Abstract
Drug activation of the human nuclear pregnane X receptor (PXR) induced gluconeogenic genes and increased glucose production. In this study, we have determined that serum- and glucocorticoid-regulated kinase 2 (SGK2) is an essential factor that mediates this PXR-regulated glucose 6-phosphatase (G6Pase) induction and glucose production. Both SGK2 and G6Pase mRNAs were increased in rifampicin-treated HepG2 cells stably expressing human PXR. Reporter and chromatin immunoprecipitation assays delineated PXR activation of the SGK2 gene to a distal and proximal DNA sequence within its promoter: distal PXR response element (−2587/−2209) and proximal PXR response element (−115/−75), respectively. Small interfering RNA (siRNA) knockdown of SGK2 severely attenuated PXR-regulated induction of G6Pase as well as glucose production. SGK2 constitutes an insulin-independent signal pathway to regulate gluconeogenesis because siRNA knockdown of the insulin-responsive transcription factor forkhead box protein O1 did not affect rifampicin induction of G6Pase. Rifampicin treatment of two different samples of human primary hepatocytes revealed that PXR induces G6Pase in the presence of high levels of SGK2, whereas PXR represses G6Pase in its absence. Mediating PXR activation of the G6Pase gene is the first biological role found for hepatic SGK2 and might have therapeutic implications for side effects, such as diabetes, caused by drugs that activate PXR.
Introduction
Rifampicin is a macrolide antibiotic that has been used to treat tuberculosis patients. Recent clinical studies have reported that rifampicin treatment increases blood glucose levels during oral glucose tolerance tests in both tuberculosis patients and healthy volunteers (Takasu et al., 1982; Rysä et al., 2013). Cotreatment with rifampicin is known to diminish the effects of antidiabetic drugs that reduce blood glucose levels, such as glyburide, gliclazide, and repaglinide (Surekha et al., 1997; Niemi et al., 2000, 2001; Park et al., 2003). Similarly, treatment with statins is also reported to increase blood glucose levels in patients (Sukhija et al., 2009; Sattar and Taskinen, 2012). The Food and Drug Administration has recently added a new safety warning to the statin label: statins may increase the risk of developing type 2 diabetes. Because rifampicin and statins are prototypical activators of the nuclear pregnane X receptor (PXR; NR1I2), PXR activation has been considered to mediate drug-induced development of hyperglycemia in humans, the mechanism of which has been an important subject of investigations. Contrary to what is observed in clinical studies, activation of mouse PXR by pregnenolone-16α-carbonitrile (PCN) results in decreased blood glucose levels in mice (Kodama et al., 2007). The lowering of blood glucose levels in mice is attributed to PXR-mediated repression of genes such as glucose 6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase 1 (PEPCK1), carnitine palmitoyltransferase 1A, and 3-hydroxy-3-methylglutaryl-CoA synthase 2 genes (Kodama et al., 2004, 2007; Nakamura et al., 2007; Konno et al., 2008). As to the molecular mechanism of this repression, PXR interacts with transcription factors such as forkhead box protein O1 (FOXO1) and forkhead box protein A2 (FOXA2) and cAMP response element-binding protein and prevents them from activating these genes (Kodama et al., 2004, 2007; Nakamura et al., 2007; Konno et al., 2008). However, these PXR-mediated regulations and their mechanism in mice have not been confirmed in humans.
Our present investigation first found that rifampicin-activated PXR increases G6Pase and PEPCK1 mRNAs. Moreover, it also found that serum- and glucocorticoid-regulated kinase 2 (SGK2) mRNA is concomitantly induced after rifampicin treatment, a member of the SGK family (Kobayashi and Cohen, 1999b). Although the biological functions of SGKs are presently not well characterized, there has been some speculation that they might be involved in glucose homeostasis. This is due to the fact that SGKs share an approximately 50% amino acid sequence homology in these kinase domains with insulin-activated AKT, a key kinase that regulates hepatic gluconeogenesis. The SGK family consists of three members; SGK1 and SGK3 are ubiquitously expressed in all tissues. In contrast, SGK2 is primarily expressed only in liver, kidney, and pancreas (Kobayashi et al., 1999a). This tissue-specific pattern of SGK2 expression raises an intriguing question: because these tissues coordinately regulate glucose metabolism, is SGK2 involved in the regulation of glucose metabolism? However, there is no study that associates SGK2 as a regulatory factor with glucose metabolism in these tissues at the present time. Our findings that G6Pase and SGK2 mRNAs were coinduced after rifampicin treatment in human liver cells prompted us to investigate whether SGK2 plays an essential role in rifampicin induction of G6Pase mRNA and PXR activation of the G6Pase gene in HepG2 cells as well as human primary hepatocytes.
In this study, we determined the molecular mechanism by which PXR activates the SGK2 promoter in HepG2 cells. Subsequently, small interfering RNAs (siRNAs) were employed to confirm that SGK2 is essential for PXR to activate the G6Pase gene as well as increase glucose production. Cotreatment with insulin and rifampicin revealed that SGK2 does not use an insulin-FOXO1 pathway to mediate PXR activation of the G6Pase gene. In human primary hepatocytes, rifampicin treatment resulted in induced G6Pase mRNA in the presence of SGK2, whereas in its absence rifampicin treatment represses this mRNA. Taken together, our present investigations characterized SGK2 as an essential regulatory factor for drug-induced increase of glucose production through PXR activating the G6Pase gene and provided us with new insights into understanding hepatic glucose metabolism in humans during drug treatments.
Materials and Methods
Rifampicin, atorvastatin, simvastatin, fluvastatin, pravastatin, bovine insulin, and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO); restriction endonucleases and DNA-modifying enzymes from New England Biolabs (Beverly, MA); anti-PXR antibody from Perseus Proteomics Inc. (Tokyo, Japan); anti-SGK2 antibody and anti-FOXO1 antibody from Cell Signaling (Danvers, MA); and normal mouse IgG and anti–β-actin antibody (C4), goat anti-rabbit IgG–horseradish peroxidase (HRP), and goat anti-mouse IgG-HRP from Santa Cruz Biotechnology (Santa Cruz, CA).
Cells and Cultures.
HepG2 cells and ShP51 cells, a HepG2-derived stable cell line that expresses human PXR (Kodama and Negishi, 2011), were cultured in minimum essential medium supplemented with 10% fetal bovine serum, antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), and 2 mM l-glutamine in an atmosphere of 5% CO2 at 37°C. Human primary hepatocytes were gifts from Invitrogen (Grand Island, NY) and cultured in Williams' medium E with phenol red supplemented with 5% fetal bovine serum, antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), 1 μM dexamethasone, 4 μg/ml insulin, and 2 mM l-glutamine in an atmosphere of 5% CO2 at 37°C.
Animal Treatment.
C3H/HeNCrlBR male mice (Charles River) at 9 weeks of age were maintained with 12-hour light/12-hour dark cycle (7:00 AM to 7:00 PM). Mice were randomly housed into two groups (3 mice per group). Rifampicin (100 mg/kg) or DMSO was administrated by intraperitoneal injection followed by 9-hour treatment. After treatment, mice were killed, and mice liver cDNAs were prepared for qRT-PCR.
Plasmids.
Various SGK2 promoter constructs (−3606/+223, −2587/+223, −2208/+223, −115/+223, −75/+223 bp) were amplified by polymerase chain reaction (PCR) using genomic DNA prepared from HepG2 cells as a template and appropriate pairs of primers and were purified and cloned into KpnI/XhoI-digested pGL4.17 vector (Promega, Madison, WI). The upstream promoter fragment (−2587/−2208 bp) was amplified using the SGK2 promoter plasmid (−3606/+223 bp) as template and was placed in the front of the SGK2 (−115/+223 bp) and (−75/+223 bp) promoters. The DNA sequences of these promoter constructs were verified using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) and proper pairs of primers.
Rapid Amplification of 5′-Complementary DNA Ends.
Total RNA from rifampicin-treated ShP51 cells were used for rapid amplification of 5′-complementary DNA ends (5′-RACE) using an Invitrogen 5′-RACE kit, according to the manufacturer’s instructions. Three GSP primer sequences used are as follows: GSP1, 5′-TTCTATGCAGTGAAGGTACTACAGA-3′; GSP2, 5′-ACTTCGACTTCCTCAAAGTCAT-3′; and GSP3, 5′-ACTTCGACTTCCTCAAAGTCAT-3′, which were designed on the basis of coding sequence of the SGK2 gene. Final PCR products were purified by gel filtration, cloned into pCR2.1-TOPO (Invitrogen) for subsequent DNA sequencing using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and the following primers: T7 primer, 5′-TAATACGACTCACTATAGGG-3′ and M13 reverse primer, 5′-CAGGAAACAGCTATGACC-3′.
Real-Time PCR.
Total RNAs were isolated from HepG2 cells, ShP51 cells, human primary hepatocytes, and mouse livers using TRIzol reagent (Invitrogen), from which cDNAs were synthesized using MultiScribe reverse transcriptase (Applied Biosystems). Real-time PCR was performed using an ABI prism 7700 sequence detection system (Applied Biosystems). Assays-on-Demand probes (Applied Biosystems) for PCR with the TaqMan PCR Master Mix (Applied Biosystems) were as follows: human CYP3A4 gene, Hs00430021_m1; human G6Pase gene, Hs00609178_m1; human PEPCK1 gene, Hs00159918_m1; mouse G6Pase gene, Mm00839363_m1; and mouse CYP3A11 gene, Mm00731567_m1. To measure the expression of human SGK1, SGK2, and SGK3 and mouse SGK2 genes, SYBR Green PCR Master Mix (Applied Biosystems) was used with the following primers: human SGK1, 5′-CACCACCAGTCCACAGTCC-3′ and 5′-CAACAGCACAACATCCACCT-3′; human SGK2, 5′-CGGAAAGAGCCTTATGATCGA-3′ and 5′-CATCTGGGATACATCTTGGCTG-3′; human SGK3, 5′-GTTTGGCCCCCGCAGGGAG-3′ and 5′-GAATGGGAAGCGCCGCCACT-3′; and mouse SGK2, 5′-CGGGCCCGGTTCTACAC-3′ and 5′-GTGACCCTGGCAGTCCAAGA-3′. The TaqMan human and mouse glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems) was used as internal control.
siRNA Knockdown.
ShP51 cells and human primary hepatocytes were transfected with various siRNAs using Lipofectamine RNAiMAX (Invitrogen), ON-TARGETplus SMART pool human SGK2 siRNA, human NR1I2 siRNA, ON-TARGETplus Non-Targeting pool (Dharmacon Research, Lafayette, CO), and Signal Silence FOXO1 siRNA (Cell Signaling), according to the manufacturer’s instructions. After 36 hours, cells were treated with rifampicin (100 μM) or simvastatin (10 μM) for 3 hours. Efficiency of knockdowns was confirmed by reverse-transcription PCR as well as Western blots.
Reporter Assays.
ShP51 cells were transiently cotransfected with various human SGK2 promoter luciferase reporter vectors and the internal control pRL-CMV (Promega) using FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN), according to the manufacturer’s instructions. After 24 hours, these cotransfected cells were treated with rifampicin (10 μM) for an additional 24 hours. Luciferase assays were performed by using a Dual-Luciferase Assay System (Promega). Firefly luciferase activities were used to normalize Renilla reniformis luciferase activities.
Chromatin Immunoprecipitation Assays.
Chromatin immunoprecipitation (ChIP) assays were performed by using a ChIP-IT Express kit (Active Motif, Carlsbad, CA), according to the manufacturer’s instructions. Briefly, ShP51 cells treated with rifampicin (100 μM) or simvastatin (10 μM) for 1 hour were cross-linked by formaldehyde. Pellets of these cross-linked cells were lysed and sonicated to shear chromatin DNA. Each aliquot of chromatin solution was immunoprecipitated by a PXR antibody or a normal mouse IgG at 4°C overnight. Immunoprecipitates were washed and eluted. After decross-linked and protease digested, these final DNA samples were PCR amplified with the pairs of primers (dPRE, 5′-GGGAGCTGAAGTCAGTTGCCCAT-3′ and 5′-CAAACCGGCTCAAGTTCAACCTCCC-3′; proximal PXR response element (pPRE), 5′-CGCATATCTGCAGTGGCCTATCTAC-3′ and 5′-CCTGTGGCCTTCCGCTAGTATTTC-3′) and were analyzed by an agarose gel electrophoresis as well as DNA sequencing.
Western Blots.
Total cell extracts were prepared in a NuPAGE LDS sample buffer (Invitrogen). Protein concentrations were determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein were separated on a SDS-polyacrylamide gel electrophoresis and were transferred onto polyvinylidene difluoride membrane. These membranes were blocked with 5% skim milk in 10 mM Tris-buffered saline containing 0.1% Tween 20, incubated overnight at 4°C with given primary antibodies, washed with Tris-buffered saline containing 0.1% Tween 20, and finally incubated with HRP-conjugated secondary antibodies. The proteins were visualized using ECL plus Western blotting detection reagents (GE Healthcare, Pittsburgh, PA).
Quantitation of Glucose.
ShP51 cells were transfected with siRNAs in minimum essential medium supplemented with 10% fetal bovine serum, antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), and 2 mM l-glutamine, according to the manufacturer’s instruction. After 36 hours, the transfected cells were cultured in glucose- and phenol red-free Dulbecco's modified Eagle medium, pH 7.4, containing 2 mM sodium pyruvate and treated with simvastatin (10 μM) for 4 hours prior to collecting the medium. Glucose concentrations in this medium were determined utilizing a glucose colorimetric/fluorometric assay kit (BioVision, Milpitas, CA), according to the manufacturer’s instructions. Cells were collected, and total cellular protein contents were measured using a Bio-Rad protein assay kit. Values, normalized by dividing the glucose levels by the total protein contents, are presented.
Statistical Analysis.
Data were analyzed with Student's t test or one-way analysis of variance (ANOVA) by using GraphPad Prism software. Significance values are represented as *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Rifampicin-activated PXR induces SGK2.
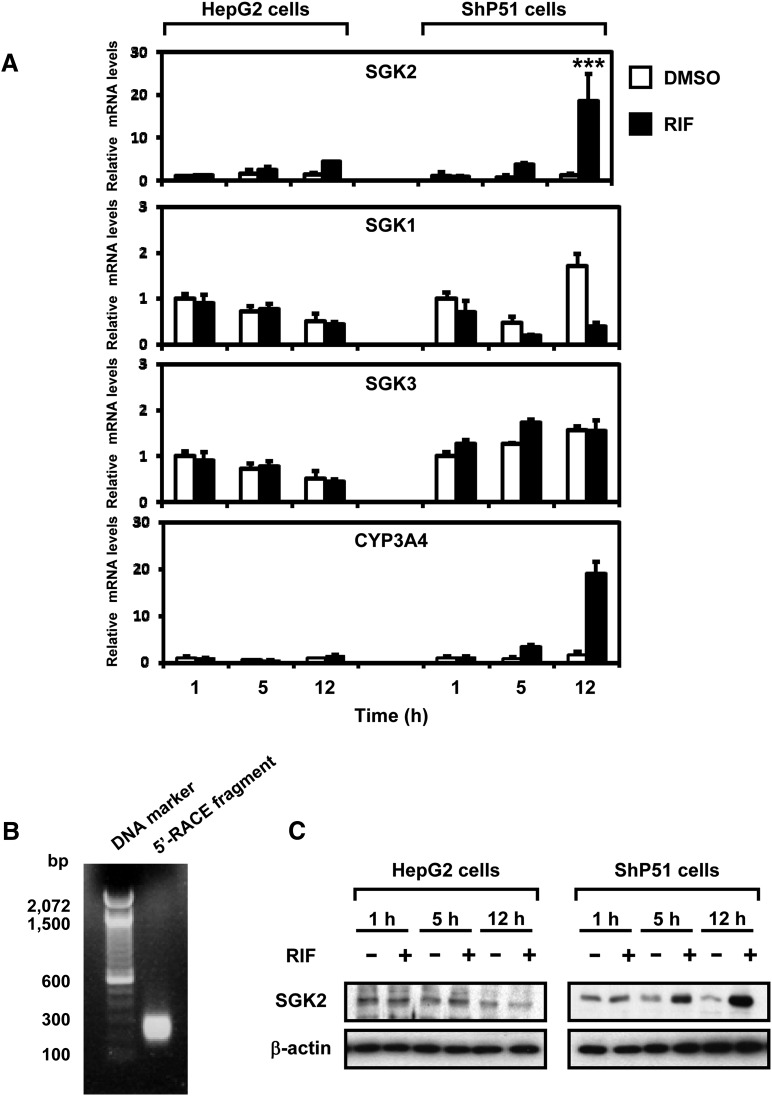
Given that DNA microarray analysis indicated an increase in SGK2 mRNA in ShP51 cells after treatment with a classic human PXR ligand rifampicin, quantitative RT-PCR (qRT-PCR) was used to further examine SGK2 as well as the other SGK members SGK1 and SGK3 (Fig. 1A). SGK2 mRNA was markedly increased in a time-dependent manner in human PXR-expressing ShP51 cells after rifampicin treatment, but not in parental HepG2 cells (Fig. 1A). In contrast, SGK1 and SGK3 mRNA levels remained constant after rifampicin treatment. In the case of SGK1, basal expression levels, which fluctuated by times, was elevated at 12-hour time point. However, rifampicin treatment decreased the SGK 1 mRNA levels at these three time points. Therefore, rifampicin treatment may have prevented a basal increase of SGK1 mRNA. Accordingly, SGK2 was the only SGK member induced when PXR was activated by rifampicin. Based on the induction rates, this SGK2 induction was as strong as that of CYP3A4 mRNA, the classic target of human PXR. Similar induction of SGK2 was also observed in ShP51 cells after treatments with various statins, which are known to activate human PXR (Supplemental Fig. 1).
Fig. 1.
Drug-activated PXR induces the human SGK2 gene. (A) mRNA levels: HepG2 cells and ShP51 cells were treated with rifampicin (RIF, 100 μM) or DMSO (control) for 1, 5, and 12 hours (n = 3), and total RNAs were prepared for qRT-PCR to measure mRNA levels of SGK1, SGK2, SGK3, and CYP3A4. These were normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to 1-hour DMSO-treated cells. Statistical analysis was assessed by one-way ANOVA; ***P < 0.001. (B) 5′-RACE: the size of the 5′-RACEed SGK2 transcript from ShP51 cells treated with rifampicin (RIF, 100 μM) was analyzed by agarose gel electrophoresis. Subsequent sequence analysis determined that this transcript is the variant α. (C) SGK2 protein levels: whole extracts prepared from these same cells were subjected to Western blots using anti-SGK2 and anti–β-actin antibody.
Human SGK2 gene is known to transcribe three variants, as follows: 1, 2, and 3, among which transcripts 1 and 3 encode SGK2 isoforms α, whereas transcript 2 encodes SGK2 isoform β. The 5′-RACE analysis was performed to determine which SGK2 isoform was induced in rifampicin-treated ShP51 cells (Fig. 1B). DNA sequence analysis of amplified fragments from several colonies verified that SGK2 transcript variant 1 encoding SGK2 isoform α was the SGK2 induced. Subsequent Western blot analysis with an anti-SGK2 antibody showed a time-dependent increase of SGK2 protein in ShP51 cells after treatment with rifampicin, but not in parental HepG2 cells (Fig. 1C).
PXR Directly Activates the SGK2 Promoter.
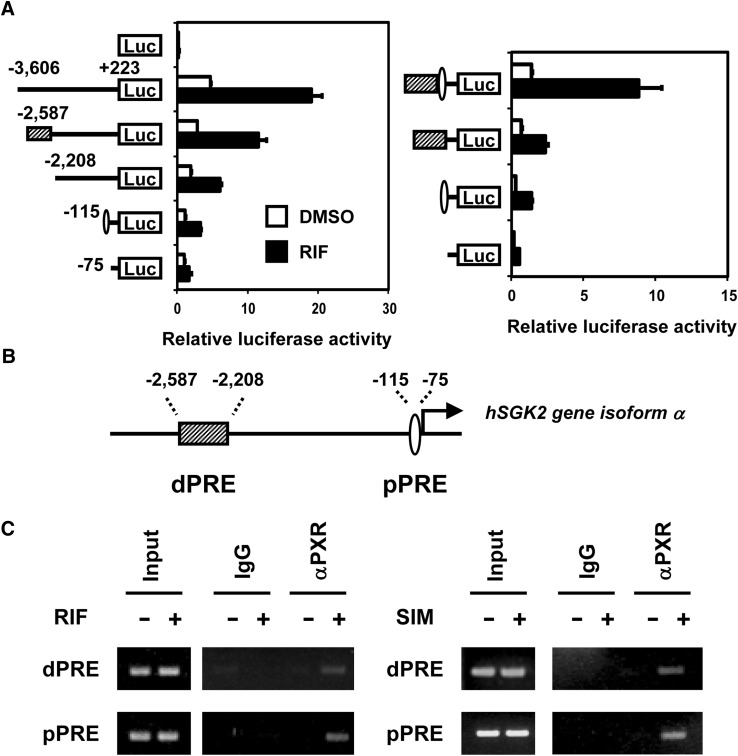
To identify the mechanism by which PXR activates the human SGK2 gene in ShP51 cells, a series of deletion promoters of the SGK2 transcript variant 1 were constructed, and the activity of these luciferase reporter genes was determined in ShP51 cells. The −3606/+223-bp SGK2 promoter was strongly activated after rifampicin treatment (Fig. 2A). The first reduction in activation rate was observed when the −2587/−2202-bp region was deleted, whereas the −115/−75-bp region was responsible for a second reduction. As can be seen in the figure on the right, the SGK2 promoter retained full activity when the −2587/−2202-bp region was placed in front of the −115/−75-bp region, whereas the −2587/−2202-bp region exhibited reduced enhancer activity in the absence of the −115/−75-bp region. Thus, these reporter assays delineated rifampicin activation of the SGK2 promoter to these two regions: −2587/−2208 and −115/−75, now designated dPRE and pPRE, respectively (Fig. 2B). Subsequently, ChIP assays were performed to examine the binding of PXR to dPRE and pPRE in ShP51 cells before or after rifampicin treatment. Treatment with rifampicin resulted in a robust increase of PXR binding to both the dPRE and pPRE (Fig. 2C). Similarly, this increased PXR binding was also observed after simvastatin treatment (the figure on the right). Simvastatin was most effective in the induction of SGK2 mRNA among the statins tested (Supplemental Fig. 1). Taken together, these results indicate that PXR directly activates the human SGK2 gene through the binding to dPRE and pPRE within the human SGK2 promoter in response to rifampicin and simvastatin.
Fig. 2.
PXR binds to and activates the SGK2 promoter. (A) Map is to locate the two PXR response elements, dPRE and pPRE, within the SGK2 isoform α promoter. (B) Reporter assays: ShP51 cells were transiently transfected with a series of human SGK2 promoter luciferase reporter plasmids (n = 3). After treatment with rifampicin (RIF, 10 μM) or DMSO (control) for 24 hours, luciferase activities were measured and normalized by using activities of cotransfected Renilla luciferase. The values of their promoter activities are expressed relative to that of pGL4.17 empty vector. (C) ChIP assays: ShP51 cells were treated with rifampicin (RIF, 100 μM), simvastatin (SIM, 10 μM), and/or DMSO (control) for 1 hour and subjected to ChIP assays using an anti-PXR antibody. Immunoprecipitated DNAs were used to amplify the regions dPRE (−2587/−2209) and pPRE (−115/−75) of the SGK2 promoter by PCR. The PCR products were analyzed by an agarose gel electrophoresis.
SGK2 Determines PXR-Mediated Activation of the G6Pase and PEPCK1 Genes.
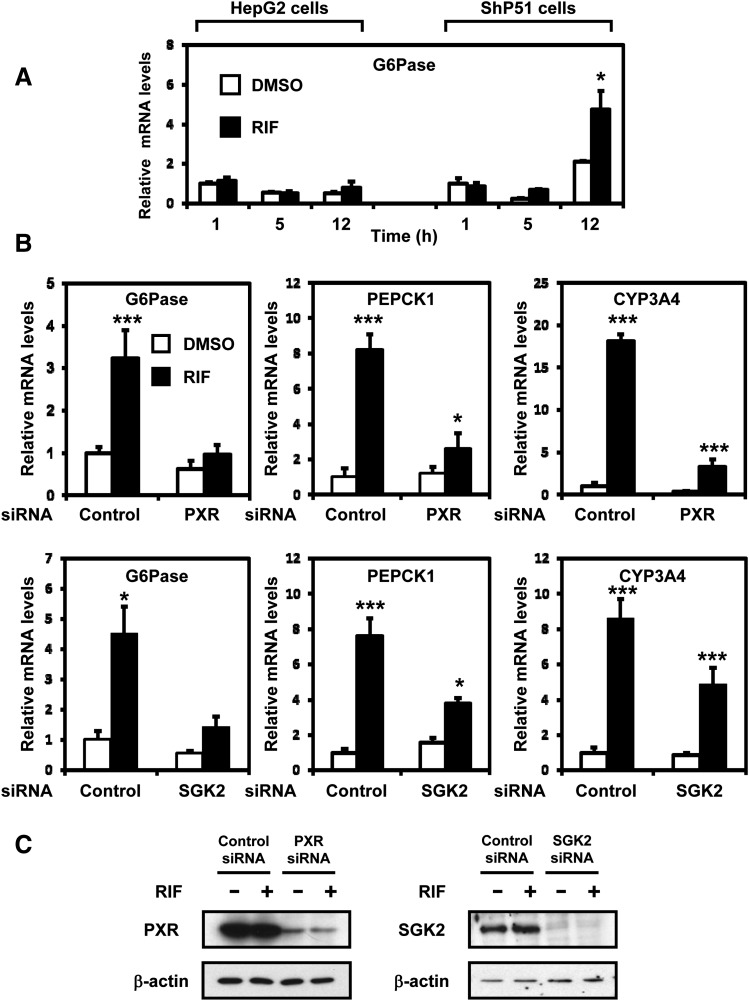
Unlike the G6Pase gene, which was repressed in mouse liver after treatment with the mouse PXR activator PCN (Kodama et al., 2007), rifampicin treatment resulted in an increased expression of G6Pase mRNA in ShP51 cells in a time-dependent manner, but not in parental HepG2 cells (Fig. 3A). First, siRNA-mediated knockdown of PXR was used to confirm that PXR regulated the activation of G6Pase and PEPCK1 genes in ShP51 cells after rifampicin treatment (Fig. 3B). siRNA knockdown of SGK2 also reduced the increase by rifampicin of both G6Pase and PEPCK 1 mRNAs by approximately 3- to 4-fold (Fig. 3B). As expected by the fact that CYP3A4 gene is the classic PXR target, the loss of PXR greatly reduced induction levels of CYP3A4 by rifampicin treatment (Fig. 3B). Although this induction appeared to be downregulated in the SGK2 knockdown, repeated experiments indicated that the effects by the knockdown were insignificant. Western blots with anti-PXR or anti-SGK2 antibody confirmed siRNA-mediated knockdown of these proteins (Fig. 3C).
Fig. 3.
PXR is required for SGK2 in rifampicin induction of G6Pase and PEPCK1. (A) HepG2 cells and ShP51 cells were treated with rifampicin (RIF, 100 μM) or DMSO (control) for 1, 5, and 12 hours (n = 3). The relative expression of G6Pase mRNA was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to 1-hour DMSO-treated cells. (B) ShP51 cells were transfected with PXR siRNA, SGK2 siRNA, or control siRNA, followed by treatment with rifampicin (RIF, 100 μM) or DMSO (control) for 3 hours (n = 3). The relative expression of G6Pase, PEPCK1, and CYP3A4 mRNAs was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to DMSO control siRNA. (C) The knockdown efficiencies of PXR and SGK2 were confirmed by Western blot using anti-PXR antibody and anti-SGK2 antibody. Statistical analysis was assessed by one-way ANOVA; *P < 0.05; ***P < 0.001.
SGK2 Uses a FOXO1-Independent Pathway for G6Pase Gene Activation.
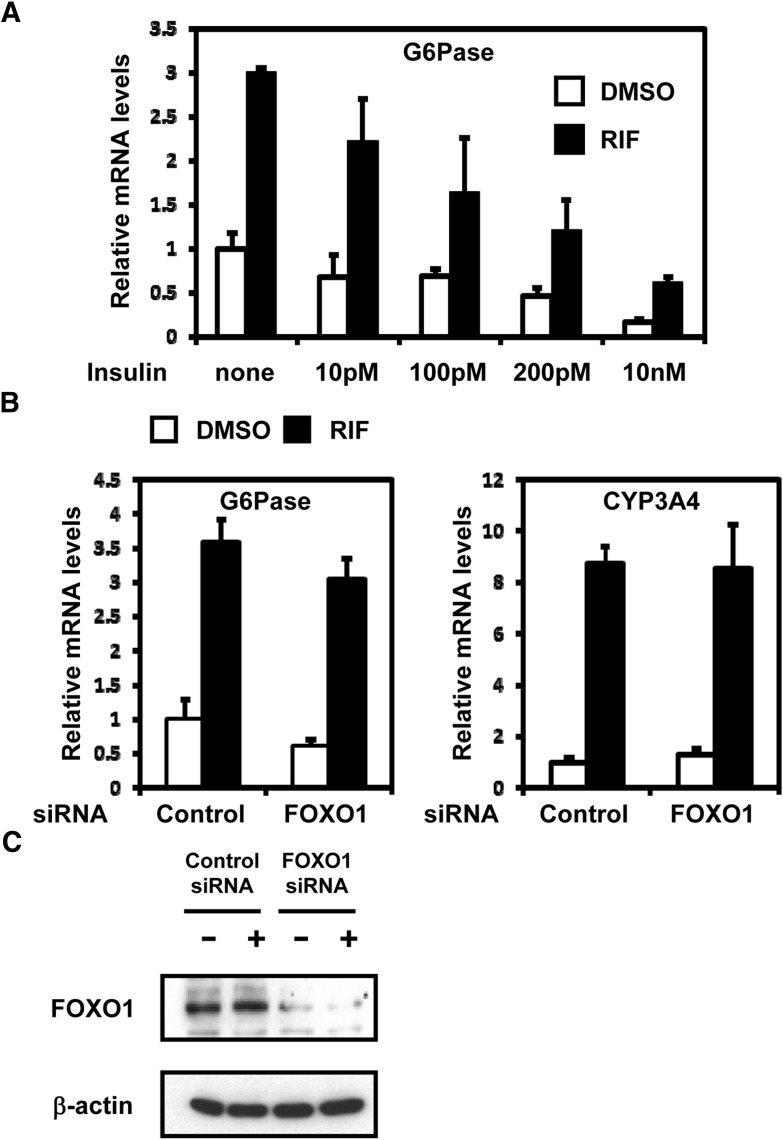
Insulin represses the G6Pase gene by eliciting cell signaling to phosphorylate and inactivate the insulin-regulated transcription factor FOXO1. Drug-activated PXR directly binds to FOXO1, thereby preventing FOXO1 binding to the G6Pase promoter and repressing the G6Pase gene in the liver of mice after PCN treatment (Kodama et al., 2004). Therefore, we examined whether SGK2 also used the insulin-FOXO1 pathway to regulate PXR-mediated activation of the G6Pase gene. ShP51 cells were pretreated with rifampicin prior to cotreatment with various concentrations of insulin. Insulin competed with rifampicin and repressed rifampicin induction of G6Pase mRNA in a dose-dependent manner (Fig. 4A). However, the rates of rifampicin induction remained constant, whereas the induction levels decreased. Then siRNA was used to knockdown FOXO1. G6Pase induction was fully retained in FOXO1 knockdowned ShP51 cells (Fig. 4B). As expected, the knockdown did not affect induction of CYP3A4 mRNA. Western blots were performed to confirm knockdown levels of FOXO1 proteins (Fig. 4C). These results indicated that SGK2 does not use the insulin-FOXO1 pathway to regulate PXR-mediated activation of the G6Pase gene in ShP51 cells.
Fig. 4.
SGK2 does not use the insulin-FOXO1 pathway to mediate PXR-regulated activation of the G6Pase gene. (A) ShP51 cells were pretreated with rifampicin (RIF, 100 μM) for 15 minutes prior to cotreatment with various doses of insulin (10 pM, 100 pM, 200 pM, and 10 nM) for an additional 6 hours (n = 3). The relative expression of G6Pase mRNA was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to 1-hour DMSO-treated cells. (B) ShP51 cells were transfected with FOXO1 siRNA or control siRNA, followed by treatment with rifampicin (RIF, 100 μM) or DMSO (control) for 3 hours (n = 3). The relative expression of G6Pase and CYP3A4 mRNAs was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to DMSO control siRNA. (C) The knockdown efficiency of FOXO1 was confirmed by Western blot using anti-FOXO1 antibody.
SGK2 Determines G6Pase Induction in Human Primary Hepatocytes.
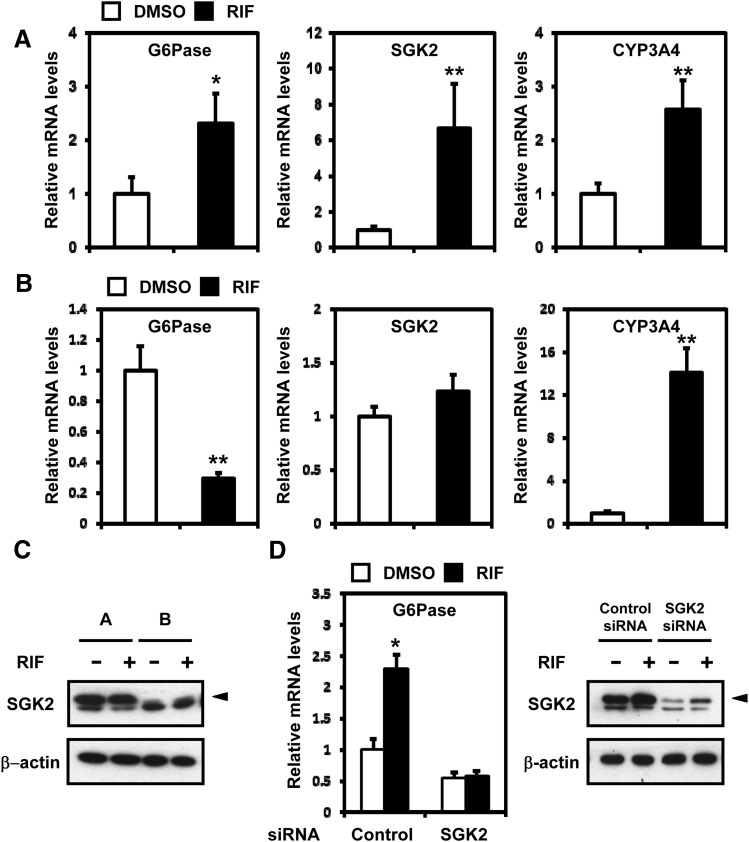
We examined whether SGK2 also regulated PXR-mediated G6Pase activation in human primary hepatocytes as observed in ShP51 cells. Two different samples of human primary hepatocytes were treated with rifampicin to measure the mRNA levels of G6Pase, SGK2, and CYP3A4 (Fig. 5, A and B). An SGK2 antibody detected two bands on Western blots, as follows: the upper band corresponded to SGK2, whereas the lower band appeared to be nonspecific (Fig. 5C). Sample A contained high levels of basal SGK2 protein; basal SGK2 protein was not present in sample B (Fig. 5C). G6Pase, SGK2, and CYP3A4 mRNAs were increased in sample A after treatment with rifampicin (Fig. 5A). In sample B, rifampicin treatment decreased G6Pase mRNA, whereas SGK2 mRNA remained unchanged (Fig. 5B). PXR was activated in sample B because CYP3A4 mRNA increased after rifampicin treatment. As a result, rifampicin treatment increased G6Pase mRNA in human primary hepatocytes when SGK2 was present, whereas it decreased it in the absence of SGK2. To further support the role of SGK2 in the activation of the G6Pase gene in human primary hepatocytes, SGK2 was knock down in the first sample A of human primary hepatocytes. No increase of G6Pase mRNA was observed after rifampicin treatment in human primary hepatocytes after SGK2 knockdown (Fig. 5D, left). SGK2 siRNA effectively silenced SGK2 protein with only residual levels of protein being detected (Fig. 5D, right). CYP3A4 mRNA increased in both of these human primary hepatocytes after rifampicin treatment even after the SGK2 knockdown (Fig. 5, A and B). As a result, it appeared that the degrees of PXR-mediated induction of G6Pase depend on the levels of SGK2 in human primary hepatocytes.
Fig. 5.
G6Pase regulation by SGK2 in human primary hepatocytes. (A) Human primary hepatocytes (age, 72 years; female; body mass index, 40; smoking; no alcohol use) were treated with rifampicin (RIF, 100 μM) or DMSO (control) for 6 hours (n = 3). The relative expression of G6Pase, SGK2, and CYP3A4 mRNAs was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as fold-change relative to DMSO-treated hepatocytes. (B) Human primary hepatocytes (age, 61 years; male; body mass index, 33; no smoking; alcohol use) were treated with rifampicin (RIF, 10 μM) or DMSO (control) for 48 hours (n = 4). The relative expression of G6Pase, SGK2, and CYP3A4 mRNAs was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to DMSO-treated hepatocytes. (C) Western blots were performed on whole extracts prepared from these same cells using an anti-SGK2 or an anti–β-actin antibody. (D) Human primary hepatocytes [same as (A)] were transfected with SGK2 siRNA or control siRNA, followed by treatment with rifampicin (RIF, 100 μM) or DMSO (control) for 6 hours (n = 4). The relative expression of G6Pase mRNA was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as a fold-change relative to DMSO control siRNA. The knockdown efficiencies of SGK2 were confirmed by Western blots using an anti-SGK2 antibody. Statistical analysis was done by one-way ANOVA; *P < 0.05; **P < 0.01.
SGK2 Determines an Increase in Glucose Production.
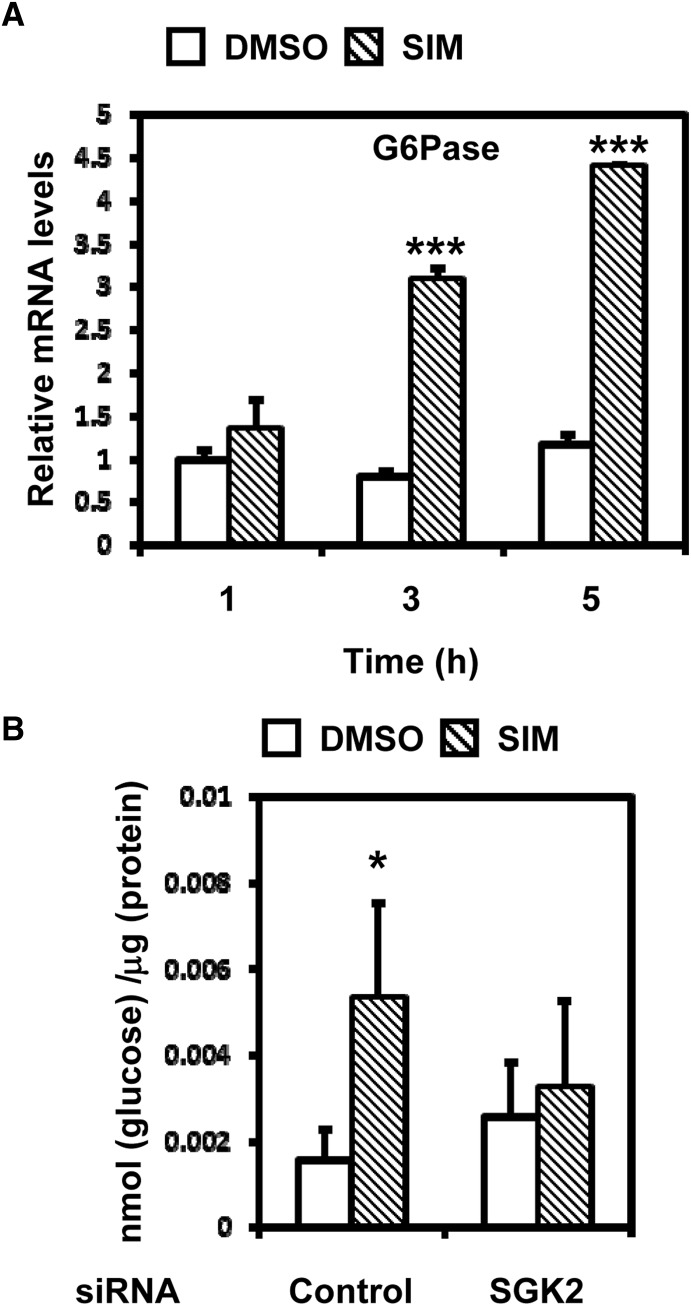
Our present study has demonstrated that activation of PXR by rifampicin or statins resulted in an increase in G6Pase mRNA and that SGK2 mediated this increase. Therefore, glucose production should be expected to increase in a SGK2-dependent manner after treatment with rifampicin or statins. To confirm this, siRNA knockdown of SGK2 was used to determine glucose levels in culture medium in ShP51 cells after treatment with simvastatin. This experiment was bound to use simvastatin because rifampicin interfered with glucose measurement. G6Pase mRNA already started to increase 3 hours after treatment and continued to increase in a time-dependent manner (Fig. 6A). Culture media were collected from siRNA-transfected ShP51 cells after simvastatin treatment of 4 hours and subjected to assays. Glucose levels increased 3-fold in the media prepared from control siRNA-transfected ShP51 cells, whereas this increase was not observed with SGK2 knockdown ShP51 cells (Fig. 6B). These findings indicate that drug treatment, in fact, results in an increased production of glucose and SGK2 mediates this production.
Fig. 6.
SGK2-dependent glucose production. (A) ShP51 cells were treated with simvastatin (SIM, 10 μM) or DMSO (control) for 1, 3, and 5 hours (n = 3). The relative expression of G6Pase mRNA was measured by qRT-PCR and was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase using the comparative cycle threshold method. Values are scored as fold-changes relative to 1-hour DMSO-treated cells. (B) ShP51 cells were transfected with SGK2 siRNA or control siRNA, followed by treatment with simvastatin (SIM, 10 μM) or DMSO (control) for 4 hours (n = 4). The total glucose contents were measured using a glucose colorimetric/fluorometric assay kit and were normalized to the total protein contents. Statistical analysis was done by one-way ANOVA; *P < 0.05; ***P < 0.001.
Discussion
Our present study has demonstrated that SGK2 mediates drug-activated PXR to induce G6Pase and PEPCK1 and increase glucose production in HepG2 cells. SGK2 determines how PXR regulates the G6Pase gene in response to therapeutic drugs; therefore, SGK2 may be a risk factor for the side effect of increasing blood glucose levels and possibly developing type 2 diabetes after treatment with drugs such as rifampicin and statins in humans. SGK2 is not involved in PXR activation of the classic target CYP3A4 gene. Mechanistically, drug-induced glucose production begins by PXR binding to two response elements (dPRE and pPRE) within the SGK2 promoter, thereby activating the SGK2 gene. Subsequently, induced SGK2 mediates PXR activation of the G6Pase gene, thereby increasing glucose production. SGK2 does not use the insulin-AKT-FOXO1 pathway to mediate PXR activation of the G6Pase gene.
Treatment with PCN, a classic mouse PXR activator, represses G6Pase mRNA in the mouse liver. Although rifampicin is a human PXR activator, at a high dose (>20 mg/kg) it has also been reported to activate mouse PXR in the mouse liver (Scheer et al., 2008). We found that this high dose of rifampicin represses both G6Pase and SGK2 mRNAs in mouse livers, unlike what was observed in human liver cells (Supplemental Fig. 2). Therefore, this repression of G6Pase is PXR dependent but not the types of its activators. In contrast, because rifampicin treatment induces SGK2 mRNA in human liver cells, how rifampicin regulates the mouse and human SGK2 genes appears to differ. Sequence alignment of human and mouse SGK2 promoter regions up to −4 kb reveals that the mouse promoter conserves the pPRE but not dPRE. Although the possibility may exist that the lack of dPRE may explain why the mouse SGK2 gene was regulated differently from the human counterpart, the promoter of mouse SGK2 gene should be analyzed in the future to understand the mechanism by which the human and mouse promoter respond differently.
The biological role of SGK enzymes has been primarily investigated utilizing SGK1 (Loffing et al., 2006). Sgk1-null mice revealed that SGK1 regulates renal Na+ homeostasis through the ion transport (ENaC/Na, K-ATPase) (Wulff et al., 2002). In addition to this regulation of ion transport, overexpressed SGK1 inactivated glycogen synthase kinase 3 in human embryonic kidney-derived 293 cells (Kobayashi et al., 1999a) and regulated intestinal glucose transport (Rexhepaj et al., 2007) and glucose transporter SGLT1 in Xenopus laevis oocytes (Dieter et al., 2004). Moreover, high glucose induced SGK1 in human umbilical vein endothelial cells (Khan et al., 2005). Although these functions have not been confirmed in organs or animals in vivo, there is the possibility that SGK1 may be involved in glucose metabolism. Compared with SGK1, SGK2 has been poorly investigated and no biological role has yet been determined for SGK2. Therefore, the regulatory role for the G6Pase gene is the first biologic function found for SGK2 in the liver. Despite structural resemblance to an insulin-regulated AKT, SGK2 is not regulated by insulin; insulin treatment increased phosphorylation of AKT but did not alter SGK2 phosphorylation in HepG2 cells (unpublished observation). The fact that SGK2, but not FOXO1, knockdown repressed G6Pase activation indicates that SGK2 does not use FOXO1 to regulate PXR activation of the G6Pase gene. Thus, SGK2 is involved in cell signaling distinct from the insulin-AKT-FOXO1 pathway to regulate hepatic gluconeogenesis. What constitutes this SGK2 signaling and how PXR interacts with this signaling remain to be investigated in the future. One potential mechanism for this interaction is to activate the SGK2 gene, which is observed in HepG2 cells. Another mechanism may occur at the level of protein-protein interactions between PXR and SGK2, as indicated by the finding that rifampicin treatment only slightly increased SGK2 protein levels but increased G6Pase mRNA when human primary hepatocytes contained high basal SGK2. SGK2 is a protein kinase, and its activity can be altered by secondary modifications such as phosphorylation. In response to drug treatments, PXR directly interacts with SGK2 to regulate such modifications to activate the G6Pase gene, as observed with FOXO1, to which PXR binds to repress FOXO1 transactivation activity (Kodama et al., 2004). SGK2 signaling can be regulated by an endogenous stimulus. Glucagon and glucose are possibilities because they are known to regulate gluconeogenesis. Nevertheless, identifying the endogenous stimulus is now a current subject of our investigations.
PXR can be activated by numerous therapeutic drugs and xenobiotics, among which activation by drugs such as rifampicin and statins might have clinical implications. Large studies on patients treated with statins have suggested that statins can cause the side effect of increased serum glucose levels (Sukhija et al., 2009; Sattar and Taskinen, 2012). Because of these and other studies, the Food and Drug Administration has recently added new safety warnings to statins, noting increased risks for type 2 diabetes. The PXR-SGK2-G6Pase pathway has now provided us with an excellent experimental basis for investigating the molecular mechanism by which statin treatment may cause this side effect. In particular, if it is found to be polymorphic in the human population, SGK2 may become a critical risk factor because SGK2 levels determine whether PXR activation results in either the induction or repression of G6Pase. In addition to drug treatment, exposures to environmental chemicals such as nicotine and bisphenol A that activate human PXR should also be highlighted as risk factors in developing diabetes (Thayer et al., 2012). In conclusion, the SGK2-G6Pase pathway presents novel regulatory signaling in hepatic gluconeogenesis. PXR interactions with this pathway provide the experimental basis for understanding how drug treatment and exposure to xenobiotics can interfere with human health.
Supplementary Material
Acknowledgments
The authors thank the National Institute of Environmental Health Sciences DNA sequencing core for performing DNA sequencing.
Abbreviations
- ANOVA
analysis of variance
- ChIP
chromatin immunoprecipitation
- DMSO
dimethylsulfoxide
- dPRE
distal pregnane X receptor response element
- FOXO1
forkhead box protein O1
- G6Pase
glucose 6-phosphatase
- HRP
horseradish peroxidase
- PCN
pregnenolone-16α-carbonitrile
- PCR
polymerase chain reaction
- PEPCK1
phosphoenolpyruvate carboxykinase 1
- pPRE
proximal pregnane X receptor response element
- PXR
pregnane X receptor
- qRT-PCR
quantitative reverse-transcription PCR
- 5′-RACE
rapid amplification of 5′-complementary DNA ends
- SGK
serum- and glucocorticoid-regulated kinase
- siRNA
small interfering RNA
Authorship Contributions
Participated in research design: Negishi, Gotoh.
Conducted experiments: Gotoh.
Contributed new reagents or analytic tools: Gotoh.
Performed data analysis: Gotoh.
Wrote or contributed to the writing of the manuscript: Negishi, Gotoh.
Footnotes
This work was supported by the Intramural Research Program of National Institutes of Health National Institute of Environmental Health Sciences [Grant Z01ES1005-01].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. (2004) Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 12:862–870 [DOI] [PubMed] [Google Scholar]
- Khan ZA, Barbin YP, Farhangkhoee H, Beier N, Scholz W, Chakrabarti S. (2005) Glucose-induced serum- and glucocorticoid-regulated kinase activation in oncofetal fibronectin expression. Biochem Biophys Res Commun 329:275–280 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Cohen P. (1999b) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339:319–328 [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P. (1999a) Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344:189–197 [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24:7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Moore R, Yamamoto Y, Negishi M. (2007) Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J 407:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Negishi M. (2011) Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem 286:3570–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno Y, Negishi M, Kodama S. (2008) The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet 23:8–13 [DOI] [PubMed] [Google Scholar]
- Loffing J, Flores SY, Staub O. (2006) Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68:461–490 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T. (2007) Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem 282:9768–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. (2000) Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther 68:495–500 [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. (2001) Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther 69:400–406 [DOI] [PubMed] [Google Scholar]
- Park JY, Kim KA, Park PW, Park CW, Shin JG. (2003) Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther 74:334–340 [DOI] [PubMed] [Google Scholar]
- Rexhepaj R, Artunc F, Metzger M, Skutella T, Lang F. (2007) PI3-kinase-dependent electrogenic intestinal transport of glucose and amino acids. Pflugers Arch 453:863–870 [DOI] [PubMed] [Google Scholar]
- Rysä J, Buler M, Savolainen MJ, Ruskoaho H, Hakkola J, Hukkanen J. (2013) Pregnane X receptor agonists impair postprandial glucose tolerance. Clin Pharmacol Ther 93:556–563 [DOI] [PubMed] [Google Scholar]
- Sattar N, Taskinen MR. (2012) Statins are diabetogenic—myth or reality? Atheroscler Suppl 13:1–10 [DOI] [PubMed] [Google Scholar]
- Scheer N, Ross J, Rode A, Zevnik B, Niehaves S, Faust N, Wolf CR. (2008) A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest 118:3228–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhija R, Prayaga S, Marashdeh M, Bursac Z, Kakar P, Bansal D, Sachdeva R, Kesan SH, Mehta JL. (2009) Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med 57:495–499 [DOI] [PubMed] [Google Scholar]
- Surekha V, Peter JV, Jeyaseelan L, Cherian AM. (1997) Drug interaction: rifampicin and glibenclamide. Natl Med J India 10:11–12 [PubMed] [Google Scholar]
- Takasu N, Yamada T, Miura H, Sakamoto S, Korenaga M, Nakajima K, Kanayama M. (1982) Rifampicin-induced early phase hyperglycemia in humans. Am Rev Respir Dis 125:23–27 [DOI] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, and Gallo MA (2012) Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120:779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Vallon V, Huang DY, Völkl H, Yu F, Richter K, Jansen M, Schlünz M, Klingel K, Loffing J, et al. (2002) Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.