Abstract
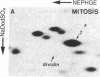
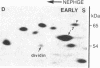
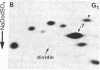
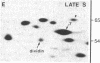
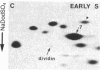
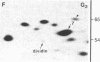
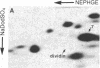
Studies of the polypeptides synthesized by normal and transformed human cultured cells under a variety of physiological conditions have revealed a basic 54-kDa protein NEPHGE 10a, whose rate of synthesis is sensitive to changes in the rate of cell proliferation. This nuclear phosphoprotein, which we have termed "dividin" (present only in populations of cells committed to divide), is synthesized almost exclusively during the S phase of the cell cycle of transformed human amnion cells (AMA). Dividin synthesis is first detected late in G1 near the G1/S transition border, reaches a maximum late in S phase, and declines thereafter. As expected for an S phase-specific protein, no detectable synthesis of dividin was observed in growth-arrested normal human cultured cells of epithelial and fibroblast origin. These findings suggest a role for this protein in events leading to DNA replication and cell division.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barque J. P., Lagaye S., Bendayan M., Puvion-Dutilleul F., Danon F., Larsen C. J. PSL, an S phase-related p55 nuclear antigen, associates transiently with chromatin. Exp Cell Res. 1985 Mar;157(1):8–14. doi: 10.1016/0014-4827(85)90147-8. [DOI] [PubMed] [Google Scholar]
- Bravo R., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from HELA cells. Coordinates and percentage of some major polypeptides. Cell Biol Int Rep. 1981 Jan;5(1):93–96. doi: 10.1016/0309-1651(81)90162-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Changes in the nuclear distribution of cyclin (PCNA) during S-phase are not triggered by post-translational modifications that are expected to moderately affect its charge. FEBS Lett. 1985 Mar 25;182(2):435–440. doi: 10.1016/0014-5793(85)80349-5. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Gene expression in normal and virally transformed mouse 3T3b and hamster BHK21 cells. Exp Cell Res. 1980 Jun;127(2):249–260. doi: 10.1016/0014-4827(80)90430-9. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Human proteins sensitive to neoplastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982 Apr;28(4 Pt 2):949–954. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Bravo R. Coordinated synthesis of the nuclear protein cyclin and DNA in serum-stimulated quiescent 3T3 cells. FEBS Lett. 1984 Apr 24;169(2):185–188. doi: 10.1016/0014-5793(84)80315-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Small J. V., Larsen P. M., Celis J. E. Coexistence of three major isoactins in a single sarcoma 180 cell. Cell. 1981 Jul;25(1):195–202. doi: 10.1016/0092-8674(81)90244-0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Bravo R. Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp Cell Res. 1986 Apr;163(2):287–293. doi: 10.1016/0014-4827(86)90059-5. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R., Larsen P. M., Fey S. J. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8(2):143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R. Synthesis of the nuclear protein cyclin in growing, senescent and morphologically transformed human skin fibroblasts. FEBS Lett. 1984 Jan 2;165(1):21–25. doi: 10.1016/0014-5793(84)80006-x. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Individual nuclei in polykaryons can control cyclin distribution and DNA synthesis. EMBO J. 1985 May;4(5):1187–1192. doi: 10.1002/j.1460-2075.1985.tb03758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Fey S. J., Larsen P. M., Celis A. Expression of the transformation-sensitive protein "cyclin" in normal human epidermal basal cells and simian virus 40-transformed keratinocytes. Proc Natl Acad Sci U S A. 1984 May;81(10):3128–3132. doi: 10.1073/pnas.81.10.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P., Nielsen S., Celis A. Nuclear patterns of cyclin (PCNA) antigen distribution subdivide S-phase in cultured cells--some applications of PCNA antibodies. Leuk Res. 1986;10(3):237–249. doi: 10.1016/0145-2126(86)90021-4. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Regulation of thymidine kinase activity in the cell cycle by a labile protein. J Cell Physiol. 1985 Aug;124(2):269–274. doi: 10.1002/jcp.1041240215. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Pardee A. B. Enhanced synthesis and stabilization of Mr 68,000 protein in transformed BALB/c-3T3 cells: candidate for restriction point control of cell growth. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4699–4703. doi: 10.1073/pnas.80.15.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase, DNA synthesis and cancer. Mol Cell Biochem. 1976 Jun 15;11(3):161–182. doi: 10.1007/BF01744997. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Madsen P., Celis J. E. S-phase patterns of cyclin (PCNA) antigen staining resemble topographical patterns of DNA synthesis. A role for cyclin in DNA replication? FEBS Lett. 1985 Nov 25;193(1):5–11. doi: 10.1016/0014-5793(85)80068-5. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Song M. K., Adolph K. W. Phosphorylation of nonhistone proteins during the HeLa cell cycle. Relationship to DNA synthesis and mitotic chromosome condensation. J Biol Chem. 1983 Mar 10;258(5):3309–3318. [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Tunón P., Johansson K. E. Yet another improved silver staining method for the detection of proteins in polyacrylamide gels. J Biochem Biophys Methods. 1984 May;9(2):171–179. doi: 10.1016/0165-022x(84)90008-3. [DOI] [PubMed] [Google Scholar]
- Wang E. A 57,000-mol-wt protein uniquely present in nonproliferating cells and senescent human fibroblasts. J Cell Biol. 1985 Feb;100(2):545–551. doi: 10.1083/jcb.100.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]