
The standard DA indirect-agonists (WIN35,428, d-amphetamine) produced dose-related leftward shifts in the dose-effect curve for d-methamphetamine self-administration (Fig. 2, A and D). For both standard indirect agonists, the pretreatment dose, d-methamphetamine dose, and their interactions were statistically significant (Table 5). In addition, the highest doses of WIN35,428 and d-amphetamine increased rates of responding maintained during the EXT component (Fig. 2, A and D, ▽ above EXT). Presession treatments with these drugs also dose-dependently left-shifted the self-administration dose-effect curves for heroin (Fig. 2, B and E) and ketamine (Fig. 2, C and F). The statistical analyses indicated significance of each dose factor and their interactions (Table 5). In contrast to the effects obtained with d-methamphetamine self-administration, the highest doses of WIN35,428 and d-amphetamine did not increase the rates of responding maintained in the EXT components when either heroin and ketamine were the training drugs (Fig. 2, B and C, E and F).
Fig. 2.
Effects of presession treatments with standard DA indirect-agonists WIN35,428 or d-amphetamine on self-administration of d-methamphetamine, heroin, or ketamine. Ordinates: responses per second; abscissae: drug dose (mg/kg/injection or μg/kg/injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. A specified dose of 0 mg/kg of each test compound indicates saline vehicle pretreatments. (A–C) Effects of the DA-uptake inhibitor WIN35,428 on self-administration of d-methamphetamine, heroin, or ketamine, respectively. (D–F) Effects of the DA releaser d-amphetamine on self-administration of d-methamphetamine, heroin, or ketamine, respectively.
TABLE 5.
Statistical analyses of effects of pretreatments on the self-administration of d-methamphetamine, heroin, ketamine, and remifentanil substitution for heroin as shown in Figs. 2–6.
| Treatment | Self-Administered Drug Dose (i.v.) | Treatment Dose (i.p.) | Interaction | Post Hoc Tests |
|---|---|---|---|---|
| WIN35,428 pretreatments (i.p.) | ||||
| d-Methamphetamine self-administration | F4,60 = 5.29; P = 0.005 | F3,60 = 7.40; P = 0.003 | F12,60 = 9.77; P < 0.001 | 1.0 mg/kg (i.p.) at EXT, t = 9.11, P < 0.001 |
| Heroin self-administration | F4,60 = 24.9; P < 0.001 | F3,60 = 17.7; P < 0.001 | F12,60 = 20.4; P < 0.001 | 0.32 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 8.65, P < 0.001 |
| 1.0 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 12.6, P < 0.001 | ||||
| 0.32 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 3.52, P = 0.005 | ||||
| 0.32 mg/kg (i.p.) at 10 µg/kg/inj, t = 3.44, P = 0.006 | ||||
| 1.0 mg/kg (i.p.) at 10 µg/kg/inj, t = 3.71, P = 0.002 | ||||
| Ketamine self-administration | F4,60 = 11.3; P < 0.001 | F3,60 = 9.17; P = 0.001 | F12,60 = 8.75; P < 0.001 | 1.0 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 5.07, P < 0.001 |
| 0.32 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 4.25, P < 0.001 | ||||
| 0.32 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 4.20, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 5.45, P < 0.001 | ||||
| d-Amphetamine pretreatments (i.p.) | ||||
| d-Methamphetamine self-administration | F3,60 = 4.43; P = 0.010 | F3,60 = 6.83; P = 0.004 | F12,60 = 8.75; P < 0.001 | 1.0 mg/kg (i.p.) at EXT, t = 8.60, P < 0.001 |
| Heroin self-administration | F4,60 = 24.6; P < 0.001 | F3,60 = 5.41; P = 0.010 | F12,60 = 22.6; P < 0.001 | 0.32 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 8.18, P < 0.001 |
| 1.0 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 11.9, P < 0.001 | ||||
| 0.32 mg/kg (i.p.) at 10 µg/kg/inj, t = 5.03, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 10 µg/kg/inj, t = 7.13, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 32 µg/kg/inj, t = 3.18, P = 0.013 | ||||
| Ketamine self-administration | F4,60 = 11.2; P < 0.001 | F3,60 = 9.18; P = 0.001 | F12,60 = 7.97; P < 0.001 | 1.0 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 5.85, P < 0.001 |
| 0.32 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 4.70, P < 0.001 | ||||
| 0.32 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 3.07, P = 0.019 | ||||
| 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 4.63, P < 0.001 | ||||
| JHW007 pretreatments (i.p.) | ||||
| d-Methamphetamine self-administration | F4,60 = 29.5; P < 0.001 | F3,60 = 20.7; P < 0.001 | F12,60 = 18.8; P < 0.001 | 3.2 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 5.61, P < 0.001 |
| 10 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 7.39, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 8.91, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 12.0, P < 0.001 | ||||
| Heroin self-administration | F4,60 = 30.7; P < 0.001 | F3,60 = 0.771; P = 0.528 | F12,60 = 1.75; P = 0.078 | 3.2 mg/kg (i.p.) at 32 µg/kg/inj, t = 3.95, P = 0.001 |
| Ketamine self-administration | F4,60 = 12.1; P < 0.001 | F3,60 = 5.35; P = 0.001 | F12,60 = 4.99; P < 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 7.28, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 5.46, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 7.67, P < 0.001 | ||||
| AHN2-005 pretreatments (i.p.) | ||||
| d-methamphetamine self-administration | F4,60 = 20.0; P < 0.001 | F3,60 = 15.4; P < 0.001 | F12,60 = 10.3; P < 0.001 | 3.2 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 2.76, P = 0.046 |
| 10 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 6.20, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 4.25, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 9.73, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 3.11, P = 0.017 | ||||
| Heroin self-administration | F4,60 = 28.4; P < 0.001 | F3,60 = 0.131; P = 0.940 | F12,60 = 1.32; P = 0.234 | NS (versus vehicle, i.p.) |
| Ketamine self-administration | F4,60 = 11.7; P < 0.001 | F3,60 = 3.64; P = 0.038 | F12,60 = 4.11; P < 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 4.25, P < 0.001, |
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 6.37, P < 0.001 | ||||
| AHN1-055 pretreatments (i.p.) | ||||
| d-Methamphetamine self-administration | F4,60 = 6.29; P = 0.002 | F3,60 = 9.97; P < 0.001 | F12,60 = 11.6; P < 0.001 | 3.2 mg/kg (i.p.) at EXT, t = 9.35, P < 0.001 |
| 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.37, P = 0.007 | ||||
| 10 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.09, P = 0.017 | ||||
| Heroin self-administration | F4,60 = 26.6; P < 0.001 | F3,60 = 1.55; P = 0.243 | F12,60 = 2.43; P = 0.013 | 1.0 mg/kg (i.p.) at 10 µg/kg/inj, t = 3.08, P = 0.019 |
| Ketamine self-administration | F4,60 = 11.5; P < 0.001 | F3,60 = 8.58; P = 0.001 | F12,60 = 5.82; P < 0.001 | 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.34, P = 0.008 |
| 3.2 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 6.76, P < 0.001 | ||||
| dl-Methadone pretreatment (i.p.) | ||||
| Heroin self-administration | F4,60 = 29.2; P < 0.001 | F3,60 = 25.4; P < 0.001 | F12,60 = 8.22; P < 0.001 | 1.0 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 3.99, P < 0.001 |
| 3.2 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 6.20, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 10 µg/kg/inj, t = 5.69, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 10 µg/kg/inj, t = 9.17, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 32 µg/kg/inj, t = 3.78, P = 0.002 | ||||
| Remifentanil self-administration | F4,60 = 12.1; P < 0.001 | F3,60 = 7.60; P = 0.003 | F12,60 = 5.27; P < 0.001 | 3.2 mg/kg (i.p.) at 0.32 µg/kg/inj, t = 3.25, P = 0.011 |
| 10 mg/kg (i.p.) at 0.32 µg/kg/inj, t = 4.94, P < 0.001 | ||||
| 1.0 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 2.86, P = 0.035 | ||||
| 3.2 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 5.20, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 7.40, P < 0.001 | ||||
| d-Methamphetamine self-administration | F4,60 = 35.3; P < 0.001 | F3,60 = 0.895; P = 0.467 | F12,60 = 3.12; P = 0.002 | 10 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 2.88, P = 0.036 |
| Ketamine self-administration | F4,60 = 11.2; P < 0.001 | F3,60 = 6.63; P = 0.005 | F12,60 = 5.79; P < 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 5.84, P < 0.001 |
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 7.42, P < 0.001 | ||||
| Morphine pretreatments (i.p.) | ||||
| Heroin self-administration | F4,60 = 29.1; P < 0.001 | F3,60 = 23.4; P < 0.001 | F12,60 = 26.1; P < 0.001 | 3.2 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 6.74, P < 0.001 |
| 10 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 7.79, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 10 µg/kg/inj, t = 8.33, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 10 µg/kg/inj, t = 12.0, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 32 µg/kg/inj, t = 4.40, P < 0.001 | ||||
| Remifentanil self-administration | F4,60 = 5.62; P = 0.003 | F3,60 = 6.83; P = 0.004 | F12,60 = 5.27; P < 0.001 | 3.2 mg/kg (i.p.) at 0.32 µg/kg/inj, t = 3.39, P = 0.007 |
| 10 mg/kg (i.p.) at 0.32 µg/kg/inj, t = 4.70, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 4.27, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 7.03, P < 0.001 | ||||
| Ketamine self-administration | F4,60 = 8.92; P < 0.001 | F3,60 = 4.74; P = 0.016 | F12,60 = 4.53; P < 0.001 | 1.0 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 6.35, P < 0.001 |
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 3.92, P = 0.001 | ||||
| d-Methamphetamine self-administration | F4,60 = 29.0; P < 0.001 | F3,60 = 1.10; P = 0.381 | F12,60 = 2.09; P = 0.031 | 1.0 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.06, P = 0.019 |
| (+)-MK-801 pretreatments (i.p.) | ||||
| Ketamine self-administration | F4,60 = 10.8; P < 0.001 | F3,60 = 7.92; P = 0.002 | F12,60 = 9.50; P < 0.001 | 0.1 mg/kg (i.p.) at EXT, t = 3.07, P = 0.019 |
| 0.1 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 5.71, P < 0.001 | ||||
| 0.032 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.18, P = 0.014 | ||||
| 0.032 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 5.69, P < 0.001 | ||||
| 0.1 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 4.27, P < 0.001 | ||||
| d-Methamphetamine self-administration | F4,60 = 27.7; P < 0.001 | F3,60 = 9.42; P < 0.001 | F12,60 = 24.8; P < 0.001 | 0.032 mg/kg (i.p.) at 0.01 mg/kg/inj, t = 5.67, P < 0.001 |
| 0.1 mg/kg (i.p.) at 0.01 mg/kg/inj, t = 9.86, P < 0.001 | ||||
| 0.01 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 3.38, P = 0.007 | ||||
| 0.032 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 3.71, P = 0.002 | ||||
| 0.032 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 5.07, P < 0.001 | ||||
| 0.1 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 9.05, P < 0.001 | ||||
| Heroin self-administration | F4,60 = 27.4; P < 0.001 | F3,60 = 11.1; P < 0.001 | F12,60 = 1.44; P = 0.175 | NS (vs. vehicle, i.p.) |
| Remifentanil self-administration | F4,20 = 5.39; P = 0.004 | F1,20 = 9.12; P = 0.029 | F4,20 = 7.01; P = 0.001 | 0.1 mg/kg (i.p.) at 0.32 µg/kg/inj, t = 5.06, P < 0.001 |
| 0.1 mg/kg (i.p.) at 1.0 µg/kg/inj, t = 3.62, P = 0.002 | ||||
| Memantine pretreatments (i.p.) | ||||
| Ketamine self-administration | F4,60 = 12.5; P < 0.001 | F3,60 = 12.2; P < 0.001 | F12,60 = 10.5; P < 0.001 | 10 mg/kg (i.p.) at EXT, t = 3.97, P = 0.001 |
| 3.2 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 3.12, P = 0.016 | ||||
| 10 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 7.01, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 3.32, P = 0.009 | ||||
| 3.2 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 5.01, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 6.18, P < 0.001 | ||||
| Heroin self-administration | F4,60 = 7.36; P < 0.001 | F3,60 = 4.95; P = 0.014 | F12,60 = 3.92; P < 0.001 | 1.0 mg/kg (i.p.) at 3.2 µg/kg/inj, t = 4.03, P < 0.001 |
| 1.0 mg/kg (i.p.) at 10 µg/kg/inj, t = 5.16, P < 0.001 | ||||
| Remifentanil self-administration | F4,20 = 4.54; P = 0.009 | F1,20 = 0.797; P = 0.413 | F4,20 = 0.789; P = 0.546 | NS (vs. vehicle, i.p.) |
| 0.1 mg/kg WIN35,428 and BD1008 pretreatments (i.p.) | ||||
| d-Methamphetamine self-administration in rats trained with d-methamphetamine | F4,100 = 28.5; P < 0.001 | F5,100 = 34.5; P < 0.001 | F20,100 = 22.2; P < 0.001 | 3.2 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 6.42, P < 0.001 |
| 10 mg/kg (i.p.) at 0.032 mg/kg/inj, t = 8.38, P < 0.001 | ||||
| 3.2 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 9.57, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.10 mg/kg/inj, t = 13.4, P < 0.001 | ||||
| 10 mg/kg (i.p.) at 0.32 mg/kg/inj, t = 3.47, P = 0.011 | ||||
| Heroin self-administration in rats trained with heroin | F4,20 = 30.0; P < 0.001 | F1,20 = 7.11; P = 0.045 | F4,20 = 1.72; P = 0.185 | NS (vs. vehicle, i.p.) |
| Ketamine self-administration in rats trained with ketamine | F4,20 = 11.2; P < 0.001 | F1,20 = 2.59; P = 0.169 | F4,20 = 4.02; P = 0.015 | NS (vs. vehicle, i.p.) |
NS, not statistically significant; inj, injection.
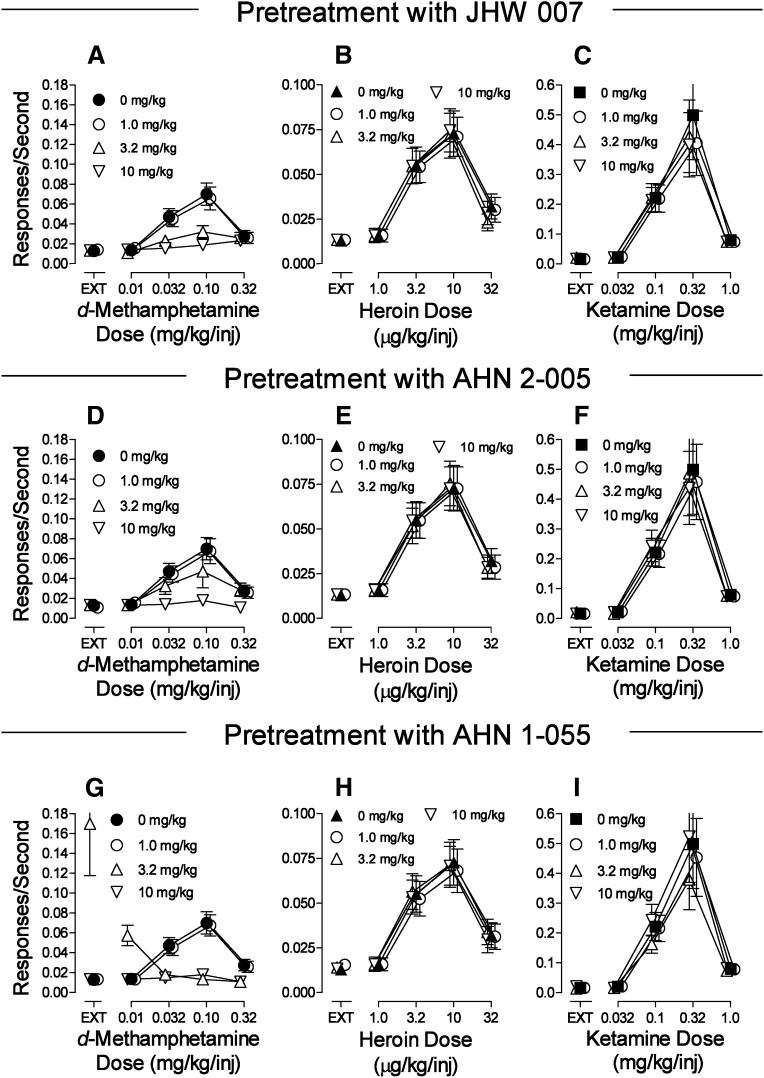
Pretreatment with JHW007 produced a dose-related decrease in the maximal rate of d-methamphetamine self-administration (Fig. 3A) with statistical significance of each dose factor and their interactions (Table 5). In contrast, none of the doses of JHW007 that were active in altering d-methamphetamine self-administration significantly altered the self-administration of heroin (Fig. 3B; Table 5). JHW007 significantly decreased ketamine self-administration at the dose of ketamine that maintained maximal rates of responding (Fig. 3C; Table 5). However, the decreases were small, of the same slight magnitude (averaging 0.088 responses per sec with an SEM of about 10%), and were independent of JHW007 dose (Fig. 3C; Table 5).
Fig. 3.
Effects of presession treatments with N-substituted BZT analogs on self-administration of d-methamphetamine, heroin, or ketamine. Ordinates: responses per second; abscissae: drug dose (mg/kg/injection or μg/kg/injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. A dose of 0 mg/kg of each test compound indicates saline vehicle injections. (A–C) Effects of JHW007 on self-administration of d-methamphetamine, heroin, or ketamine, respectively. (D–F) Effects of AHN2-005 on self-administration of d-methamphetamine, heroin, or ketamine, respectively. (G–I) Effects of AHN1-055 on self-administration of d-methamphetamine, heroin, or ketamine, respectively.
Pre-treatments with AHN2-005 also produced dose-related decreases in the maximal rate of d-methamphetamine self-administration (Fig. 3D; Table 5). At the highest dose of AHN2-005, no dose of d-methamphetamine maintained responding at levels above those maintained in the EXT component (Fig. 3D). Across the range of AHN2-005 doses that decreased d-methamphetamine self-administration, there were no significant effects on heroin self-administration (Fig. 3E; Table 5), though there were small but significant effects on ketamine self-administration at the 0.32 mg/kg/injection dose, averaging 0.051 responses per second at doses of 1.0 and 10.0 mg/kg of AHN2-005 (Fig. 3F; Table 5).
The effects of AHN1-055 pretreatment differed somewhat from those of the other BZT analogs. The intermediate dose of AHN1-055 shifted the d-methamphetamine self-administration dose-effect curve leftward, and increased rates of responding obtained in the EXT component (Fig. 3G, △; Table 5). However, the highest dose of AHN1-055 produced an insurmountable antagonism over the entire range of d-methamphetamine doses (Fig. 3G, ▽; Table 5). As with the other BZT analogs, no dose of AHN1-055 significantly altered heroin self-administration dose-effect curves (Fig. 3H; Table 5), though there were small but significant effects of 3.2 mg/kg of AHN1-055 on self-administration of 0.1 and 0.32 mg/kg/injection of ketamine that, as previously, were small, averaging 0.090 responses per second (Fig. 3I; Table 5).
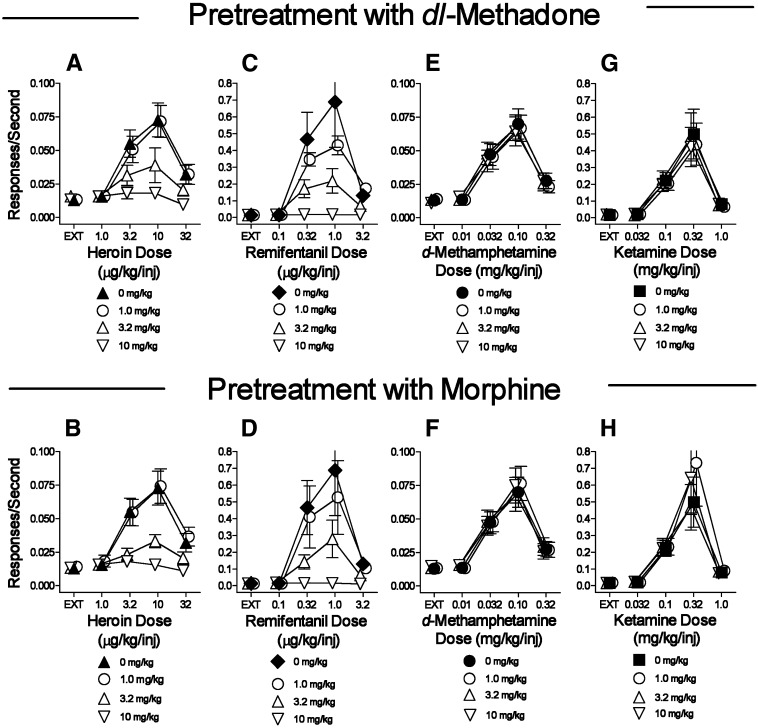
To put the distinct effects of pretreatments with the BZT analogs and standard DA indirect agonists in pharmacologic context, the effects of pretreatments both within and across class were assessed with µ-opioid agonists and ketamine self-administration. Pretreatments with µ-opioid agonists (dl-methadone, morphine) dose-dependently decreased the maximal self-administration of heroin and remifentanil (Fig. 4, A–D; Table 5). At the highest doses of the µ-agonist pretreatments, no dose of heroin or remifentanil maintained responding at levels above those obtained in the EXT component (Fig. 4, A–D). In contrast, neither dl-methadone nor morphine had effects on d-methamphetamine self-administration dose-effect curves (Fig. 4, E and F; Table 5) across the same range of doses that decreased µ-agonist self-administration. With ketamine self-administration, dl-methadone produced small but significant increases, whereas morphine produced small but significant increases in response rates at doses of 1.0 and 10.0 mg/kg at the 0.32 mg/kg ketamine dose/injection (Fig. 3, G and H; Table 5).
Fig. 4.
Effects of presession treatments with dl-methadone or morphine on self-administration of d-methamphetamine, heroin, remifentanil substitution for heroin, or ketamine. Ordinates: responses per second; abscissae: drug dose (mg/kg/injection or μg/kg/injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. A dose of 0 mg/kg of each test compound indicates saline vehicle injections. (A, C, E, G) Effects of dl-methadone on self-administration of heroin, remifentanil, d-methamphetamine, or ketamine, respectively. (B, D, F, H) Effects of morphine on self-administration of heroin, remifentanil, d-methamphetamine, or ketamine, respectively.
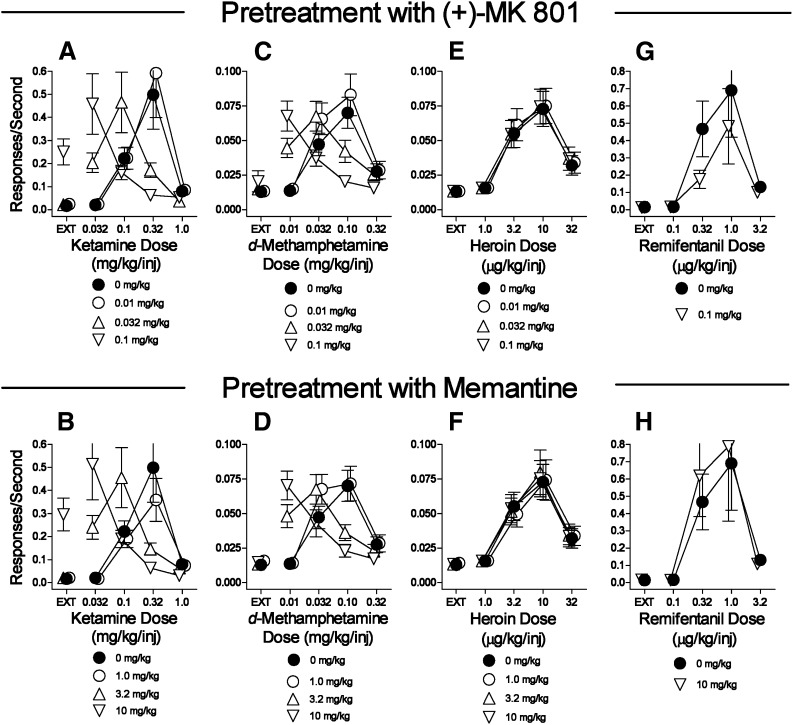
Pretreatments with the noncompetitive NMDA receptor antagonists (+)-MK-801 and memantine dose-dependently shifted the dose-effect curves for ketamine self-administration leftward (Fig. 5, A and B; Table 5). Further, the highest doses of (+)-MK-801 and memantine increased the rates of responding maintained during the EXT component (Fig. 5, A and B). Similarly, presession treatments with (+)-MK-801 or memantine dose-dependently shifted the d-methamphetamine self-administration dose-effect curve to the left, but without effects on rates of responding during the EXT component (Fig. 5, C and D; Table 5). Both (+)-MK-801 and memantine at doses that were active against d-methamphetamine and ketamine self-administration were inactive against self-administration of either µ-opioid agonist (Fig. 5, E–H; Table 5).
Fig. 5.
Effects of presession treatments with (+)-MK-801 or memantine on self-administration of d-methamphetamine, heroin, remifentanil substitution for heroin, or ketamine. Ordinates: responses per second; abscissae: drug dose (mg/kg/injection or μg/kg/injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. A dose of 0 mg/kg of each test compound indicates saline vehicle injections. (A, C, E, G) Effects of (+)-MK-801 on self-administration of ketamine, d-methamphetamine, heroin, or remifentanil, respectively. (B, D, F, H) Effects of memantine on self-administration of ketamine, d-methamphetamine, heroin, or remifentanil, respectively.
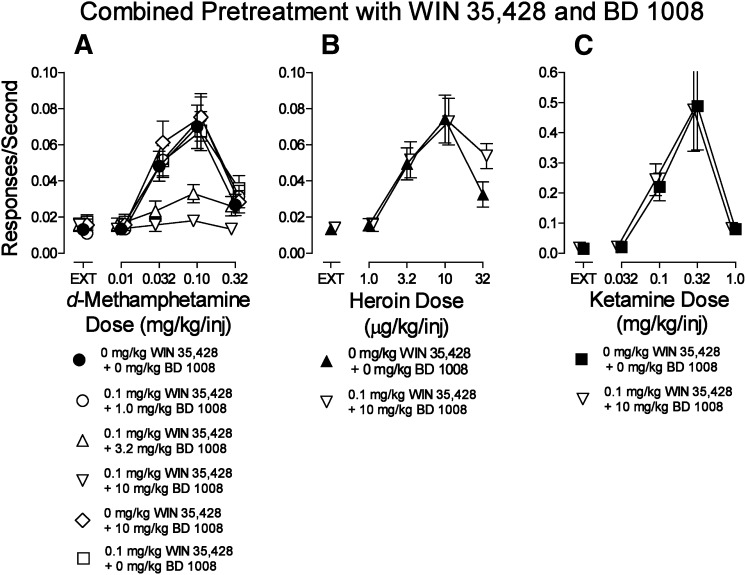
The present radioligand binding studies indicated that the N-substituted BZT analogs tested have affinities for σRs as well as the DAT; a previous study had indicated that combinations of these effects can selectively decrease cocaine self-administration (Hiranita et al., 2011). Therefore, we assessed the effects on d-methamphetamine self-administration of combinations of the DA uptake inhibitor WIN35,428 and the σR antagonist BD1008. Pretreatment with 10.0 mg/kg of BD1008 alone did not affect d-methamphetamine self-administration (Fig. 6A, compare ⬤ with ◊). Additionally, 0.1 mg/kg of WIN35,428 alone did not alter d-methamphetamine self-administration (Fig. 6A, compare ⬤ with □; Fig. 2A). In contrast, when the drugs were administered in combination, the self-administration of d-methamphetamine was decreased in a BD1008 dose-dependent manner (Fig. 6A). Statistical analysis (Table 5) indicated significant effects of the d-methamphetamine dose, pretreatment (BD1008 dose in combination with 0.1 mg/kg WIN35,428), and their interaction. The dose-combination that was most effective against d-methamphetamine self-administration was ineffective in the subjects that self-administered either heroin (Fig. 6B) or ketamine (Fig. 6C).
Fig. 6.
Effects of presession treatments with WIN35,428 (0.1 mg/kg) combined with the σR antagonist BD1008 on self-administration of d-methamphetamine, heroin, or ketamine. Ordinates: responses per second; abscissae: drug dose (mg/kg/injection or μg/kg/injection), log scale. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects. A 0 mg/kg dose of each test compound indicates saline vehicle injections. (A) d-Methamphetamine self-administration. (B) Heroin self-administration. (C) Ketamine self-administration.
Effects of Prefeeding on Responding Maintained by Food Reinforcement.
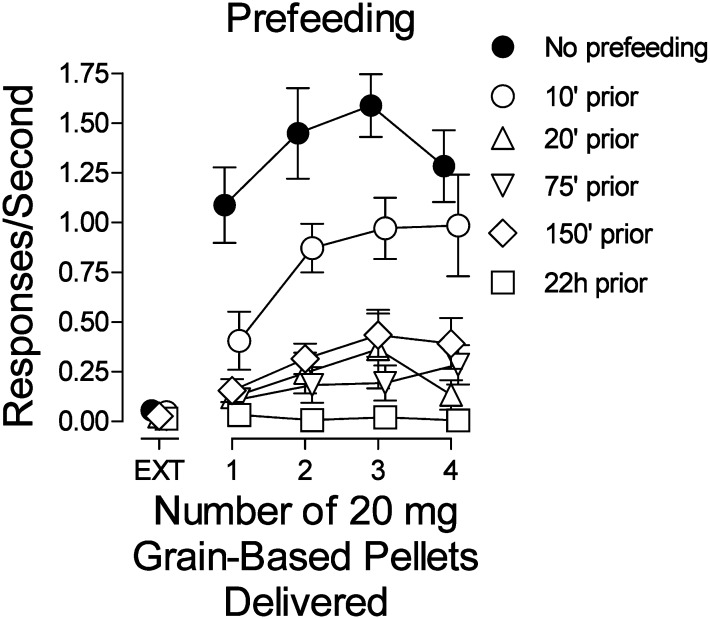
In subjects studied with food reinforcement, the rates of responding were low during the first component in which food was not delivered (EXT). In the subsequent components, the response rates increased to a maximum with three pellets delivered and decreased with four pellets delivered on completion of each FR 5 (Fig. 7, ⬤). Feeding subjects before sessions produced a decrease in response rates, primarily in the components in which one to three pellets were delivered. Longer times with food before sessions (20–150 minutes) produced further but comparable decreases across the four components in which food was available. With food continuously available (22 hours), response rates were virtually eliminated throughout the session (Fig. 7, compare □ with ⬤). An ANOVA indicated that prefeeding time (F3,60 = 33.3, P < 0.001), number of pellets delivered (F4,60 = 37.7, P < 0.001), and their interaction (F12,60 = 9.01, P < 0.001) were statistically significant.
Fig. 7.
Effects of presession unrestricted access to 1.0 g chocolate pellets on responding maintained by response-dependent presentation of 20-mg grain-based food pellets. Ordinates: responses per second; abscissae: number of 20-mg grain-based food pellet delivered per reinforcement. Each point represents the mean ± S.E.M. of response rates on the active lever in six subjects.
Discussion
Substitution for the Self-Administration Training Drugs.
In the present study, standard DA indirect agonists (d-methamphetamine, cocaine, WIN35,428, and d-amphetamine) were self-administered at rates greater than those obtained with vehicle injections (compare Yokel and Pickens, 1973). In contrast and as reported previously (Hiranita et al., 2009), the BZT analogs AHN2005 and JHW007 failed to maintain self-administration in subjects trained to self-administer d-methamphetamine, whereas AHN1-055 was self-administered above saline levels, though at rates less than those maintained by the standard DA indirect agonists. The differences between BZT analogs and standard DA indirect agonists were obtained in all subjects with each training drug. Combined with the absence of place conditioning with these (Li et al., 2005; Velázquez-Sánchez et al., 2009, 2010) or other BZT analogs (Li et al., 2011; Tanda et al., 2013), the results are consistent with reduced or minimal abuse liability of BZT analogs.
Subjects trained to self-administer abused drugs generally self-administer other drugs from the same class. For example, the heroin-trained subjects in our study self-administered other µ-opioid agonists, with potencies related to affinities for µ-opioid receptors (e.g., Woods et al., 1981; France et al., 1995) and maximal effects related to duration of action (Panlilio and Schindler, 2000; Ko et al., 2002). Similarly, ketamine-trained subjects self-administered other NMDA antagonists with potencies related to their NMDA receptor affinities (e.g., Bresink et al., 1995) and maximal rates related to their durations of action (Winger et al., 2002). These findings in other systems, along with the affinities for the DAT comparing favorably among the standard DA-uptake inhibitors and the BZT analogs, suggest that differences in their substitution profiles are a reflection of fundamental differences in their mechanisms of action.
The lack of cross-substitution among µ-opioid agonists and NMDA antagonists is not without precedent. Young and Woods (1981) showed that several NMDA antagonists substitute in rhesus monkeys trained with ketamine but not codeine self-administration. Thus, experience with self-administration can influence which other drugs substitute. However, it appears that some DA indirect agonists have broader substitution profiles. Importantly, the substitution profile obtained with the standard DA indirect agonists was more circumscribed, particularly with the BZT analogs JHW007 and AHN2-005.
Pretreatment Effects on Self-Administration.
The BZT analogs decreased maximal rates of responding maintained by d-methamphetamine, whereas standard DA indirect agonists shifted the d-methamphetamine self-administration dose-effect curve leftward, an effect reported previously with cocaine self-administration (e.g., Schenk, 2002; Barrett et al., 2004; Ferragud et al., 2009; Hiranita et al., 2011; Li et al., 2013). To better understand these effects, our study compared pretreatments on the self-administration of other classes of abused drugs as well as to effects of pretreatments with drugs from within and across pharmacologic class.
The standard DA indirect-agonists each produced dose-dependent leftward shifts in the dose-effect curves for each self-administered drug. In contrast, decreases in maximal self-administration produced by BZT analogs were specific to stimulant self-administration, as there were no appreciable effects on heroin or ketamine self-administration. That effects of the standard DA indirect agonists were similar across self-administered drug classes suggests a general effect, such as the stimulation of operant response rates (e.g., Dews, 1958). In contrast, the BZT analogs did not increase response rates maintained by d-methamphetamine at any doses, consistent with earlier reports that these compounds generally produce blunted stimulant-like effects compared with the standard DA indirect agonists (e.g., Katz et al., 1999, 2004; Ferragud et al., 2009). However, the decreases in self-administration were selective for d-methamphetamine and cocaine self-administration and were not obtained with self-administration of other drugs or food reinforcement (Hiranita et al., 2009; Li et al., 2013). Taken together, these findings indicate that the BZT analogs produced specific decreases in stimulant self-administration.
The effects of other pretreatments varied across classes of self-administered drugs. Both dl-methadone and morphine decreased maximal self-administration of µ-opioid agonists without effects on self-administration of d-methamphetamine and ketamine. Previous studies showed similar results in rhesus monkeys (Harrigan and Downs, 1981; Winger et al., 1992). In addition, dl-methadone dose-dependently decreased heroin self-administration in human opioid-dependent volunteers in a similar manner (Donny et al., 2005). In contrast, (+)-MK-801 and memantine shifted the self-administration dose-effect curve for ketamine (and d-methamphetamine) leftward but had no effect on the dose-effect curves for the µ-opioid agonists. Pretreatments with the NMDA antagonists were similar to the effects of the standard DA indirect agonists in that dose-effect curves for at least some drugs of abuse from outside their restricted class were similarly affected. However, the NMDA antagonists were inactive against µ-opioid agonist self-administration whereas the DA indirect agonists had a more general effect.
Thus, there were two types of effects of drug pretreatments on drug self-administration. Dose-related leftward shifts were obtained with pretreatments both from within and outside the pharmacologic class of the self-administered drug. Decreases in maximal self-administration were also obtained, and this particular effect was only obtained with pretreatments from within pharmacologic class. Nonspecific decreases in self-administration can occur with any pretreatment administered at sufficiently high doses (Mello and Negus, 1996). However, the present decreases in the maximal self-administration of drugs from within class produced by both BZT analogs and µ-opioid agonists were obtained at doses that did not affect behaviors maintained by other drugs.
The effects of BZT analogs on stimulant and µ-opioid agonists on opioid self-administration showed similarities to the effects of prior feeding on responding maintained by food reinforcement. The resultant similarity suggests the hypothesis that the effects are due to behaviorally similar processes. The effect of prefeeding with food on subsequent responding maintained by food reinforcement has been called satiation. Whether pretreatments with µ-opioid agonists decrease subsequent µ-agonist self-administration through an analogous mechanism is not clear, though the effect has been suggested as one that underlies preclinical effects (e.g., Cooper et al., 2008) as well as the clinical effectiveness of methadone maintenance (Dole and Nyswander, 1967).
Pretreatment Effects on Extinguished Responding.
An increase in extinguished responding previously maintained by drug injections has been used to model relapse to drug use. The present increases in response rates during EXT produced by standard DA indirect agonists were specific to d-methamphetamine self-administration. The increase in responding during EXT may be due, at least in part, to a discriminative-stimulus effect of the indirect agonists acquired during the many sessions of self-administration (Katz and Higgins, 2003) as reinforcing stimuli can acquire discriminative-stimulus effects in addition to their reinforcing effects (e.g., Reid, 1958). Further supporting that interpretation is the finding that many standard DA indirect agonists share discriminative-stimulus effects, whereas the BZT analogs which did not increase response rates in EXT typically do not share discriminative effects with standard DA indirect agonists (Katz et al., 2004).
The NMDA antagonists also produced increases in responding during the EXT component only in subjects self-administering a drug from within that class, again suggesting the contribution of discriminative-stimulus effects to the increases in responding during EXT. However, limits to the contribution of discriminative-stimulus effects to responding during EXT were indicated by results with µ-opioid agonists which generally share discriminative-stimulus effects with other µ-opioid agonists (Woods et al., 1988) but did not increase EXT responding. Possibly effects similar to those produced by satiation dampen the induction of responding by the discriminative effects of µ-opioid agonists.
Pharmacologic Mechanisms.
Several mechanisms have been suggested for the antagonism by BZT analogs of the effects of cocaine (Hiranita et al., 2009; Tanda et al., 2009a). Previous studies examined the potential role of muscarinic or histaminic antagonist effects, though studies conducted to date provide little evidence that those actions can account for the antagonism of cocaine (Tanda et al., 2009b), and presumably that of d-methamphetamine. Nonetheless, specific binding of radiolabeled JHW007 has shown that it binds to sites other than the DAT, and that binding is not fully displaceable by M1 or H1 ligands, indicating still other sites as potentially contributing to the antagonist effects of BZT analogs (Kopajtic et al., 2010). Our study established that the BZT analogs examined had affinity for σRs that compared with their DAT affinity (see also Li et al., 2011).
Recent studies have focused on a role for σRs in the antagonism of the effects of stimulant drugs (Katz et al., 2011). A previous study demonstrated that σR antagonism along with DA-uptake inhibition can selectively block cocaine self-administration (Hiranita et al., 2011). In our present study, the σR antagonist BD1008 combined with a low dose of WIN35,428 dose-dependently decreased the maximum self-administration of d-methamphetamine without affecting self-administration of heroin or ketamine, indicating the specificity of the effects of dual inhibition of the DAT and σRs.
In summary, our study characterized the effects of N-substituted BZT analogs on d-methamphetamine self-administration and placed those effects in a pharmacologic context. The insurmountable antagonism produced by the BZT analogs was similar to effects of µ-opioid agonists against opioid self-administration and the effects of food satiation on food-reinforced behavior. Further, the inhibition of DA transport along with σR antagonism can produce a selective insurmountable antagonism of stimulant self-administration and may be involved in a behavioral mechanism like that of satiation with food-reinforced behavior. Finally, these results suggest potential development of the N-substituted BZT analogs as medications specific for stimulant abuse.
Acknowledgments
The authors thank Maryann Carrigan for administrative assistance, Jianjing Cao, in the Medicinal Chemistry Section, for synthesizing the N-substituted BZTs used in this study, and Dr. Amy H. Newman for the compounds and several suggestions regarding the manuscript. We dedicate this article to the memory of Dr. William L. Woolverton, friend and colleague, who made innumerable contributions to behavioral pharmacology and was our collaborator in some early studies on BZT analogs. “Too soon gone”—The Band.
Abbreviations
- AHN1-055
3α-[bis(4′-fluorophenyl)methoxy]-tropane hydrochloride
- AHN2-005
N-allyl-3α-[bis(4′-fluorophenyl)methoxy]-tropane oxalate
- BD1008
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- BZT
benztropine
- DA
dopamine
- DAT
dopamine transporter
- DTG
1,3-di-o-tolylguanidine
- EXT
extinction (no injection)
- FR
fixed ratio
- GBR 12909
1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine
- inj
injection
- JHW007
N-(n-butyl)-3α-[bis-(4′-fluorophenyl)methoxy]-tropane hydrochloride
- LED
light-emitting diode
- (+)-MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine)
- NMDA
N-methyl-d-aspartate
- OWW
original wet weight
- WIN 35,428
(−)-3β-(4-fluorophenyl)-tropan-2-β-carboxylic acid methyl ester tartrate
- σR
sigma (σ) receptor
- σ1R
σ1 receptor
- σ2R
σ2 receptor
Authorship Contributions
Participated in research design: Hiranita, Katz.
Conducted experiments: Hiranita, Kohut, Kopajtic.
Performed data analysis: Hiranita, Katz.
Wrote or contributed to the writing of the manuscript: Hiranita, Kohut, Soto, Tanda, Kopajtic, Katz.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
Portions of this work were presented as follows: Hiranita T, Soto PL, Tand G, and Katz JL (2013) Specificity of cocaine-induced dopamine-independent sigma agonist self-administration. Experimental Biology annual meeting; 2013 Apr 20–24; Boston, MA; and Hiranita T, Soto PL, Tanda G, Newman AH, and Katz JL (2013) Abuse liability assessment and preclinical indicators of N-substituted benztropine (BZT) analogs as medications for stimulant abuse; Katz JL, Zou MF, Kopajtic TA, Soto PL, Lupica CR, Newman AH, and Hiranita T (2013) Abuse liability and potential of 3-substituted phenyltropane dopamine uptake inhibitors as medications for cocaine abuse. Neuroscience Annual Meeting; 2013 Nov 9–13; San Diego, CA. Society for Neuroscience, Washington, D.C.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. (1997) Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40:4329–4339 [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47(Suppl 1):256–273 [DOI] [PubMed] [Google Scholar]
- Bresink I, Danysz W, Parsons CG, Mutschler E. (1995) Different binding affinities of NMDA receptor channel blockers in various brain regions—indication of NMDA receptor heterogeneity. Neuropharmacology 34:533–540 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN, Shi YG, Woods JH. (2008) Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther 326:920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. (2005) Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25:1889–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB. (1958) Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther 122:137–147 [PubMed] [Google Scholar]
- Dole VP, Nyswander M. (1965) A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA 193:646–650 [DOI] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME. (1967) Heroin addiction—a metabolic disease. Arch Intern Med 120:19–24 [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. (2005) Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction 100:1496–1509 [DOI] [PubMed] [Google Scholar]
- Ferragud A, Velázquez-Sánchez C, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) A dopamine transport inhibitor with markedly low abuse liability suppresses cocaine self-administration in the rat. Psychopharmacology (Berl) 207:281–289 [DOI] [PubMed] [Google Scholar]
- France CP, Gerak LR, Flynn D, Winger GD, Medzihradsky F, Bagley JR, Brockunier LL, Woods JH. (1995) Behavioral effects and receptor binding affinities of fentanyl derivatives in rhesus monkeys. J Pharmacol Exp Ther 274:17–28 [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, et al. (2011) Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry 69:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FH, Matecka D, Bacher JD, Mansbach RS, Balster RL, Rice KC. (1995) Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: I. Dependence on unit dose of cocaine. Exp Clin Psychopharmacol 3:219–231 DOI: 10.1037/1064-1297.3.3.219. [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. (2004a) Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology 29:969–981 [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. (2004b) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464 [DOI] [PubMed] [Google Scholar]
- Harrigan SE, Downs DA. (1981) Pharmacological evaluation of narcotic antagonist delivery systems in rhesus monkeys. NIDA Res Monogr 28:77–92 [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. (2011) Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and σ receptors. J Pharmacol Exp Ther 339:662–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. (2013) Stimulants as specific inducers of dopamine-independent σ agonist self-administration in rats. J Pharmacol Exp Ther 347:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, et al. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 168:21–30 [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. (1999) Novel 3α-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288:302–315 [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. (2004) Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309:650–660 [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. (2011) A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 4:880–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. (2002) Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301:698–704 [DOI] [PubMed] [Google Scholar]
- Kopajtic TA, Liu Y, Surratt CK, Donovan DM, Newman AH, Katz JL. (2010) Dopamine transporter-dependent and -independent striatal binding of the benztropine analog JHW 007, a cocaine antagonist with low abuse liability. J Pharmacol Exp Ther 335:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hiranita T, Hayashi S, Newman AH, Katz JL. (2013) The stereotypy-inducing effects of N-substituted benztropine analogs alone and in combination with cocaine do not account for their blockade of cocaine self-administration. Psychopharmacology (Berl) 225:733–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Kopajtic TA, O’Callaghan MJ, Agoston GE, Cao J, Newman AH, Katz JL. (2011) N-substituted benztropine analogs: selective dopamine transporter ligands with a fast onset of action and minimal cocaine-like behavioral effects. J Pharmacol Exp Ther 336:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Newman AH, Katz JL. (2005) Place conditioning and locomotor effects of N-substituted, 4′,4″-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313:1223–1230 [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424 [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. (2009) Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. (2003) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 167:324–332 [DOI] [PubMed] [Google Scholar]
- Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. (1995) Novel 4′-substituted and 4′,4″-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J Med Chem 38:3933–3940 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. (2000) Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 150:61–66 [DOI] [PubMed] [Google Scholar]
- Reid RL. (1958) The role of the reinforcer as a stimulus. Br J Psychol 49:202–209 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. (1989) Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther 248:1010–1017 [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223 [DOI] [PubMed] [Google Scholar]
- Schenk S. (2002) Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self-administration and cocaine seeking in rats. Psychopharmacology (Berl) 160:263–270 [DOI] [PubMed] [Google Scholar]
- Tam SW. (1983) Naloxone-inaccessible sigma receptor in rat central nervous system. Proc Natl Acad Sci USA 80:6703–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Li SM, Mereu M, Thomas AM, Ebbs AL, Chun LE, Tronci V, Green JL, Zou MF, Kopajtic TA, et al. (2013) Relations between stimulation of mesolimbic dopamine and place conditioning in rats produced by cocaine or drugs that are tolerant to dopamine transporter conformational change. Psychopharmacology (Berl) 229:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. (2009a) Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther 330:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. (2009b) Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57:253–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) The dopamine uptake inhibitor 3 alpha-[bis(4′-fluorophenyl)metoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology 34:2497–2507 [DOI] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Murga J, Cardá M, Canales JJ. (2010) The high affinity dopamine uptake inhibitor, JHW 007, blocks cocaine-induced reward, locomotor stimulation and sensitization. Eur Neuropsychopharmacol 20:501–508 [DOI] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, García-Verdugo JM, Murga J, Canales JJ. (2013) The atypical dopamine transport inhibitor, JHW 007, prevents amphetamine-induced sensitization and synaptic reorganization within the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry 44:73–80 [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. (2002) Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther 301:690–697 [DOI] [PubMed] [Google Scholar]
- Winger G, Skjoldager P, Woods JH. (1992) Effects of buprenorphine and other opioid agonists and antagonists on alfentanil- and cocaine-reinforced responding in rhesus monkeys. J Pharmacol Exp Ther 261:311–317 [PubMed] [Google Scholar]
- Woods JH, Bertalmio AJ, Young AM, Essman WD, Winger G. (1988) Receptor mechanisms of opioid drug discrimination, in Transduction Mechanisms of Drug Stimuli (Colpaert FC, Balster RL, eds) pp 95–106, Springer-Verlag, Berlin: [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Young AM, Medzihradsky F, Smith CB. (1981) Correlations among certain behavioral, physiological, and biochemical effects of narcotic agonists. NIDA Res Monogr 34:43–57 [PubMed] [Google Scholar]
- Yokel RA, Pickens R. (1973) Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther 187:27–33 [PubMed] [Google Scholar]
- Young AM, Woods JH. (1981) Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther 218:720–727 [PubMed] [Google Scholar]