Abstract
A plethora of research has implicated the cingulate cortex in the processing of social information (i.e., processing elicited by, about, and directed toward others) and reward-related information that guides decision-making. However, it is often overlooked that there is variability in the cytoarchitectonic properties and anatomical connections across the cingulate cortex, which is indicative of functional variability. Here we review evidence from lesion, single-unit recording and functional imaging studies. Taken together, these support the claim that the processing of information that has the greatest influence on social behavior can be localized to the gyral surface of the midcingulate cortex (MCCg). We propose that the MCCg is engaged when predicting and monitoring the outcomes of decisions during social interactions. In particular, the MCCg processes statistical information that tracks the extent to which the outcomes of decisions meet goals when interacting with others. We provide a novel framework for the computational mechanisms that underpin such social information processing in the MCCg. This framework provides testable hypotheses for the social deficits displayed in autism spectrum disorders and psychopathy.
Keywords: social reward, autism spectrum disorders (ASD), psychopathy, prediction error, midcingulate cortex, anterior cingulate cortex, social cognition, empathy
Primates live in social environments that require individuals to understand the complex behavior of conspecifics. A plethora of research implicates the dorsal Anterior Cingulate Cortex (ACC) as playing a vital role in processing “social” information (i.e., processing elicited by, about, or directed toward others) (Amodio and Frith, 2006; Somerville et al., 2006; Rudebeck et al., 2008; Behrens et al., 2009; Apps et al., 2012; Hillman and Bilkey, 2012). Indeed, individuals with lesions to the ACC display social deficits so severe that they are said to have “acquired sociopathy” (Anderson et al., 1999). However, the ACC is also engaged by rewards (Doya, 2008), attention and salience (Davis et al., 2005), conflict, and during decision-making (Botvinick et al., 1999; Botvinick, 2007) which are inherently non-social processes. How can the same region be engaged by such a distinct set of processes? It is often overlooked that the area labeled as “ACC” by functional imaging research comprises multiple sub-regions, each with distinct cytoarchitecture and anatomical connections (Vogt et al., 1995; Palomero-Gallagher et al., 2008; Beckmann et al., 2009). Thus, some of the processes that have been reported to elicit an ACC response may in fact be localized to distinct sub-regions.
Here, we draw attention to anatomical tracer, neurophysiology, lesion and neuroimaging studies investigating the anatomical and functional properties of the dorsal ACC. Taken together this research highlights one sub-region which processes information about the outcomes of others' decisions and about the decisions made by others during social interactions. This region in fact lies on the gyral surface of the midcingulate cortex (MCCg) and not in the anatomically defined ACC. We contend that whilst the sulcal (MCCs) and gyral (MCCg) regions of the MCC can be differentiated in terms of processing first-person and social information respectively, the two areas process similar information about rewards that guide decision-making. By drawing parallels between the role of the MCCs in processing first-person rewards, and that of the MCCg in processing rewards in social contexts, we provide a new framework for investigating the contribution of the MCC to social decision-making.
Anatomy of the cingulate cortex
The cingulate cortex consists of four zones: retrosplenial, posterior (PCC), mid (MCC), and anterior (ACC) (Vogt et al., 1987, 1995; Palomero-Gallagher et al., 2008). Often the MCC is labeled as “dorsal” ACC and the actual ACC as “rostral” ACC. Unfortunately, the use of ACC as a “catch-all” terminology, has led many to inaccurately discuss the functional properties of an MCC result in relation to the functional and anatomical properties of the ACC. The ACC and MCC can be further subdivided by their cytoarchitecture (Palomero-Gallagher et al., 2008). In both the MCC and ACC there are differences in cytoarchitecture between the sulcus and the gyrus (see Figure 1A), indicative of distinct functional properties. Notably in this article we are discussing only regions within the cingulate cortex and not the region lying at the borders of the paracingulate sulcus and the superior frontal gyrus (“paracingulate cortex”) that is well known for its role in processing social information.
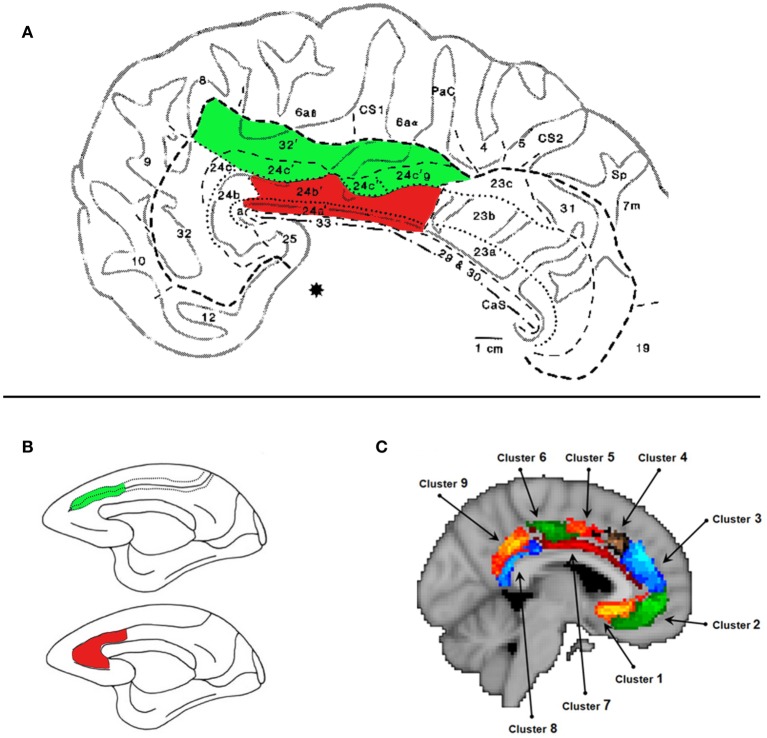
Figure 1.
The Midcingulate Cortex (MCC). (A) Cytoarchitecture of the MCC taken from Vogt et al. (1995). The areas shaded in green lie in the MCCs. The areas shaded in red lie on the MCCg. We argue that this area is engaged when processing information about others' decisions. Specifically we argue that areas 24a' and 24b', which lie on gyral surface of the cingulate cortex, extending on average 22 mm posterior to and 30 mm anterior to the anterior commisure denoted by (*). (B) Lesion site of the MCCg and ACCg (red) and the MCCs and the ACCs (green) from Rudebeck et al. (2006). The lesions that affected the gyrus caused disruptions to social behavior and disrupted the processing of social stimuli. (C) Subdivisions of the MCC and ACC according resting-state connectivity (Beckmann et al., 2009). Cluster 7 shown in dark red corresponds, broadly, to the MCCg.
Each cytoarchitectonic region has a different connectional fingerprint (Vogt and Pandya, 1987; Vogt et al., 1987; Devinsky et al., 1995; Margulies et al., 2007; Beckmann et al., 2009; Torta and Cauda, 2011). The MCCg shows a connectional profile that suggests involvement in processing information about others. This region has been shown to have strong connections with posterior portions of the superior temporal sulcus (pSTS) (Pandya et al., 1981; Seltzer and Pandya, 1989), temporal poles (TPs) (Markowitsch et al., 1985; Barbas et al., 1999) and paracingulate cortex (Vogt and Pandya, 1987; Petrides and Pandya, 2006). These areas have been consistently linked to processing information about others' mental states and intentions (Frith and Frith, 2003; Ramnani and Miall, 2004; Amodio and Frith, 2006; Hampton et al., 2008). There is minimal overlap between these connections and those of other portions of the ACC and MCC to the TPs, the pSTS and paracingulate cortex. Furthermore, the tracer studies listed above suggest that connections between the MCCg and these areas may be stronger than the connections from other ACC and MCC sub-regions. This profile leads us to propose that the MCCg is the sub-region of the cingulate cortex that plays the most significant role in social behavior.
Interestingly, the MCCg has connections which overlap with the MCCs to areas that are engaged during reward-based decision-making. Both areas project to medial and lateral portions of the orbitofrontal cortex (Morecraft et al., 1992; Morecraft and Van Hoesen, 1998) and to the nucleus accumbens (Kunishio and Haber, 1994; Haber et al., 1995). Anterior portions of both MCC sub-regions also receive dopaminergic input from the ventral tegmental area (VTA) (Hollerman and Schultz, 1998; Schultz, 1998; Williams and Goldman-Rakic, 1998). The connections of both the MCCg and MCCs to areas engaged when processing rewards (Schultz, 2006; Rushworth and Behrens, 2008) are indicative of a shared sensitivity to information that guides decision-making. Thus, we suggest that the MCCg plays an important role in processing information about the rewards others will receive and the decisions that lead to others' rewarding outcomes.
The MCCg and social information processing
Is there functional evidence for a role of the MCCg in processing reward-related information that guides decisions during social interactions? Chang et al. (2013) recorded from single-neurons during a task where monkeys received rewards or when they observed another monkey receiving reinforcement. They found a class of neurons lying on the gyral surface putatively in the MCC (although without histology it is not possible to localize accurately) that showed a change in spike-frequency when the monkeys observed another receiving the reward. The same neurons did not respond on trials when the monkeys received a reward themselves. Only a small proportion of neurons in the MCCs showed this same profile. This response profile highlights the MCCg as signaling information related to outcomes experienced by others (i.e., it contains a class of neurons that respond exclusively to others' reward receipt). Whilst only one study, this supports our claim that the MCCg processes information about rewards that others will receive.
Evidence from lesion studies also supports the notion that the MCCg processes social information. Lesions to the gyrus of the MCC and ACC of macaques have been shown to reduce the execution of social behaviors, such as the time spent in proximity with others and vocalizations, and also the processing of social stimuli (Hadland et al., 2003; Rudebeck et al., 2006). Unoperated monkeys or those with lesions to the MCCs or to the OFC, show delays in responding to a food item in the presence of social stimuli. Monkeys with lesions to the MCCg (Figure 1) show a reduced delay, suggesting a reduction in the value assigned to the social information (Rudebeck et al., 2006).
A small number of neuroimaging studies in humans have tested the claim that it is the MCCg and not the MCCs which processes information about others' decision-making. In Behrens et al. (2008) participants learned the probability of receiving a rewarding outcome from two options associated with different reward levels. On each trial participants received advice from a confederate about which option to choose. To maximize financial return subjects had to track how volatile the environment was (how rapidly the better option was shifting between the two) and also the volatility of the confederate advice. Whilst MCCs activity covaried with the environmental volatility, activity in the MCCg covaried with the volatility of the advice at the time of every trial outcome (Figure 2A).
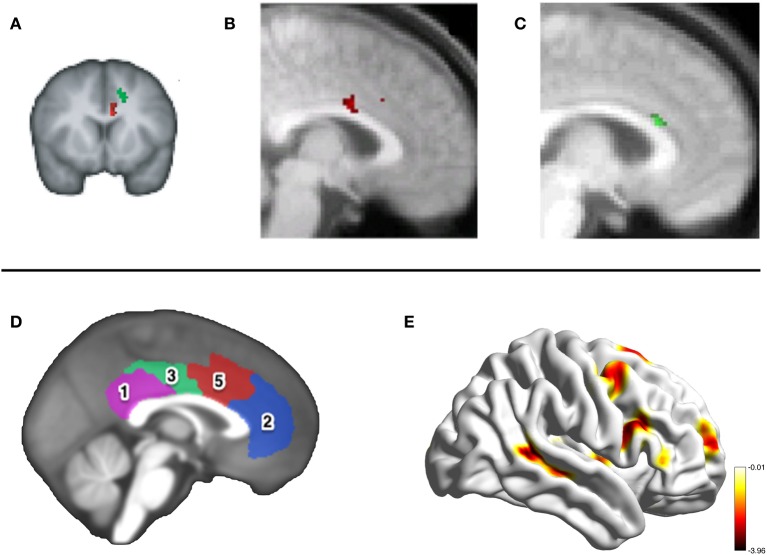
Figure 2.
Neuroimaging the MCC. The top panel shows activity in the same portion of the MCCg in three fMRI studies investigating reward processing during social interactions. (A) Activity in the MCCg (the cluster in red, MNI coordinate: −6, 12, 26) correlating with the volatility of advice given by a social confederate on a reward-based decision-making task, taken from Behrens et al. (2008). Activity in this cluster correlated with individual differences in the influence that the advice had on the subjects' own decision-making. (B) Activity in the MCCg [taken from Apps et al. (2013)] signaling a prediction error when the outcome of another's decision was unexpectedly positive (coordinate: 0, 8, 28), but not to the expected or unexpected outcomes of a computer's responses. (C) Activity shown in the MCCg (coordinate: 4, 22, 20) correlating with the anticipated net-value (benefit-cost) of a reward to be received by another person, but not rewards that will be received one's self [taken from Apps and Ramnani (under review)]. The bottom panel shows the results of resting-state connectivity analysis in Autism Spectrum Disorders by Balsters et al. (in prep). Connectivity between the MCC, cluster 5 shown in red (D), and the pSTS (E) was reduced in ASD compared to control participants.
Apps et al. (2013) examined activity when participants monitored the decisions and outcomes of a confederate and a computer, when the outcomes were sometimes unexpectedly either positive or negative. They examined activity at the time of a cue that revealed the outcome of the trial to the subject before it was revealed to the confederate or computer. Whilst the MCCs signaled when the outcome of either the computer or confederate's response was unexpectedly positive, the MCCg signaled the same information but only when the choice was made by another person and not by the computer (Figure 2B). Unpublished data from Apps and Ramnani (under review), also found that the MCCg signaled the net-value of rewards others will receive (benefit-cost) and not the net-value of one's own rewarding outcomes. These findings support the claim that the MCCg is engaged when processing information about the rewards others receive (Figure 2C).
The MCCs, decision-making and response-outcome monitoring
Whilst there has been considerable theoretical discussion of the functional properties of the MCC (or “dorsal ACC”), this literature largely ignores the contribution of this region to social cognition and is based on studies that find activation that lies predominantly, or exclusively, in the MCCs. As a result, there is a an absence of a theory of MCCg function. However, it is notable that the studies discussed in the previous section are consistent with a claim that the MCCg processes similar information to the MCCs. Here, we discuss a theoretical account of MCCs function, in order to draw parallels with the MCCg in the next section.
Recent theoretical accounts suggest that the MCCs is engaged when predictions are made about the outcomes of decisions and when the outcomes of decisions are monitored (Alexander and Brown, 2011; Silvetti et al., 2013). When outcomes are discrepant from those that were predicted, neurons in the MCCs signal prediction errors (PE), equating to the surprise evoked by the outcome (Matsumoto et al., 2007; Holroyd and Coles, 2008; Quilodran et al., 2008; Jocham et al., 2009; Kennerley et al., 2011; Nee et al., 2011). Furthermore, it has been argued that such a response-outcome functional property allows the region to play a role in monitoring the extent to which behaviors are meeting higher order needs or goals (Behrens et al., 2007; Botvinick, 2012; Holroyd and Yeung, 2012; Kolling et al., 2012). That is, the MCC tracks response-outcome contingencies within the context of how actions are meeting temporally abstract goals. Although there is not scope to discuss studies in detail here, there is evidence that MCC prediction and outcome processing is modulated by the extent to which behaviors are meeting contextually driven goals (Behrens et al., 2007; Rushworth and Behrens, 2008; Kolling et al., 2012).
It has been suggested that information processing in the MCC conforms to the principles of hierarchical reinforcement learning theory (HRL). In HRL, learning is not simply between stimulus-response and outcome [as in classic reinforcement learning (RL)], but learning occurs in a hierarchical framework where multiple actions (or sub-goals) must be performed and monitored in order to reach the higher-order goal (e.g., stimulus-response-response-response-outcome learning) (Botvinick, 2012). As such, each performed action is aimed at meeting a sub-goal that does not lead to a rewarding outcome on its own, but the performance of each action is crucial in order to achieve the higher order goal of the rewarding outcome. In HRL PE signals drive learning and occur when an outcome is unexpected as in RL. There are a considerable number of neurophysiological and neuroimaging studies have shown that neurons in the MCCs signal when the outcomes of decisions are unexpected (Matsumoto et al., 2007; Holroyd and Coles, 2008; Quilodran et al., 2008; Jocham et al., 2009; Kennerley et al., 2011; Nee et al., 2011). However, unlike in standard RL, in HRL PEs occur when actions fail to achieve sub-goals. These are sometimes referred to as pseudo-prediction errors (PPE) as they are not directly linked to the receipt of a rewarding outcome. Ribas-Fernandes et al. (2011) showed that the MCCs signal occurs when a PPE would be processed and not at the time when a classic PE would be signaled. This suggests that the PE signals in the MCCs may operate to track the extent to which an action is meeting an organism's goals by signaling the surprise at the time of the outcome of a decision. These surprise signals may take the form of PPEs as proposed in HRL.
The MCCg : predictions and errors during social interactions
We argue that the MCCg processes similar information to the MCCs but does so during social interactions [i.e., information is processed in an “other” reference frame (Hunt and Behrens, 2011)]. That is, the MCCg signals predictions and monitors outcomes during social interactions when the outcome will be received by another. We suggest that social behavior can be organized into a HRL framework, whereby a subject's own goal of how to interact with another acts as a higher-order policy. The actions of others (or one's own actions impacting upon another) will therefore serve as sub-goals to that policy. The outcome of each action (or sub-goal) will be monitored during a social exchange, in relation to the prior predictions instantiated by the higher-order goal. Thus, we suggest the MCCg will be engaged when processing the value of each action during a social exchange. In addition, it will be involved in processing information about whether actions or choices meet current, overarching goals in a social environment. When a sub-goal is not met, a “social” prediction error (SPE) will signal the discrepancy between the predicted and actual consequences of the choice, whether self or other, updating the agent's own policy. Simply put, the MCCg will signal predictions and monitor the outcomes of each action when interacting with another. However, the nature of the predictions will be influenced by the context within which each action and outcome are being processed. Thus, the context of a social interaction will influence the manner in which the MCCg codes information about others' rewarding outcomes.
For this theoretical account to hold true,the MCCg must be sensitive to rewards that others receive, MCCg activity must be related to higher level statistical properties of others' behavior (e.g., volatility) and it must signal prediction errors when the outcomes of others' choices are unexpected. These three properties were demonstrated in studies outlined above, where we highlighted that the MCCg contained neurons that responded when another receives a reward (Chang et al., 2013), MCCg activity tracked the volatility of another's choices (Behrens et al., 2008) and also this area signalled when the outcome of another's decision was unexpected (Apps et al., 2013). Furthermore, this account would also allow for considerable flexibility and individual differences in how reward-related information is processed in different social contexts, and therefore the extent to which MCCg influences behavior.
The MCCg and disorders of social cognition
What predictions can be made for behavioral consequences of MCCg damage? We suggest that disruptions to the MCCg will have two main effects: first, this account would be a multi-faceted impact on motivation for engagement in social interactions may decline as decreased sensitivity to others' rewards will diminish the influence of such outcomes on the higher-order goals of an agent. Furthermore, when presented with the possibility of interacting with another, the motivation for attending to sub-goals will not be maintained and agents may become apathetic toward social engagement. In addition, even when engaged in a social interaction, a failure to maintain motivation for attending to sub-goals would result in unsustained social interaction. Second, we contend that MCCg dysfunction may cause a failure in individuals to update the value of a policy when an unexpected outcome of a sub-goal fails to evoke a SPE. As a result, an agent may become insensitive to an outcome of a sub-goal that reduces the value of a reward another will receive (i.e., a reduction in empathy), or to the outcomes of their own actions that reduce the value of a rewarding outcome for another (e.g., a failure to maintain prosocial behaviors).
The first prediction fits with existing theories of social deficits displayed in Autism Spectrum Disorders (ASD) (Dawson et al., 2005; Chevallier et al., 2012). Social Motivation Theory (Chevallier et al., 2012) proposes that individuals with ASD are unable to form stimulus-reward contingencies for social stimuli, resulting in reduced social attention and engagement. Chevallier et al. (2012) focused on an orbitofrontal-striatal-amygdala circuit; we propose that the MCCg may play a key role in ASD. Previous studies have shown disturbed cytoarchitecture specifically in the MCCg in individuals with ASD(Simms et al., 2009). Similarly, Delmonte et al. (2013) showed hyperconnectivity between the caudate and MCCg in children with ASD, the strength of which was negatively correlated with neural responses to social rewards (Delmonte et al., 2012). Unpublished data by Balsters et al. (in prep) suggests a reduction in connectivity between the MCC and the pSTS, an area that is engaged when processing others' mental states, in individuals with ASD (see Figure 2).
A meta-analysis of fMRI studies examining social processing in ASD compared to controls (Di Martino et al., 2009). They showed consistent group differences in anterior and posterior regions of the cingulate cortex in the processing of social stimuli, but not in the MCCg for either the social or non-social tasks. However, our theoretical perspective would suggest that differences in MCCg function in ASD will only be observed when processing others' decisions or outcomes during social interactions. To date, studies examining social processing in ASD and those reviewed in the meta-analysis, have largely focused on the perception of social stimuli and not required subjects to interact with another and monitor decision-outcome contingencies. Future research should therefore test the tenets of our theory specifically when subject are engaged in a social interaction.
The second prediction above matches behavioral deficits seen in individuals with psychopathy, who are suggested to be insensitive to rewards that others will receive, leading to increased competitive behaviors (Mokros et al., 2008; Koenigs et al., 2010; Curry et al., 2011). Similarly, individuals with psychopathy have been shown to display a reduced error related negativity, measured using Electroencephalography, when observing other's outcomes during a social interaction (Brazil et al., 2011). This signal is putatively sourced in the MCC. Recent studies also indicate that gray matter volume and activity in the MCCg correlate with psychopathic and callous traits (De Brito et al., 2009; Anderson and Kiehl, 2012; Cope et al., 2012; Lockwood et al., 2013). Thus, whilst only preliminary evidence, these studies highlight the putative role that differences in MCCg function may have to psychopathy and psychopathic traits and particularly to the choices they make when interacting others.
Summary
Based on anatomical connectivity, neurophysiology and neuroimaging evidence, we suggest that the region of the cingulate cortex that plays the most important role in social cognition and social behavior lies in the MCCg. Our model highlights this region as playing an important role in predicting and monitoring the outcomes one's own and others' decisions when the outcomes will be experienced by another. Future research should examine the extent to which the MCCg is engaged when monitoring the outcomes of others' decisions and how deficits in MCCg function lead to deficits in using social information to guide one's behavior.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alexander W. H., Brown J. W. (2011). Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–U163 10.1038/nn.2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Anderson N. E., Kiehl K. A. (2012). The psychopath magnetized: insights from brain imaging. Trends Cogn. Sci. 16, 52–60 10.1016/j.tics.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. W., Bechara A., Damasio H., Tranel D., Damasio A. R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 2, 1032–1037 10.1038/14833 [DOI] [PubMed] [Google Scholar]
- Apps M. A. J., Balsters J. H., Ramnani N. (2012). The anterior cingulate cortex: monitoring the outcomes of others' decisions. Soc. Neurosci. 7, 424–435 10.1080/17470919.2011.638799 [DOI] [PubMed] [Google Scholar]
- Apps M. A. J., Green R., Ramnani N. (2013). Reinforcement learning signals in the anterior cingulate cortex code for others' false beliefs. Neuroimage 64, 1–9 10.1016/j.neuroimage.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Barbas H., Ghashghaei H., Dombrowski S. M., Rempel-Clower N. L. (1999). Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J. Comp. Neurol. 410, 343–367 [DOI] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M. F. S. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T. E. J., Hunt L. T., Rushworth M. F. S. (2009). The computation of social behavior. Science 324, 1160–1164 10.1126/science.1169694 [DOI] [PubMed] [Google Scholar]
- Behrens T. E. J., Hunt L. T., Woolrich M. W., Rushworth M. F. S. (2008). Associative learning of social value. Nature 456, 245–249 10.1038/nature07538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T. E. J., Woolrich M. W., Walton M. E., Rushworth M. F. S. (2007). Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221 10.1038/nn1954 [DOI] [PubMed] [Google Scholar]
- Botvinick M. M. (2012). Hierarchical reinforcement learning and decision making. Curr. Opin. Neurobiol. 22, 956–962 10.1016/j.conb.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L. E., Fissell K., Carter C. S., Cohen J. D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- Botvinick M. M. (2007). Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356–366 10.3758/CABN.7.4.356 [DOI] [PubMed] [Google Scholar]
- Brazil I. A., Mars R. B., Bulten B. H., Buitelaar J. K., Verkes R. J., De Bruijn E. R. A. (2011). A neurophysiological dissociation between monitoring one's own and others' actions in psychopathy. Biol. Psychiatry 69, 693–699 10.1016/j.biopsych.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Chang S. W. C., Gariepy J. F., Platt M. L. (2013). Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 16, 243–250 10.1038/nn.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E. S., Schultz R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239 10.1016/j.tics.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L. M., Shane M. S., Segall J. M., Nyalakanti P. K., Stevens M. C., Pearlson G. D., et al. (2012). Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Res. 204, 91–100 10.1016/j.pscychresns.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry O., Chesters M. J., Viding E. (2011). The psychopath's dilemma: the effects of psychopathic personality traits in one-shot games. Pers. Individ. Dif. 50, 804–809 10.1016/j.paid.2010.12.036 [DOI] [Google Scholar]
- Davis K. D., Taylor K. S., Hutchison W. D., Dostrovsky J. O., McAndrews M. P., Richter E. O., et al. (2005). Human anterior cingulate cortex neurons encode cognitive and emotional demands. J. Neurosci. 25, 8402–8406 10.1523/JNEUROSCI.2315-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Webb S. J., McPartland J. (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- De Brito S. A., Mechelli A., Wilke M., Laurens K. R., Jones A. P., Barker G. J., et al. (2009). Size matters: increased gray matter in boys with conduct problems and callousunemotional traits. Brain 132, 843–852 10.1093/brain/awp011 [DOI] [PubMed] [Google Scholar]
- Delmonte S., Balsters J. H., McGrath J., Fitzgerald J., Brennan S., Fagan A. J., et al. (2012). Social and monetary reward processing in autism spectrum disorders. Mol. Autism 3, 7–7 10.1186/2040-2392-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmonte S., Gallagher L., O'Hanlon E., McGrath J., Balsters J. H. (2013). Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front. Hum. Neurosci. 7:430 10.3389/fnhum.2013.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Morrell M. J., Vogt B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118, 279–306 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Di Martino A., Ross K., Uddin L. Q., Sklar A. B., Castellanos F. X., Milham M. P. (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry 65, 63–74 10.1016/j.biopsych.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. (2008). Modulators of decision making. Nat. Neurosci. 11, 410–416 10.1038/nn2077 [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C. D. (2003). Development and neurophysiology of mentalizing. Philos. Trans. R. Soc B. Biol. Sci. 358, 459–473 10.1098/rstb.2002.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. N., Kunishio K., Mizobuchi M., Lyndbalta E. (1995). The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 15, 4851–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland K. A., Rushworth M. F. S., Gaffan D., Passingham R. E. (2003). The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 41, 919–931 10.1016/S0028-3932(02)00325-1 [DOI] [PubMed] [Google Scholar]
- Hampton A. N., Bossaerts P., O'Doherty J. P. (2008). Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc. Natl. Acad. Sci. U.S.A. 105, 6741–6746 10.1073/pnas.0711099105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman K. L., Bilkey D. K. (2012). Neural encoding of competitive effort in the anterior cingulate cortex. Nat. Neurosci. 15, 1290–1297 10.1038/nn.3187 [DOI] [PubMed] [Google Scholar]
- Hollerman J. R., Schultz W. (1998). Dopamine neurons report an error in the temporal prediction of reward during learning. Nat. Neurosci. 1, 304–309 10.1038/1124 [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Coles M. G. H. (2008). Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behaviour. Cortex 44, 548–559 10.1016/j.cortex.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Yeung N. (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 16, 122–128 10.1016/j.tics.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Behrens T. E. J. (2011). Frames of reference in human social decision making. Neural Basis Motiv.Cogn. Control 1, 409–424 10.7551/mitpress/9780262016438.003.0022 [DOI] [Google Scholar]
- Jocham G., Neumann J., Klein T. A., Danielmeier C., Ullsperger M. (2009). Adaptive Coding of Action Values in the Human Rostral Cingulate Zone. J. Neurosci. 29, 7489–7496 10.1523/JNEUROSCI.0349-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley S. W., Behrens T. E. J., Wallis J. D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat. Neurosci. 14, 1581–1589 10.1038/nn.2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Kruepke M., Newman J. P. (2010). Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia 48, 2198–2204 10.1016/j.neuropsychologia.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N., Behrens T. E. J., Mars R. B., Rushworth M. F. S. (2012). Neural Mechanisms of Foraging. Science 336, 95–98 10.1126/science.1216930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K., Haber S. N. (1994). Primate cingulostriatal projection - limbic striatal versus sensorimotor striatal input. J. Comp. Neurol. 350, 337–356 10.1002/cne.903500302 [DOI] [PubMed] [Google Scholar]
- Lockwood P. L., Sebastian C. L., McCrory E. J., Hyde Z. H., Gu X., De Brito S. A., et al. (2013). Association of callous traits with reduced neural response to others' pain in children with conduct problems. Curr. Biol. 23, 901–905 10.1016/j.cub.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D. S., Kelly A. M. C., Uddin L. Q., Biswal B. B., Castellanos F. X., Milham M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588 10.1016/j.neuroimage.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Markowitsch H. J., Emmans D., Irle E., Streicher M., Preilowski B. (1985). Cortical and subcortical afferent connections of the primates temporal pole - a study of rhesus-monkeys, squirrel-monkeys, and marmosets. J. Comp. Neurol. 242, 425–458 10.1002/cne.902420310 [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Matsumoto K., Abe H., Tanaka K. (2007). Medial prefrontal cell activity signaling prediction errors of action values. Nat. Neurosci. 10, 647–656 10.1038/nn1890 [DOI] [PubMed] [Google Scholar]
- Mokros A., Menner B., Eisenbarth H., Alpers G. W., Lange K. W., Osterheider M. (2008). Diminished cooperativeness of psychopaths in a prisoner's dilemma game yields higher rewards. J. Abnorm. Psychol. 117, 406–413 10.1037/0021-843X.117.2.406 [DOI] [PubMed] [Google Scholar]
- Morecraft R. J., Geula C., Mesulam M. M. (1992). Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J. Comp. Neurol. 323, 341–358 10.1002/cne.903230304 [DOI] [PubMed] [Google Scholar]
- Morecraft R. J., Van Hoesen G. W. (1998). Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res. Bull. 45, 209–232 10.1016/S0361-9230(97)00344-4 [DOI] [PubMed] [Google Scholar]
- Nee D. E., Kastner S., Brown J. W. (2011). Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage 54, 528–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Mohlberg H., Zilles K., Vogt B. (2008). Cytology and receptor architecture of human anterior cingulate cortex. J. Comp. Neurol. 508, 906–926 10.1002/cne.21684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D. N., Vanhoesen G. W., Mesulam M. M. (1981). Efferent connections of the cingulate gyrus in the rhesus-monkey. Exp. Brain Res. 42, 319–330 10.1007/BF00237497 [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D. N. (2006). Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J. Comp. Neurol. 498, 227–251 [DOI] [PubMed] [Google Scholar]
- Quilodran R., Rothe M., Procyk E. (2008). Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron 57, 314–325 [DOI] [PubMed] [Google Scholar]
- Ramnani N., Miall R. C. (2004). A system in the human brain for predicting the actions of others. Nat. Neurosci. 7, 85–90 10.1038/nn1168 [DOI] [PubMed] [Google Scholar]
- Ribas-Fernandes J. J. F., Solway A., Diuk C., McGuire J. T., Barto A. G., Niv Y., et al. (2011). A Neural Signature of Hierarchical Reinforcement Learning. Neuron 71, 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P. H., Bannerman D. M., Rushworth M. F. S. (2008). The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn. Affect. Behav. Neurosci. 8, 485–497 10.3758/CABN.8.4.485 [DOI] [PubMed] [Google Scholar]
- Rudebeck P. H., Buckley M. J., Walton M. E., Rushworth M. F. S. (2006). A role for the macaque anterior cingulate gyrus in social valuation. Science 313, 1310–1312 10.1126/science.1128197 [DOI] [PubMed] [Google Scholar]
- Rushworth M. F. S., Behrens T. E. J. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 11, 389–397 10.1038/nn2066 [DOI] [PubMed] [Google Scholar]
- Schultz W. (1998). Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 57, 87–115 10.1146/annurev.psych.56.091103.070229 [DOI] [PubMed] [Google Scholar]
- Seltzer B., Pandya D. N. (1989). Frontal-lobe connections of the superior temporal sulcus in the rhesus-monkey. J. Comp. Neurol. 281, 97–113 10.1002/cne.902810108 [DOI] [PubMed] [Google Scholar]
- Silvetti M., Alexander W., Verguts T., Brown J. (2013). From conflict management to reward-based decision making: actors and critics in primate medial frontal cortex. Neurosci. Biobehav. Rev. [Epub ahead of print]. 10.1016/j.neubiorev.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Simms M. L., Kemper T. L., Timbie C. M., Bauman M. L., Blatt G. J. (2009). The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathol. 118, 673–684 10.1007/s00401-009-0568-2 [DOI] [PubMed] [Google Scholar]
- Somerville L. H., Heatherton T. F., Kelley W. M. (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 9, 1007–1008 10.1038/nn1728 [DOI] [PubMed] [Google Scholar]
- Torta D. M., Cauda F. (2011). Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage 56, 2157–2172 10.1016/j.neuroimage.2011.03.066 [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Nimchinsky E. A., Vogt L. J., Hof P. R. (1995). Human cingulate cortex - surface-features, flat maps, and cytoarchitecture. J. Comp. Neurol. 359, 490–506 10.1002/cne.903590310 [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Pandya D. N. (1987). Cingulate cortex of the rhesus-monkey.2. Cortical afferents. J. Comp. Neurol. 262, 271–289 10.1002/cne.902620208 [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Pandya D. N., Rosene D. L. (1987). Cingulate cortex of the rhesus-monkey.1. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 262, 256–270 10.1002/cne.902620207 [DOI] [PubMed] [Google Scholar]
- Williams S. M., Goldman-Rakic P. S. (1998). Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex 8, 321–345 10.1093/cercor/8.4.321 [DOI] [PubMed] [Google Scholar]