Abstract
Objective
Thromboxane A2 receptor (TPr) has been reported to trigger vascular inflammation. Nuclear factor κ B (NF-κB) is a known transcription factor. The aims of the present study were to determine the contributions of NF-κB activation to TPr-triggered vascular inflammation and elucidate the mechanism(s) underlying TPr activation of NF-κB.
Approach and Results
The effects of TPr activators, I-BOP and U46619, on NF-κB activation, phosphorylation of rhoA/ rho-associated kinases and liver kinase B1, cell adhesion and migration, proliferation, and endothelium-dependent vasorelaxation were assayed in cultured human umbilical vein endothelial cells, human monocytes, or isolated mouse aortas. Exposure of human umbilical vein endothelial cells to TPr agonists I-BOP and U46619 induced dose-dependent and time-dependent phosphorylation of inhibitor of κB α in parallel with aberrant expression of inflammatory markers cyclooxygenase-2, inducible nitric oxide synthase, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1. Inhibition of NF-κB by pharmacological or genetic means abolished TPr-triggered expression of inflammatory markers. Consistently, exposure of human umbilical vein endothelial cells to either I-BOP or U46619 significantly increased phosphorylation of inhibitor of κB α, IkappaB kinase, rhoA, rho-associated kinases, and liver kinase B1. Pretreatment of human umbilical vein endothelial cells with the TPr antagonist SQ29548 or rho-associated kinases inhibitor Y27632 or silencing of the LKB1 gene blocked TPr-enhanced phosphorylation of inhibitor of κB α and its upstream kinase, IkappaB kinase. Finally, exposure of isolated mouse aortas to either U46619 or I-BOP enhanced NF-κB activation and vascular inflammation in parallel with reduced endothelium-dependent relaxation in intact vessels.
Conclusions
TPr stimulation instigates aberrant inflammation and endothelial dysfunction via rho-associated kinases/liver kinase B1/IkappaB kinase-dependent NF-κB activation in vascular endothelial cells.
The inflammatory response is a protective reaction to all acute and chronic infection. However, chronic or aberrant inflammatory response is considered a key pathological event in cardiovascular diseases, including endotoxic shock, hypertension, and coronary heart disease.1 Nuclear factor κ B (NF-κB) is a key transcription factor and is essential to the initiation and development of inflammatory response. In mammals, the NF-κB family consists of 5 members, RelA/ p65, RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). Under normal conditions, the NF-κB dimers are maintained in inactive form in the cytoplasm in a complex with inhibitor of κB (IκB). After phosphorylation, IκB undergoes ubiquitination and degradation by the proteasome. IκB-free NF-κB dimers translocate into the nucleus and regulate the transcription of downstream genes.2–4 Among genes regulated by NF-κB, cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), and cell adhesion molecules, including vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, are well known and play significant roles in endothelial cell activation and dysfunction.5–7
Thromboxane A2 (TXA2) is an eicosanoid produced by thromboxane synthase. Local and systemic elevations in TXA2 have been reported in several thrombotic and vascular diseases.8 TXA2 binds the tTXA2 receptor (TPr), a G protein–linked receptor, which occurs as 2 alternatively spliced subtypes, TPα and TPβ, in humans.9–11 TPr is expressed in a number of tissues, including platelets, placenta, vascular endothelial cells, and vascular smooth muscle cells.10,12,13 Activation of TPr by TXA2 is reported to inhibit endothelial cell migration, intercellular communication, and vascular tube formation.14–16 Moreover, activation of TPr induces apoptosis in endothelial cells through inhibition of Akt phosphorylation.17 TXA2 can also attenuate endothelial insulin signaling through the rho-associated kinase (ROCK)/liver kinase B (LKB)1/ phosphatase and tensin homolog pathway.18 TPr has also been reported to increase the expression of ICAM-1 and VCAM-1 in human endothelial cells through induction of NF-κB activation.6,19 However, how TPr activates NF-κB remains poorly understood. In this study, we investigated the mechanism underlying TPr activation of NF-κB in vascular cells. Our results demonstrate that LKB1 is required for TPr-dependent NF-κB activation.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
U46619 and I-BOP Induce Expression of COX-2 in HUVECs
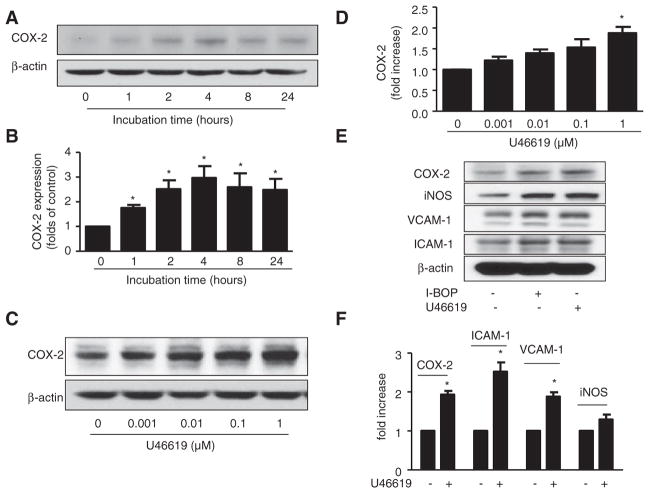
COX-2 plays a key role in the process of inflammation.20,21 To determine whether TPr agonists alter COX-2 expression, human umbilical vein endothelial cells (HUVECs) were starved overnight and treated with TPr agonist U46619 (1 μmol/L)13,22 for different periods of time. COX-2 expression was significantly increased 1 hour after U46619 treatment; expression peaked at 4 hours and decreased slightly after pro-longed (>4 hours) incubation (Figure 1A and 1B). U46619- induced COX-2 expression was also dose-dependent. Figure 1C and 1D show dose-dependent increase in COX-2 expression 4 hours after U46619 treatment, with a significant increase seen after treatment with 1 μmol/L U46619. Similarly, I-BOP, a TPr agonist that is structurally related to U46619, increased COX-2 expression in HUVECs in a time-dependent and dose-dependent manner (data not shown).
Figure 1.
Thromboxane A2 receptor (TPr) agonists induced expression of inflammatory genes. A and B, Cyclooxygenase-2 (COX-2) was increased by TPr agonist in a time-dependent manner. Human umbilical vein endothelial cells (HUVECs) were incubated with U46619 (1 μmol/L) for the indicated times. A, Western analysis of COX-2 expression. B, Quantification of COX-2 expression (n=3; *P<0.05 vs 0 hour). C and D, HUVECs were treated with the indicated doses of U46619 for 4 hours. C, Western analysis of COX-2 expression. D, Quantification of COX-2 expression (n=3; *P<0.05 vs 0 hour). E and F, HUVECs were treated with TPr agonists I-BOP or U46619 (1 μmol/L) for 4 hours. E, Western analysis of expression levels of inflammatory mRNAs, COX-2, inducible nitric oxide synthase (iNOS), vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1. F, Real-time polymerase chain reaction analysis of inflammatory mRNA levels in cells treated with U46619 (n=4; *P<0.05 vs control).
Inflammatory proteins such as cell adhesion molecules and iNOS are well-characterized markers of NF-κB activation and play an important role in inflammation in endothelial cells.23–25 To elucidate the important roles of TPr in vascular inflammation, we examined the effects of the TPr agonists, U46619 and I-BOP, on levels of ICAM-1, VCAM-1, and iNOS mRNA and protein in endothelial cells. Treatment of HUVECs with either U46619 or I-BOP for 4 hours enhanced expression of all 3 proteins (Figure 1E). Quantitative real-time polymerase chain reaction analysis consistently showed significant upregulation of ICAM-1, VCAM-1, iNOS, and COX-2 mRNAs by U46619 or I-BOP (Figure 1F).
U46619- and I-BOP–Enhanced Inflammation Is TPr Mediated
We next investigated whether TPr was required for U46619-triggered or I-BOP–triggered inflammatory responses. To this end, the TPr-specific antagonist, SQ29548 (10 μmol/L),13 was added to HUVECs 30 minutes before the addition of U46619 or I-BOP. Whereas SQ29548 alone had no effect on basal levels of COX-2, iNOS, ICAM-1, and VCAM-1, SQ29548 reduced the levels of inflammatory protein (Figure I in the online-only Data Supplement), and mRNA enhanced by U46619 (Figure IB–ID in the online-only Data Supplement) and I-BOP (data not shown). These data provide strong evidence that TPr activation promotes inflammatory response in HUVECs. Because the effects of I-BOP and U46619 on TPr-enhanced expression of proinflammatory genes were highly comparable, we examined the effects of TPr activation by interchangeably using I-BOP or U46619.
TPr Activation Activates the NF-κB Pathway
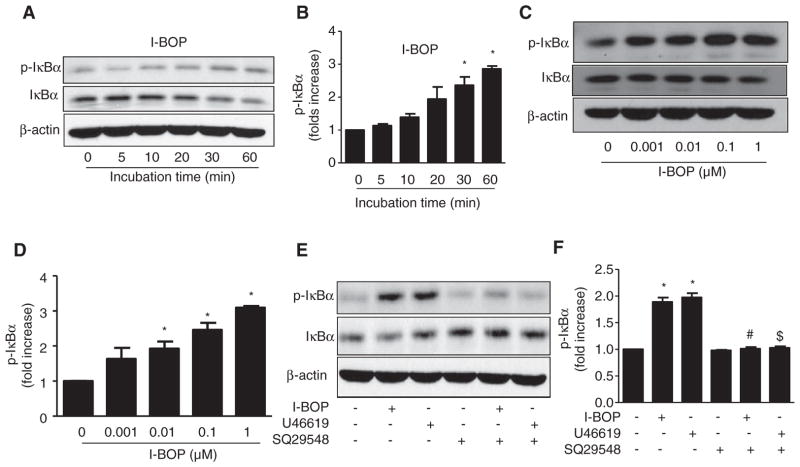
ICAM-1, VCAM-1, iNOS, and COX-2 are well-characterized downstream targets of NF-κB. IκBα phosphorylation by upstream kinases is a central event in NF-κB activation.26–28 Thus, we investigated whether TPr alters the phosphorylation of IκBα. I-BOP (1 μmol/L) increased the phosphorylation of IκBα in a time-dependent manner (Figure 2A), with a significant increase seen after 30 minutes of treatment (Figure 2B). I-BOP consistently lowered levels of total IκBα (Figure 2A), suggesting that IκBα was degraded after being phosphorylated.26–28 Similarly, I-BOP increased levels of p-IκBα in a dose-dependent manner (Figure 2C), with significant changes observed 30 minutes after treatment with a dose of at least 0.01 μmol/L I-BOP (Figure 2D).
Figure 2.
Thromboxane A2 receptor (TPr) agonists activated the nuclear factor κB pathway. A and B, TPr activation increased the phosphorylation of inhibitor of κB (IκBα) in a time-dependent manner. Human umbilical vein endothelial cells (HUVECs) were treated with I-BOP (1 μmol/L) for the indicated times. Western analysis of levels of phosphorylated and total IκBα (A) and quantitation of levels of phosphorylated IκBα (B) were performed. Data are means±SEM. (n=3; *P<0.05 vs 0 hour). C and D, HUVECs were treated with the indicated concentrations of I-BOP for 30 minutes. Western analysis of levels of phosphorylated and total IκBα (C) and quantitation of levels of phosphorylated IκBα (D) were performed. Data are means±SEM (n=3; *P<0.05 vs 0 μmol/L). E and F, HUVECs were preincubated in the presence or absence of SQ29548 for 30 minutes and treated with I-BOP or U46619 (1 μmol/L) for another 30 minutes. Western analysis of levels of phosphorylated and total IκBα (E) and quantitation of levels phosphorylated IκBα (F) were performed. Data are means±SEM (n=3; *P<0.05 vs control; #P<0.05 vs I-BOP alone; $P<0.05 vs U46619 alone).
TPr Activation of NF-κB Is TPr Dependent
To further confirm the effect of TPr activation on NF-κB activation, HUVECs were treated with SQ29548 (10 μmol/L), a TPr-specific antagonist, 30 minutes before treatment with TPr agonist (I-BOP). As expected, SQ29548 alone had no effect on phosphorylation of IκBα; however, the antagonist abolished the increase in p-IκB induced by TPr agonists (Figure 2E and 2F).
TPr Activation Increases the Expression of Inflammatory Genes via NF-κB
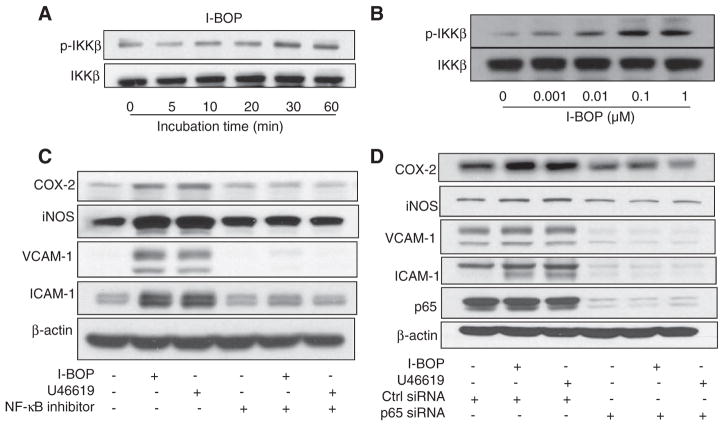
IκB kinase (IKK)α/β are well-known upstream kinases of IκB phosphorylation. Phosphorylation of these kinases at Ser 177 and Ser 181 is linked to their activation.4 Thus, we reasoned that TPr stimulation with I-BOP might activate NF-κB by phosphorylating IKK. I-BOP increased levels of p-IKKβ in a dose-dependent and time-dependent manner (Figure 3A and 3B). To further explore whether the gene expression mediated by TPr agonists was NF-κB mediated, HUVECs were pretreated with NF-κB–specific inhibitor for 30 minutes before the addition of U46619 or I-BOP. Although the inhibitor alone had no effect, it significantly attenuated TPr-enhanced inflammatory gene expression in HUVECs (Figure 3C).
Figure 3.
Thromboxane A2 receptor (TPr) agonists increase expression of inflammatory genes via activation of the nuclear factor κB (NF-κB) pathway. A, TPr activation increased phosphorylation of IkappaB kinase (IKK)β in a time-dependent manner. Human umbilical vein endothelial cells (HUVECs) were treated with I-BOP (1 μmol/L) for the indicated times, followed by Western analysis of levels of phosphorylated and total IKKβ. B, HUVECs were treated with the indicated concentrations of I-BOP for 30 minutes, followed by Western analysis of levels of phosphorylated and total IKKβ. C, HUVECs were preincubated with NF-κB inhibitor for 30 minutes before treatment with I-BOP or U46619 (1 μmol/L) for 4 hours, and expression levels of inflammatory genes were determined by Western analysis. D, HUVECs were transfected with control small interfering RNA (siRNA) or NFκB-P65 siRNA for 48 hours and treated with I-BOP or U46619 (1 μmol/L) for 4 hours, and expression levels of inflammatory genes were determined by Western analysis. COX indicates cyclooxygenase-2; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase; and VCAM, vascular cell adhesion molecule.
Translocation of NF-κB subunit p65 into nuclei is a pivotal step in NF-κB activation. Thus, p65-specific small interfering RNA (siRNA) was used to suppress the activity of NF-κB. p65 was significantly decreased by p65-specific siRNA, compared with control siRNA (Figure 3D). As expected, TPr agonist treatment increased the expression of iNOS, COX-2, VCAM-1, and ICAM-1 in cells treated with control siRNA, but not in those treated with p65 siRNA, indicating that NF-κB activation is required for TPr upregulation of COX-2, VCAM-1, ICAM-1, and iNOS expression in endothelial cells.
LKB1 Phosphorylation Is Required for TPr-Induced NF-κB Activation
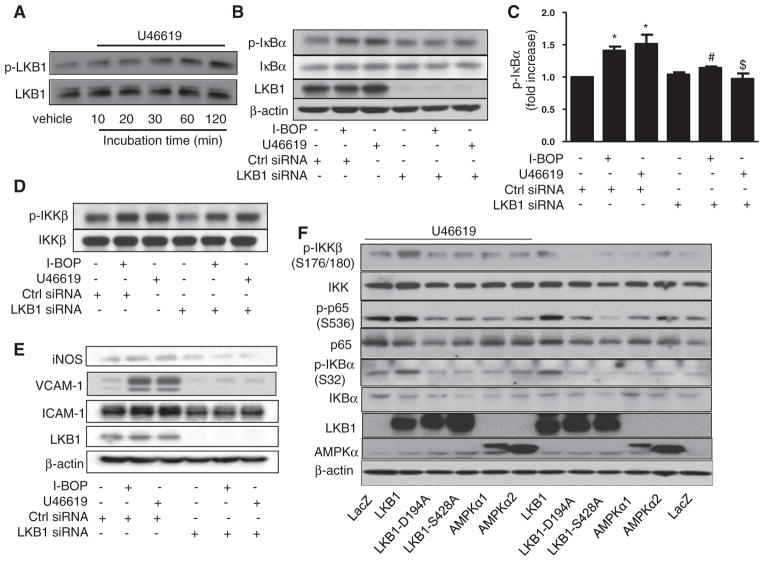
We previously reported that TPr stimulation increases the phosphorylation of LKB1 at serines 428 and 30713 in vascular smooth muscle cells.18 Thus, we investigated whether TPr stimulation alters the phosphorylation of LKB1 at Ser 428 in HUVECs. U46619 increased the level of p-LKB1 in these cells in a time-dependent manner (Figure 4A). Increased LKB1 phosphorylation was detected as early as 5 minutes and peaked at 120 minutes, after U46619 treatment. I-BOP also increased the phosphorylation of LKB1 in HUVECs (data not shown).
Figure 4.
Phosphorylation of liver kinase B (LKB)1 at Ser 428 is required for thromboxane A2 receptor (TPr)–induced nuclear factor κB (NF-κB) activation. A, TPr activation increased phosphorylation of LKB1 in a time-dependent manner. Human umbilical vein endothelial cells (HUVECs) were treated with U46691 (1 μmol/L) for the indicated times, and levels of phosphorylated and total LKB1 were determined by Western analysis. B–E, Silencing of LKB1 blocks TPr agonist–induced NF-κB activation. HUVECs were transfected with control small interfering RNA (siRNA) or LKB1 siRNA for 48 hours and treated with I-BOP or U46619 (1 μmol/L) for 4 hours. Western analysis of levels of phosphorylated and total inhibitor of κB (IκB)α and LKB1 (B) and quantitation of levels of phosphorylated IκBα (C) were performed. Data are means±SEM (n=3; *P<0.05 vs control; #P<0.05 vs I-BOP alone; $P<0.05 vs U46619 alone). D, Silencing of LKB1 reduces TPr-induced IkappaB kinase (IKK) phosphorylation. Levels of phosphorylated and total IKKβ were determined by Western analysis. E, Silencing of LKB1 abolishes TPr-enhanced expression of inducible nitric oxide synthase (iNOS), vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1. F, LKB1, but not AMP-activated protein kinase (AMPK)α, is required for TPr-induced NF-κB activation. Human THP-1 cells were transfected with plasmids encoding LacZ, LKB1, phosphorylation-defective LKB1 mutant LKB1-S428A, LKB1 mutant LKB1-D194A, AMPKα1, or AMPKα2 for 48 hours and treated with U46619 (1 μmol/L). Levels of total and phosphorylated IKK, p65, and IKBα; LKB1 and AMPKα were determined by Western analysis. Blots are representative of the results of at least 3 independent experiments.
Next, we assayed whether genetic suppression of LKB1 alters TPr-induced NF-κB activation. As expected, transfection of LKB1-specific siRNA did not affect basal levels of p-IκB; however, LKB1-specific siRNA, but not control siRNA, significantly abolished I-BOP–induced or U46619-induced increase in p-IκB (Figure 4B and 4C). Consistently, LKB1 siRNA, but not control siRNA, attenuated I-BOP–enhanced or U46619-enhanced IKKβ phosphorylation (Figure 4D). Finally, LKB1 siRNA, but not control siRNA, abolished the overexpression of VCAM-1, ICAM-1, and iNOS (Figure 4E). Taken together, these results indicate that LKB1 phosphorylation by TPr is required for the expression of inflammatory genes in HUVECs.
To further confirm the role of LKB1 in TPr-induced NF-κB activation, we tested whether overexpression of an LKB1 phosphorylation–defective mutant (LKB1-S428A, in which serine 428 is replaced with alanine) altered TPr-induced phosphorylation of IKK and consequent NF-κB activation. Because endothelial cells are difficult to transfect with plasmids, we performed the experiments using a human monocytic cell line, THP-1, which is deficient in LKB1. As shown in Figure 4F, THP-1 cells expressed no detectable LKB1. Compared with THP-1 cells transfected with LacZ, THP-1 cells overexpressing wild-type LKB1 showed increased phosphorylation of IKKβ, p65, and IκB. In contrast, overexpression of mutants LKB1-S428A or LKB1-D194A (in which Asp 194 is replaced with alanine), or of plasmids encoding AMP-activated protein kinase (AMPK)α1 or AMPKα2, had no effect on the phosphorylation of IKK, p65, or IκB (Figure 4F). Taken together, these results suggest that overexpression of LKB1, but not AMPKα1 or AMPKα2, increases NF-κB activation in THP-1 cells.
As expected, U46619 markedly increased the level of phosphorylated forms of IKK, p65, and IκB in THP-1 cells transfected with wild-type LKB1 (Figure 4F). Interestingly, U49919-enhanced phosphorylation of IKK, p65, and IκB was absent in cells overexpressing LKB1 mutants or AMPK (Figure 4F). Taken together, these results further confirm that TPr, via LKB1 phosphorylation at Ser 428, is required for AMPK-independent NF-κB activation.
Antioxidants Do Not Affect TPr-Induced NF-κB Activation
We had previously reported that TPr increased reactive oxygen species (ROS) in both endothelial cells and vascular smooth muscle cells.13,29 As expected, incubation of U46619 significantly increased ROS production in HUVECs (data not shown). To establish whether ROS was involved in TPr-enhanced NF-κB–mediated COX-2 expression, HUVECs were preincubated with tempol or mitotempol, 2 potent antioxidants, before the addition of U46619 or I-BOP. As depicted in Figure IIA in the online-only Data Supplement, tempol or mitotempol, which alone slightly increased COX-2 expression, caused a further enhancement of COX-2 in I-BOP–treated HUVECs. Consistently, tempol or mitotempol could not change the increase of p-IκBα mediated by I-BOP (Figure IIB in the online-only Data Supplement), indicating that ROS was not required for TPr-increased NF-κB activation.
Silencing of Atypical Protein Kinase C-ζ Does Not Alter TPr-Induced NF-κB Activation
Atypical protein kinase C-ζ (PKC-ζ) is reported to be responsible for LKB1-dependent AMPK activation.30,31 To further elucidate the roles of ROS and PKC-ζ, HUVECs were transfected with control or PKC-ζ–specific siRNA 48 hours before the addition of I-BOP or U46619. As expected, PKC-ζ–specific siRNA transfection lowered the levels of PKC-ζ (Figure IIC in the online-only Data Supplement). Importantly, PKC-ζ–specific siRNA, but not control siRNA, markedly increased U46619-induced or I-BOP–induced COX-2 expression (Figure IIC in the online-only Data Supplement). Taken together, these results suggested that PKC-ζ was not involved in TPr-induced NF-κB–mediated COX-2 expression.
Chelation of Exogenous Calcium With EDTA Abolishes TPr Agonist–Induced NF-κB Activation
Increase of intracellular calcium is a central event in TPr signaling. 32 Thus, it was important to investigate whether calcium chelation inhibited TPr-induced NF-κB activation. As depicted in Figure IID in the online-only Data Supplement, EDTA, a calcium chelator, abolished the phosphorylation of IκB, indicating that TPr-induced calcium increase was required for NF-κB activation in endothelial cells.
ROCK Is the Upstream Regulator of LKB1
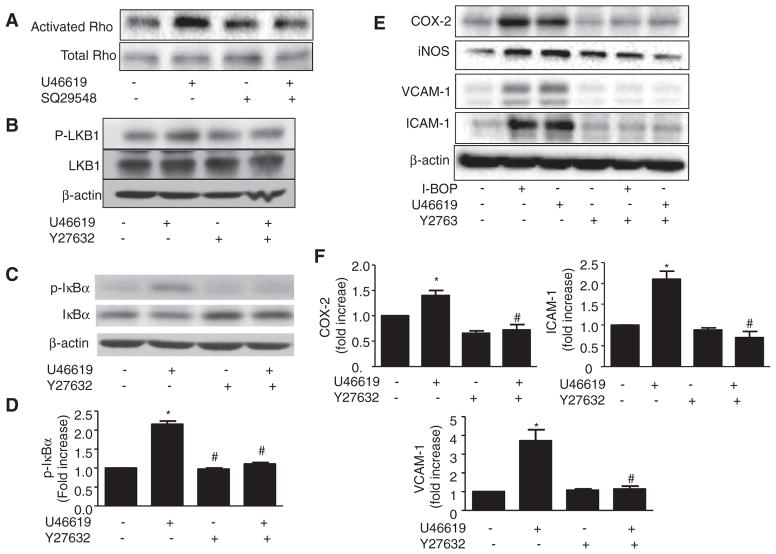
Because the results described above refuted the involvement of ROS/PKCζ in TPr-induced NF-KB activation and we had previously found that TPr alters ROCK activity,18 we reasoned that ROCK might be important for LKB1 phosphorylation and consequent induction of inflammatory genes in HUVECs. As shown in Figure 5A, U46619 activated Rho protein in a TPr-dependent manner. As expected, Rho/ROCK inhibitor Y27632 abolished U46619-enhanced phosphorylation of LKB1 (Figure 5B), suggesting that ROCK might be an upstream regulator of LKB1. We next investigated whether Y27632 alters TPr-induced NF-KB activation in HUVECs. Y27632 totally blocked the increase in phosphorylation of IκBα and the decrease in total IκBα induced by U46619 (Figure 5C and 5D), which suggests that the Rho/ ROCK pathway is the upstream regulator of NF-κB.
Figure 5.
Inhibition of rho-associated kinase (ROCK) blocks thromboxane A2 receptor (TPr)–enhanced vascular inflammation. A, Human umbilical vein endothelial cells (HUVECs) were pretreated in the presence or absence of SQ29548 and in the presence or absence of U46619 (1 μmol/L) for 2 hours. Levels of activated and total Rho were determined using a Rho activation assay kit. B–D, HUVECs were pretreated in the presence or absence of ROCK inhibitor Y27632 and treated in the presence or absence of U46619 (1 μmol/L) for 30 minutes. Levels of phosphorylated and total liver kinase B (LKB1; B) and inhibitor of κB (IκBα; C) were determined by Western analysis. Levels of phosphorylated IκBα were quantified (D). Data are means±SEM (n=3; *P<0.05 vs control; #P<0.05 vs U46619 alone). E and F, HUVECs were pretreated in the presence or absence of Y27632 for 30 minutes and treated with I-BOP or U46619 (1 μmol/L) for 4 hours. Expression of inflammatory genes was detected by Western blotting (E), and levels of inflammatory mRNAs in U46619-treated cells were quantitated by real-time polymerase chain reaction (F). Data are means±SEM (n=3–4; *P<0.05 vs control; #P<0.05 vs U46619 alone).
Inhibition of ROCK Blocks TPr-Enhanced Vascular Inflammation
To determine whether ROCK/LKB1 serves as an intermediate component of the TXA2-TPr signaling pathway regulating the inflammatory response, a specific inhibitor of ROCK, Y27632, was added to HUVECs before the addition of TPr agonists. Y27632 ablated TPr-enhanced expression of ICAM-1, VCAM-1, iNOS, and COX-2 (Figure 5E). Downregulation of ICAM-1, VCAM-1, and COX-2 by Y27632 was further confirmed by real-time polymerase chain reaction (Figure 5F).
TPr Activation Impairs Cell Migration and Increased THP-1 Adhesion Through NF-κB Activation
We next investigated whether TPr agonists affect endothelial cell migration in a Boyden chamber assay. The TPr agonist U46619 significantly impaired cell migration (Figure IIIA in the online-only Data Supplement). To further confirm whether this impairment is attributable to TPr-enhanced NF-κB activation, HUVECs were pretreated with SQ29548, Y27632, or NF-κB activation inhibitor before treatment with U46619. SQ29548, Y27632, or NF-κB activation inhibitor alone abolished U46619-impaired migration (Figure IIIA in the online-only Data Supplement), suggesting that NF-κB activation by ROCK is required for U46619 impairment of endothelial cell migration.
Next, we investigated the effects of TPr agonists on cell adhesion. HUVECs were treated with U46619 overnight and incubated with stained THP-1 cells for 30 minutes. Adhesion of THP-1 cells increased in U46619-stimulated HUVECs, and U46619-enhanced adhesion was blocked in cells pretreated with SQ29548, Y27632, or NF-κB inhibitor (Figure IIIB in the online-only Data Supplement). Taken together, these results support the essential role of ROCK-mediated NF-κB activation in endothelial adhesion activity.
TPr Activation Triggered Inflammatory Response in Mice Through NF-κB Activation
Finally, we determined whether TPr agonists triggered a whole-body inflammatory response via NF-κB activation. C57BL/6 mice that had been pretreated with NF-κB inhibitor or vehicle alone were administered U46619. Serum was collected, and cytokines were determined. U46619 significantly increased the levels of interleukin-1β, interleukin-6, and necrosis factorα (Figure IV in the online-only Data Supplement). Importantly, pretreatment with NF-κB inhibitor ablated U46619-induced elevation of the cytokines, compared with pretreatment with vehicle alone.
TPr-Induced NF-κB Activation Impaired Endothelium-Dependent Relaxation
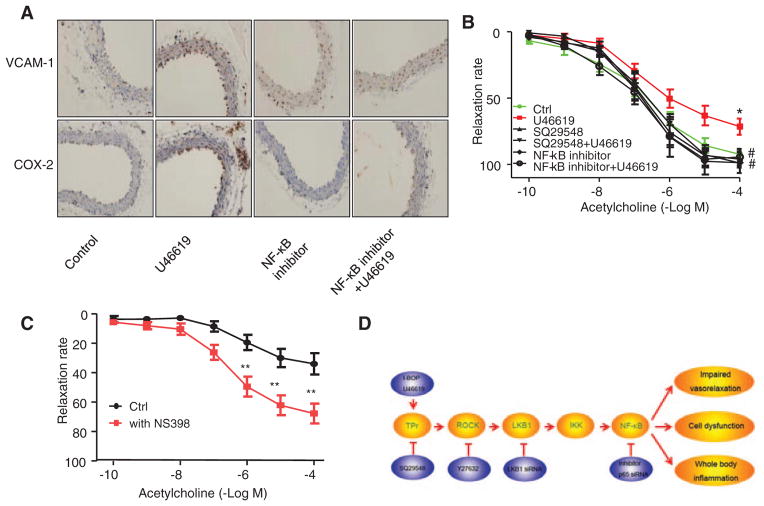
We thought it would be interesting to determine whether TPr-induced NF-κB–mediated expression of the inflammatory genes ICAM-1, VCAM-1, and COX-2 occurs in intact vascular explants and whether overexpression of inflammatory molecules impairs endothelium-dependent relaxation after U46619 treatment. To this end, mouse aortic rings were prepared and assayed for inflammatory markers and acetyl-choline-induced endothelium-dependent relaxation. U46619 markedly increased the levels of VCAM-1 and COX-2 and, importantly, pretreatment with NF-κB inhibitor abolished the U46619-induced overexpression (Figure 6A).
Figure 6.
Thromboxane A2 receptor (TPr) agonist–induced nuclear factor κB (NF-κB) activation impairs endothelium-dependent relaxation. A, Immunohistochemical staining of aortas from mice treated with U46619 and NF-κB inhibitor, alone or together, demonstrated expression of inflammatory genes. B, Aortic rings were pretreated in the presence or absence of SQ29548 or NF-κB inhibitor and treated with U46619 (2 μmol/L) overnight. Endothelium-dependent relaxation was assessed at the indicated concentrations of acetylcholine. Data are means±SEM (n=5–7; *P<0.05 vs control; #P<0.01 vs U46619 alone). C, Aortic rings were treated in the presence or absence of 10 μmol/L NS393 in Kreb’s buffer with oxygen for 1 hour. Endothelium-dependent relaxation was assessed at the indicated concentrations of acetylcholine. Data are means±SEM (n=6–7; **P<0.01 vs control). D, Proposed mechanism of TPr-induced NF-κB. IκB indicates inhibitor of κB; IKK, IkappaB; LKB1, liver kinase B1; ROCK, Rho-associated kinase; and VCAM, vascular cell adhesion molecule.
We next investigated whether U46619-enhanced NF-κB activation results in abnormal endothelium-dependent relaxation. U46619 caused a 30% reduction in endothelium-dependent vasorelaxation compared with vehicle-treated aortic rings (Figure 6B). U46619 treatment had no effect on endotheliumin-dependent relaxation (data not shown). Unlike U46619, exposure of vessels to phenylephrine, a selective α1-adrenergic receptor agonist, did not alter acetylcholine-induced vasorelaxation (data not shown). Furthermore, pretreatment with SQ29548 or NF-κB inhibitor abolished U46619-impaired vasorelaxation (Figure 6B), suggesting that TPr-mediated NF-κB activation is responsible for impaired relaxation.
Because TPr agonists markedly increased the expression of COX-2, we investigated whether inhibition of COX-2 by NS398, a selective COX-2 inhibitor, has an effect on endothelium-dependent relaxation. As expected, the addition of NS398 significantly improved acetylcholine-induced vasorelaxation (Figure 6C), suggesting that COX-2–derived factors might contribute to impaired endothelium-dependent vasorelaxation in intact vessels.
Discussion
Increasing evidence indicates that aberrant activation of TPr contributes to the initiation and progression of cardiovascular diseases, including atherosclerosis, hypertension, and diabetes mellitus. The present study has unveiled a novel mechanism, whereby TPr, via ROCK-mediated LKB1-dependent NF-κB activation, triggers aberrant vascular inflammation accompanied by impaired vasorelaxation in vivo. Consistently, inhibition of ROCK or LKB1 blocked TPr agonist–mediated NF-κB activation. Finally, inhibition of COX-2, a well-characterized downstream effector of NF-κB, normalized endothelium-dependent relaxation. Overall, our results suggest that ROCK-mediated, but LKB1-dependent, NF-κB activation by TPr might be responsible for aberrant inflammation and vascular dysfunction often seen in cardiovascular diseases (Figure 6D).
The most important finding of this study is the identification of LKB1-dependent NF-κB activation and consequent inflammation in vascular endothelial cells. Overexpression of LKB1, but not AMPK, increased the phosphorylation of IKK and IKBα with the expression of NF-κB effector genes, including ICAM-1, iNOS, and COX-2. Consequently, LKB1 knockdown decreased the phosphorylation of IKKβ mediated by TPr agonists. Interestingly, the effects of LKB1 on NF-κB activation seem to be dependent on ROCK-induced LKB1 phosphorylation. First, we found that ROS-activated PKC-ζ pathway, which is important in LKB1-dependent AMPK activation, was not involved in TPr-induced NF-κB activation, suggesting that neither PKC-ζ nor AMPK are required; Second, inhibition of ROCK with Y27632 blocked TPr-induced NF-κB activation and induction of NF-κB target genes. Finally, the most conclusive evidence, supporting an essential role of LKB1 phosphorylation in TPr-induced NF-κB activation, is that overexpression of a phosphorylation-defective LKB1 mutant (LKB1-S428A) blocked NF-κB activation. Indeed, we previously reported that TPr agonists increase LKB1 phosphorylation at Ser 428, and that TPr-activated, Rho-mediated LKB1 phosphorylation attenuates insulin signaling in endothelium.18 Because inflammation is a known risk factor for insulin resistance, it is highly anticipated that NF-κB–mediated inflammation might be involved in TPr-induced insulin resistance.
LKB1-enhanced NF-κB activation seems to be mediated by increased IKK phosphorylation. Whether IKK is phosphorylated by LKB1 or another of the LKB1 family kinases remains unclear, and this warrants further investigation. AMPK is one of 14 downstream targets of LKB1,33 and the role of AMPK in NF-κB signaling is highly controversial. For example, AMPK has been reported to directly phosphorylate IKKβ.34 However, our published work and the work of others indicate that AMPK activation inhibited NF-κB activation in endothelial cells, and mice deficient in AMPKα2 exhibited excessive activation of NF-κB.35 Although the roles of AMPK in the NF-κB pathway remain to be determined, our results suggest that LKB1-dependent IKK phosphorylation is independent of AMPK. Further investigation is warranted.
Endothelium can be considered as an initial pathogenic factor in many diseases, and endothelial cell activation and dysfunction impair cell function or cause vascular inflammation.36,37 Another important finding of this study is that aberrant NF-κB activation by TPr activation causes impairment of endothelium-dependent relaxation in vivo. We have further reported that selective inhibition of COX-2 normalizes vascular relaxation in aortic rings, supporting the idea that NF-κB–mediated induction of COX is operational in intact vascular explants. Importantly, small doses of TPr trigger whole-body inflammation, as evidenced by increased serum cytokine levels in mice in vivo. These results suggest that TPr induces systemic inflammation, and that TPr-induced inflammation extends beyond vascular walls. Thus, our results support the concept that overproduction of TPr is a potent inducer of both vascular and systemic inflammation. As a result, TPr antagonists, ROCK inhibitors, or inhibition of LKB1 are potential key targets to inhibit TXA2-induced inflammation in relevant diseases, such as diabetes mellitus, in which increased TXA2 release is evident.38
In conclusion, this study provides evidence that LKB1 is required for TPr-instigated systemic inflammation, and that inhibition of LKB1 or ROCK might be effective in preventing TPr-induced vascular dysfunction and related disorders.
Supplementary Material
Supplemental Figure I. U46619- and I-BOP-enhanced inflammation is mediated by TPr. A–D. HUVECs were treated with 10 μM TPr antagonist SQ29548 30 min prior to treatment with I-BOP (1μM ) or U46619 (1μM) for 4 h. Expression of inflammatory genes was detected by western analysis (A) and real-time PCR (B–D). Data are means ± SEM. (n=4; *, p<0.05 versus control).
Supplemental Figure II. Effects of antioxidants, calcium chelation, and PKC-ζ inhibition on TPr-induced NF-κB activation in HUVECs. A. HUVECs were treated with I-BOP(1uM) for 4 hours in the presence or absence of Tempol(10uM) or mitotempol(10uM). HUVECs were pre-incubated with Tempol or mitotempol for 30 min prior to the addition of I-BOP or U46619. Expression of COX-2 and β-actin were detected by western blots. B. HUVECs were pre-incubated with or without Tempol(10uM) or mitotempol(10uM) for 30 min prior to treat with I-BOP(1uM) for 30 min. Expression of p-IκBα, IκBα and β-actin were detected as described in Methods and Materials. C. HUVECs were transfected with PKC-ζ siRNA or control siRNA for 48 h. After the transfection, cells were treated with I-BOP or U46619 (1 μM) for 4h. Expression of COX-2 and PKC-ζ were detected by western blots. D. HUVECs were incubated with EDTA(100mM) prior to treat with I-BOP or U46619(1uM) for 30 min. Expression of p-IκBα, IκBα and β-actin were detected by western blots.
Supplemental Figure III. TPr agonist impaired cell migration and increased THP-1 adhesion through NF-κB activation. HUVECs were pretreated in the presence or absence of SQ29548, Y27632, and NF-κB inhibitor and treated with or without U46619 overnight. A. HUVEC migration was assessed using Boyden chamber assay and migrated cells were counted. Data are means ± SEM. (n=4; *, p<0.01 versus control; #, p<0.05 versus U46619 alone) B. THP-1 cells were stained and incubated with HUVECs for 30 min. Nonadherent cells were removed by washing and adherent cells were counted. Data are means ± SEM. (n=3; *, p<0.01 versus control; #, p<0.05 versus U46619 alone).
Supplemental Figure IV. TPr agonist induced inflammatory response in mice through NF-κB activation. Mice were pretreated with or without NF-κB inhibitor and treated with U46619 overnight, and levels of indicated serum cytokines were determined. Data are means ± SEM. (n=5–7; *, p<0.05 versus control; #, p<0.05 versus U46619 alone).
Supplemental Table I. Primers used in this study.
Significance.
Overwhelming evidence suggest that aberrant activation of Thromboxane A2 receptor (TPr) contributes to the initiation and progression of cardiovascular diseases, including atherosclerosis, hypertension, and diabetes mellitus. By using a combination of gain-of-function and loss-of-function approaches with pharmacological or genetic means, the present study has unveiled a novel mechanism, whereby TPr, via rho-associated kinase-mediated liver kinase B1–dependent nuclear factor κB activation, triggers aberrant vascular inflammation accompanied by impaired vasorelaxation in vivo. Consistently, inhibition of rho-associated kinase or liver kinase B1 blocked TPr agonist–mediated nuclear factor κB activation. Finally, inhibition of cyclooxygenase-2, a well-characterized downstream effector of nuclear factor κB, normalized endothelium-dependent relaxation. Overall, our results support the concept that overproduction of TPr is a potent inducer of both vascular and systemic inflammation, and that liver kinase B1 is required for TPr-instigated systemic inflammation. As a result, TPr antagonists, rho-associated kinase inhibitors, or inhibition of liver kinase B1 are potential key targets to inhibit thromboxane A2–induced inflammation in relevant diseases, such as diabetes mellitus, in which increased thromboxane A2 release is evident.
Acknowledgments
Sources of Funding
This study was supported by funding from the following agencies: the National Institutes of Health RO1 (HL074399, HL079584, HL080499, HL08920, HL096032, HL105157, and HL110488), the American Diabetes Association, and the Warren Endowed Chair of the University of Oklahoma Health Science Center (all to Dr Zou). Part of this work was also supported by an international cooperation grant from the Chinese National Science Foundation (81028002, to Y. Zhu and M.H. Zou). This work was also supported in part by grants from the Major National Basic Research Grant of China (2010CB912504, to Y. Zhu). Dr Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.301296/-/DC1.
Disclosures
None.
References
- 1.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–2142. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Lenardo MJ, Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 3.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Nf-kappab, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leceta J, Gomariz RP, Martinez C, Abad C, Ganea D, Delgado M. Receptors and transcriptional factors involved in the anti-inflammatory activity of VIP and PACAP. Ann N Y Acad Sci. 2000;921:92–102. doi: 10.1111/j.1749-6632.2000.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 6.Ashton AW, Ware GM, Kaul DK, Ware JA. Inhibition of tumor necrosis factor alpha-mediated NFkappaB activation and leukocyte adhesion, with enhanced endothelial apoptosis, by G protein-linked receptor (TP) ligands. J Biol Chem. 2003;278:11858–11866. doi: 10.1074/jbc.M210766200. [DOI] [PubMed] [Google Scholar]
- 7.Nakao S, Ogtata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002;238:11–18. doi: 10.1023/a:1019927616000. [DOI] [PubMed] [Google Scholar]
- 8.Ogletree ML. Overview of physiological and pathophysiological effects of thromboxane A2. Fed Proc. 1987;46:133–138. [PubMed] [Google Scholar]
- 9.Hirata T, Ushikubi F, Kakizuka A, Okuma M, Narumiya S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J Clin Invest. 1996;97:949–956. doi: 10.1172/JCI118518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raychowdhury MK, Yukawa M, Collins LJ, McGrail SH, Kent KC, Ware JA. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J Biol Chem. 1995;270:7011. doi: 10.1074/jbc.270.12.7011. [DOI] [PubMed] [Google Scholar]
- 11.Walsh M, Foley JF, Kinsella BT. Investigation of the role of the carboxyl-terminal tails of the alpha and beta isoforms of the human thromboxane A(2) receptor (TP) in mediating receptor:effector coupling. Biochim Biophys Acta. 2000;1496:164–182. doi: 10.1016/s0167-4889(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 12.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, Guzman M, Wu J, Zou MH. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, Ware JA. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2) J Biol Chem. 1999;274:35562–35570. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Tang S, Zhou S, Ware JA. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J Pharmacol Exp Ther. 2001;296:426–433. [PubMed] [Google Scholar]
- 16.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2) Circ Res. 2000;87:739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- 18.Song P, Zhang M, Wang S, Xu J, Choi HC, Zou MH. Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem. 2009;284:17120–17128. doi: 10.1074/jbc.M109.012583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Ishizuka T, Kawakami M, Hidaka T, Matsuki Y, Takamizawa M, Suzuki K, Kurita A, Nakamura H. Stimulation with thromboxane A2 (TXA2) receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol. 1998;112:464–470. doi: 10.1046/j.1365-2249.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mindrescu C, Le J, Wisniewski HG, Vilcek J. Up-regulation of cyclooxygenase-2 expression by TSG-6 protein in macrophage cell line. Biochem Biophys Res Commun. 2005;330:737–745. doi: 10.1016/j.bbrc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RA, Humphrey PP, Kennedy I, Levy GP, Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 1981;73:773–778. doi: 10.1111/j.1476-5381.1981.tb16814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd AW, Wawryk SO, Burns GF, Fecondo JV. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci USA. 1988;85:3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 25.Bereta M, Bereta J, Georgoff I, Coffman FD, Cohen S, Cohen MC. Methylxanthines and calcium-mobilizing agents inhibit the expression of cytokine-inducible nitric oxide synthase and vascular cell adhesion molecule-1 in murine microvascular endothelial cells. Exp Cell Res. 1994;212:230–242. doi: 10.1006/excr.1994.1139. [DOI] [PubMed] [Google Scholar]
- 26.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellits KH, Hay RT, Goodbourn S. Proteolytic degradation of MAD3 (I kappa B alpha) and enhanced processing of the NF-kappa B precursor p105 are obligatory steps in the activation of NF-kappa B. Nucleic Acids Res. 1993;21:5059–5066. doi: 10.1093/nar/21.22.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkalay I, Yaron A, Hatzubai A, Jung S, Avraham A, Gerlitz O, Pashut-Lavon I, Ben-Neriah Y. In vivo stimulation of I kappa B phosphorylation is not sufficient to activate NF-kappa B. Mol Cell Biol. 1995;15:1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:125–132. doi: 10.1161/ATVBAHA.110.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent KC, Collins LJ, Schwerin FT, Raychowdhury MK, Ware JA. Identification of functional PGH2/TxA2 receptors on human endothelial cells. Circ Res. 1993;72:958–965. doi: 10.1161/01.res.72.5.958. [DOI] [PubMed] [Google Scholar]
- 33.Katajisto P, Vallenius T, Vaahtomeri K, Ekman N, Udd L, Tiainen M, Mäkelä TP. The LKB1 tumor suppressor kinase in human disease. Biochim Biophys Acta. 2007;1775:63–75. doi: 10.1016/j.bbcan.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Bess E, Fisslthaler B, Fromel T, Fleming I. Nitric oxide-induced activation of the amp-activated protein kinase alpha2 subunit attenuates ikappab kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6:e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita M, Yano S, Yamaguchi T, Sugimoto T. Advanced glycation end products-induced reactive oxygen species generation is partly through NF-kappa B activation in human aortic endothelial cells. J Diabetes Complicat. 2013;27:11–15. doi: 10.1016/j.jdiacomp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Usui T, Okada M, Mizuno W, Oda M, Ide N, Morita T, Hara Y, Yamawaki H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;302:H1894–H1904. doi: 10.1152/ajpheart.01039.2011. [DOI] [PubMed] [Google Scholar]
- 38.Tang WH, Stitham J, Gleim S, et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest. 2011;121:4462–4476. doi: 10.1172/JCI59291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I. U46619- and I-BOP-enhanced inflammation is mediated by TPr. A–D. HUVECs were treated with 10 μM TPr antagonist SQ29548 30 min prior to treatment with I-BOP (1μM ) or U46619 (1μM) for 4 h. Expression of inflammatory genes was detected by western analysis (A) and real-time PCR (B–D). Data are means ± SEM. (n=4; *, p<0.05 versus control).
Supplemental Figure II. Effects of antioxidants, calcium chelation, and PKC-ζ inhibition on TPr-induced NF-κB activation in HUVECs. A. HUVECs were treated with I-BOP(1uM) for 4 hours in the presence or absence of Tempol(10uM) or mitotempol(10uM). HUVECs were pre-incubated with Tempol or mitotempol for 30 min prior to the addition of I-BOP or U46619. Expression of COX-2 and β-actin were detected by western blots. B. HUVECs were pre-incubated with or without Tempol(10uM) or mitotempol(10uM) for 30 min prior to treat with I-BOP(1uM) for 30 min. Expression of p-IκBα, IκBα and β-actin were detected as described in Methods and Materials. C. HUVECs were transfected with PKC-ζ siRNA or control siRNA for 48 h. After the transfection, cells were treated with I-BOP or U46619 (1 μM) for 4h. Expression of COX-2 and PKC-ζ were detected by western blots. D. HUVECs were incubated with EDTA(100mM) prior to treat with I-BOP or U46619(1uM) for 30 min. Expression of p-IκBα, IκBα and β-actin were detected by western blots.
Supplemental Figure III. TPr agonist impaired cell migration and increased THP-1 adhesion through NF-κB activation. HUVECs were pretreated in the presence or absence of SQ29548, Y27632, and NF-κB inhibitor and treated with or without U46619 overnight. A. HUVEC migration was assessed using Boyden chamber assay and migrated cells were counted. Data are means ± SEM. (n=4; *, p<0.01 versus control; #, p<0.05 versus U46619 alone) B. THP-1 cells were stained and incubated with HUVECs for 30 min. Nonadherent cells were removed by washing and adherent cells were counted. Data are means ± SEM. (n=3; *, p<0.01 versus control; #, p<0.05 versus U46619 alone).
Supplemental Figure IV. TPr agonist induced inflammatory response in mice through NF-κB activation. Mice were pretreated with or without NF-κB inhibitor and treated with U46619 overnight, and levels of indicated serum cytokines were determined. Data are means ± SEM. (n=5–7; *, p<0.05 versus control; #, p<0.05 versus U46619 alone).
Supplemental Table I. Primers used in this study.