Key Points
Human IL-6 and a viral IL-6 homolog encoded by KSHV/HHV8 can independently or together lead to flares of KSHV-associated MCD.
KSHV-MCD disease flares were more severe where both human and viral IL-6 were elevated, suggesting they jointly contribute to severity.
Abstract
Kaposi sarcoma herpesvirus (KSHV)-associated multicentric Castleman disease (MCD) is a polyclonal B-cell lymphoproliferative disorder. Human (h) IL-6 and a KSHV-encoded homolog, viral IL-6, have been hypothesized to contribute to its pathogenesis, but their relative contributions to disease activity is not well understood. We prospectively characterized KSHV viral load (VL), viral (v) and hIL-6, and other cytokines during KSHV-MCD flare and remission in 21 patients with 34 flares and 20 remissions. KSHV-VL, vIL-6, hIL-6, IL-10, and to a lesser extent TNF-α, and IL-1β were each elevated during initial flares compared with remission. Flares fell into 3 distinct IL-6 profiles: those associated with elevations of vIL6-only (2 flares, 6%), hIL-6 elevations only (17 flares, 50%), and elevations in both hIL-6 and vIL-6 (13 flares, 38%). Compared with hIL-6–only flares, flares with elevated hIL-6 plus vIL-6 exhibited higher C-reactive protein (CRP) (P = .0009); worse hyponatremia (P = .02); higher KSHV VL (P = .016), and higher IL-10 (P = .012). This analysis shows vIL-6 and hIL-6 can independently or together lead to KSHV-MCD flares, and suggests that vIL-6 and hIL-6 may jointly contribute to disease severity. These findings have implications for the development of novel KSHV-MCD therapies targeting IL-6 and its downstream signaling. This trial was registered at clinicaltrials.gov as #NCT099073.
Introduction
Multicentric Castleman disease (MCD) is a polyclonal B-lymphoproliferative disorder characterized by inflammatory flares, including fever, cachexia, lymphadenopathy, splenomegaly, cytopenias, and hypoalbuminemia.1-3 MCD was first recognized as an idiopathic condition.2,4 More recently, a form caused by Kaposi sarcoma-associated herpesvirus (KSHV), also called human herpesvirus 8 (HHV-8), has been recognized.5-8 Almost all cases of MCD in the setting of HIV are KSHV-associated.9,10 Although the symptoms of KSHV-MCD may wax and wane, the disease is almost universally fatal if untreated.8,11
KSHV is also the etiologic agent of Kaposi sarcoma (KS) and primary effusion lymphoma.12-14 Its life cycle is characterized by latent and lytic phases,15-19 and its genome is notable for having pirated several genes homologous to cellular genes, including a viral homolog of human interleukin-6 (hIL-6) called viral IL-6 (vIL-6).20-25 Viral IL-6 can activate cells by binding to the broadly expressed gp130 subunit for the IL-6 receptor without involving the specific IL-6 receptor α (CD126) chain, thus potentially activating a broader range of cells26; it may also have intracellular actions in KSHV-infected cells.27,28 Compared with hIL-6, vIL-6 is about one thousandth less potent in activating the IL-6 receptor.29 KSHV can also induce the expression of cellular cytokines, including IL-6 and IL-10.30,31
KSHV-MCD is unique among herpesvirus-associated lymphoproliferative disorders in that a proportion of the pathogenic plasmablasts express KSHV in a lytically active form.10,11,16,31 Involved lymph nodes demonstrate hypocellular germinal centers with KSHV-infected polyclonal but monotypic plasmacytoid cells predominantly in the intrafollicular area.6,11,32,33 A proportion of the KSHV-infected cells express lytic genes, in particular vIL-6.6 Notably though, B lymphocytes in affected nodes are largely uninfected plasmacytoid cells that can produce hIL-6 but not vIL-6.6,33,34
Many clinical manifestations of idiopathic MCD are thought to be caused by overexpression of hIL-6.4,35,36 Overexpression of IL-6 in murine models gives rise to a syndrome resembling MCD.37 Patients with KSHV-MCD have elevated serum levels of vIL-6, and much work on KSHV-MCD pathogenesis has focused on this cytokine.38,39 KSHV-MCD flares have also been shown to exhibit elevated KSHV viral loads (VLs) and dysregulation of other cytokines including hIL-6, resolving with symptom resolution.40-42 Although both vIL-6 and human cytokines including hIL-6 have been studied separately in small KSHV-MCD cohorts, their respective roles and relative importance remain undefined. Some studies have reported high vIL-6 levels in KSHV-MCD and hypothesized that this was the principal cause of the inflammatory symptoms.43 Others have noted that much vIL-6 remains intracellular, acting through autocrine signaling, and that it is therefore unlikely to be responsible for systemic symptoms.28 Even if both cytokines contribute, as has been suggested by 1 murine model, it is possible that their contribution may be distinct or complementary.44 We therefore explored the role of vIL-6, hIl-6, and other cytokines in the pathogenesis of KSHV-MCD within a prospective natural history study.
Methods
Study population
Twenty-one patients with KSHV-MCD confirmed by histopathology were studied. These patients were enrolled in a clinical research protocol to explore the natural history and treatment of the disease. Treatments evaluated included high-dose zidovudine in combination with valganciclovir45; rituximab in combination with liposomal doxorubicin; and rituximab in combination with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin. Two patients who enrolled on this protocol were also studied before entry on a separate protocol to study laboratory parameters. The National Cancer Institute Institutional Review Board approved both protocols. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Clinical disease and response assessment
In the absence of a consensus definition of a KSHV-MCD acute episode (or clinical flare),46 we prospectively defined a flare requiring treatment in the protocol as the presence of at least 1 clinical symptom (eg, fever, sweats, or cachexia) and 1 laboratory abnormality (eg, hyponatremia, hypoalbuminema, anemia, or thrombocytopenia) attributed to KSHV-MCD in addition to the pathological diagnosis; this definition has since been published in a pilot treatment study.45 Subsequent to the commencement of the present study, an alternate definition of a flare (or attack) was published by the French Agence Nationale de Recherche sur le SIDA (ANRS)47; for the purposes of comparison, we have retrospectively classified the clinical flares analyzed here according to the ANRS scheme and also using treatment guidelines used in 2 other recent prospective studies of MCD.48,49 Symptom severity was graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0.50
Patients with clinical flares received 1 of the investigational regimens, whereas those with subclinical disease underwent active surveillance. Glucocorticoids were also used in some patients, especially during the initial treatment, and highly active antiretroviral therapy was initiated in patients where this had not already occurred.
In the absence of a consensus definition of a clinical remission of KSHV-MCD,46 evaluation for remission was performed using clinical and biochemical response criteria as previously described.45 To be considered a remission for the current analysis, patients had to have complete responses in clinical and biochemical parameters, but not necessarily radiographic parameters; we excluded radiographic changes as we observed during a previous treatment study that in some cases minor splenomegaly and/or lymphadenopathy persisted after resolution of other abnormalities and did not clearly indicate persistent active disease.45 In some cases, mild isolated hematologic and biochemical abnormalities that were attributable to HIV, drugs, or intercurrent illness, but not KSHV-MCD, persisted at remission. Notably these criteria are more stringent than those used in other interventional KSHV-MCD studies, where normalization of laboratory abnormalities has not generally been required in determining response.47-49
KSHV quantitative real-time PCR for assessment of VL in PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated from blood and DNA was isolated using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). DNA quality and concentration were assessed by optical density using Nanodrop1000 (Thermo Scientific, Wilmington, DE). DNA concentration was adjusted to 250 ng per 10 μL for 2 quantitative real-time PCR assays developed using TaqMan (Applied Biosystems, Foster City, CA). KSHV DNA was detected using previously reported primers for the K6 gene region. Cellular equivalents were determined using a quantitative assay for human endogenous retrovirus 3 (ERV-3). Samples were tested in triplicate for both assays, averaged, and reported as viral DNA copies per million PBMCs.
Viral IL-6
vIL-6 was measured using a modified version of a sandwich enzyme-linked immunosorbent assay previously described,43 using clone v6m 31.2.4 mouse monoclonal anti-vIL-6 antibody to coat the plates and then rabbit polyclonal anti-vIL-6 antibody and goat anti-rabbit antibody conjugated to horseradish peroxidase. The limit of detection was 1560 pg/mL, and the assay did not detect hIL-6 added to serum at concentrations up to 10 000 pg/mL. Samples were tested in duplicate and the results averaged. As we were undertaking paired analyses in patients with known KSHV-MCD, we used 1560 pg/mL as the lower limit of detection consistent with a previous study using this assay.45
Human cytokines
Serum levels of hIL-6, IL-1β, IL-8, IL-10, IL-12 p70, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) were evaluated using the MSD Multiarray Proinflammatory 7-plex Assay, and serum levels of IL-5 were evaluated using the MSD Multiarray Singleplex IL-5 Assay (both Meso-Scale Discovery, Gaithersburg, MD), all with the Sector Imager. Cross-reactivity of vIL-6 was tested in the Meso-Scale IL-6 assay (which uses the same antibodies as the Multiarray Proinflammatory 7-plex assay) by diluting vIL-6 in a protein-containing kit diluent. The Meso-Scale hIL-6 assay did not detect vIL-6 at concentrations up to 20 000 pg/mL.
Other assays
Plasma HIV-1 mRNA was measured by quantitative RNA polymerase chain reaction using Roche Amplicor HIV-1 Monitoring Kits (Roche Diagnostic Systems, Branchburg, NJ). CD4 counts were assessed by fluorescent-activated cell sorting (Beckman Coulter, Brea, CA). C-reactive protein (CRP) was assayed using the Siemens Dimension Vista platform (Siemens AG, Munich, Germany). From study inception until May 2009, a standard sensitivity assay (sCRP) was used (upper limit of normal 13.0 mg/L). This was subsequently replaced by a high sensitivity assay (hsCRP) using the same platform (upper limit of normal 3.0 mg/L). CRPs were harmonized using a formula (hsCRP = [sCRP*0.93] + 0.56), based on the results of assays run by the Department of Laboratory Medicine, Clinical Center, National Institutes of Health (Bethesda, MD).
Statistical analysis
Paired analyses of the relative difference between each cytokine and KSHV VL during the first flare and during remission were performed using Wilcoxon signed rank tests. Given the relatively high threshold of the vIL-6 assay and high proportion of values at or below the detection threshold, vIL-6 values were categorized as detectable (>1560 pg/mL) or undetectable, with changes from first flare to remission assessed using the McNemar test for paired categorical data.
Univariate associations between continuous clinical parameters (hemoglobin, sodium, albumin, temperature, and platelet count), each human cytokine, and KSHV VL (log10 transformed) and between human cytokines and KSHV VL were determined using a Spearman rank correlation. Associations between dichotomous clinical parameters and human cytokines or KSHV VL were evaluated using a Wilcoxon rank sum test. For the aforementioned reasons, for association analyses involving vIL-6, values were categorized into 3 ordered categories: undetectable; detectable below 5500 pg/mL; and detectable above 5500 pg/mL (the cutoff between the 2 higher categories being approximately the median of subjects with detectable vIL-6). Then the association between ordered vIL-6 and continuous clinical parameters, human cytokines, and KSHV VL was evaluated using the Jonckheere-Terpstra test for trend, and that with dichotomous clinical parameters was evaluated using the exact Cochran-Armitage trend test.
Based on results from the univariate associations, linear regression models were developed to determine the relationships between flare-associated cytokines and individual continuous clinical parameters. Prediction of GI and respiratory symptoms was performed using logistic regression analysis after first performing a screening analysis for associations using a Wilcoxon rank sum test for the continuous cytokines and an exact Cochran-Armitage test for the categorized vIL6 values.
As this is a hypothesis-generating study, analyses were considered exploratory, with no formal correction for multiple comparisons. Given the number of comparisons, P values <.01 were interpreted as being significant, whereas P values from .01 to .05 indicated trends. All P values are 2-tailed.
Results
Patient characteristics
Twenty-one of the 27 patients enrolled on the KSHV-MCD protocol from its inception in 2004 to January 2010 had at least 1 observed flare by the study definition, and these 21 (19 male, median age 44 [range 29-52]) are the subject of the current study. Thirty-four flares were observed in total (range 1-3 per patient), including 11 second flares and 2 third flares each necessitating a change in therapy before remission was attained. Each flare was characterized at onset in terms of clinical and laboratory parameters outlined earlier, and the initial flare was followed until the first complete clinical and biochemical remission (20 patients) or until death without achieving remission (1 patient). All were HIV coinfected. Median HIV VL was undetectable at both flare and remission: 24 (71%) of flares occurred with HIV VL undetectable and a further 5 (15%) with HIV VL <1000 copies/mL, whereas 16 (80%) of remissions occurred with HIV VL undetectable and a further 2 (10%) with HIV VL <1000 copies/mL. Thirty-two flares (94%) occurred in patients receiving highly active antiretroviral therapy. Median CD4 count was 252 cells/mm3 (range 24-1319). Of the 34 flares, 18 (53%) were treated with high-dose zidovudine in combination with valganciclovir; 12 (35%) with rituximab in combination with liposomal doxorubicin; 2 (6%) with rituximab in combination with dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; 1 (3%) with rituximab alone; and 1 (3%) with valganciclovir alone.
Flare characteristics
Clinical and laboratory parameters observed during the 34 flares and 20 first remissions are shown in Table 1. The most common clinical manifestations during flares were fever and sweats; gastrointestinal disturbances including anorexia, early satiety, nausea, and xerostomia; and respiratory symptoms including cough, dyspnea, and nasal obstruction or discharge; fatigue was also common. Neurological, dermatological, and rheumatologic symptoms were observed in a minority of flares, and these symptoms included cognitive disturbance, neuropathic pain, rash and cutaneous drug hypersensitivity reactions, joint pain, and myalgias, respectively. The median grade of the most severe symptom present for each flare, including fever, by National Cancer Institute Common Toxicity Criteria for Adverse Events guidelines was 2 (range 1-3), and the median number of symptoms present was 4 (2-8). The most common laboratory abnormalities were hypoalbuminemia and anemia. Lymphadenopathy was observed in 30 of the flares (88%). Four flares occurred in patients who had had splenectomies; 20 of the remaining 30 flares (67%) were accompanied by splenomegaly. Concurrent active KS was present at the onset of 9 flares (26%). All flares analyzed would also have met criteria for therapy in 2 recent prospective treatment studies of MCD,47,48 whereas 28 of 34 flares (82%) and 16 of 21 initial flares (76%) met the ANRS definition of an MCD “attack”; of the 6 flares that did not meet ANRS criteria, in 3 the CRP was insufficiently elevated (although in each the CRP was ≥3× the upper limit of normal) and in 3 fever was not documented at our initial assessment (although some may have had intermittent fever not captured at their clinic visit).
Table 1.
Clinical and laboratory manifestations of KSHV-MCD
| Flares | Remissions* | |||
|---|---|---|---|---|
| Episodes (patients) | 34 (21) | 20 (20) | ||
| Number of symptoms present† (median, range) | 4 (2-8) | 0 | ||
| Most severe symptom grade present‡ (median, range) | 2 (1-3) | 0 | ||
| Clinical manifestations |
N present (% flares) |
CTC grade (median, range) |
N present (% remissions) |
CTC grade (median, range) |
| Fever (temperature abnormal if >38.3°C) | 21 (61.8) | 1 (0-3)§ | 0 | — |
| Sweats or rigors | 23 (67.6) | 1 (0-2) | 0 | — |
| Fatigue | 31 (91.2) | 2 (2-3) | 2 (10) | 0 (0-1) |
| Respiratory | 21 (61.8) | 1 (0-2) | 0 | — |
| Gastrointestinal | 23 (67.6) | 1 (0-3) | 0 | — |
| Neurological | 8 (23.5) | 0 (0-1) | 1 (5) | 0 (0-1) |
| Rheumatological | 4 (11.8) | 0 (0-1) | 0 | — |
| Dermatological | 4 (11.8) | 0 (0-1) | 0 | — |
| Laboratory manifestations |
N abnormal (% flares) |
Value (median, range) |
N abnormal (% remissions) |
Value (median, range) |
| Hemoglobin (g/dL) | 33 (97) | 9.9 (6.8-14.4) | 12 (60) | 12.7 (10.5-15.4) |
| (abnormal if <13.7 g/dL) | ||||
| Platelets (103 cells/μL) | 23 (67.6) | 100 (6-567) | 3 (15) | 198 (91-338) |
| (abnormal if <161 × 103 cells/μL) | ||||
| Leukocytes (103 cells/μL)‖ | 11 (32.6) | 5.4 (1.0-63.3) | 7 (35) | 4.7 (1.7-11.5) |
| (abnormal if <4.23 × 103 cells/μL) | ||||
| Sodium (mmol/L) | 23 (67.6) | 133 (127-143) | 1 (5) | 138 (133-144) |
| (abnormal if <135 mmol/L) | ||||
| Albumin (g/dL) | 33 (97) | 2.7 (1.2-3.9) | 2 (10) | 3.9 (3.2-4.4) |
| (abnormal if <3.7 mg/dL) | ||||
| HIV parameters |
N present (% flares) |
Value (median, range) |
N present (% remissions) |
Value (median, range) |
| Receiving combination antiretroviral therapy | 32 (94) | — | 20 (100) | — |
| CD4 count (cells/μL) | — | 252 (24-1319) | — | 367 (129-872) |
| HIV VL (copies/mL) | — | <50 (<50-64 100) | — | <50 (50-1150) |
| HIV VL (<50 copies/mL) | 24 (71) | — | 16 (80) | — |
Any abnormalities noted in remission were not attributable to KSHV-MCD.
Number of clinical manifestations of KSHV-MCD present; includes fever and symptoms scored in organ systems but not laboratory abnormalities.
Most severe symptom attributable to KSHV-MCD present, scored using Common Toxicity Criteria for Adverse Events, version 3.0.
Temperature 38.0°C (range 36.1-40.5).
Leukocytes were not considered in the definition of biochemical responses.
KSHV activity and cytokines during flares
We first sought to identify cytokines and viral parameters that were associated with clinical flares. To avoid statistical bias from patients with multiple flares, for this part of the analysis flare-associated parameters were identified using the first flare for each enrolled patient compared with their remission. KSHV VL was near universally elevated at flare onset (20 of 21 initial flares, 95%, and 32 of 34 flares overall, 94%; Figure 1 and Table 2). Median VL in PBMCs during flares was 22 400 copies/106 PBMCs (range 0-3 913 000; P < .0001 for initial flare compared with remission). In the 2 flares where KSHV VL was not elevated at onset, it subsequently became elevated during the flare. In a minority of instances (6, 30%), we observed detectable KSHV VLs persisting at the time of complete remission, although at lower levels than those seen in flares; median KSHV VL during remissions was 0 copies/106 PBMCs, range 0 to 2200.
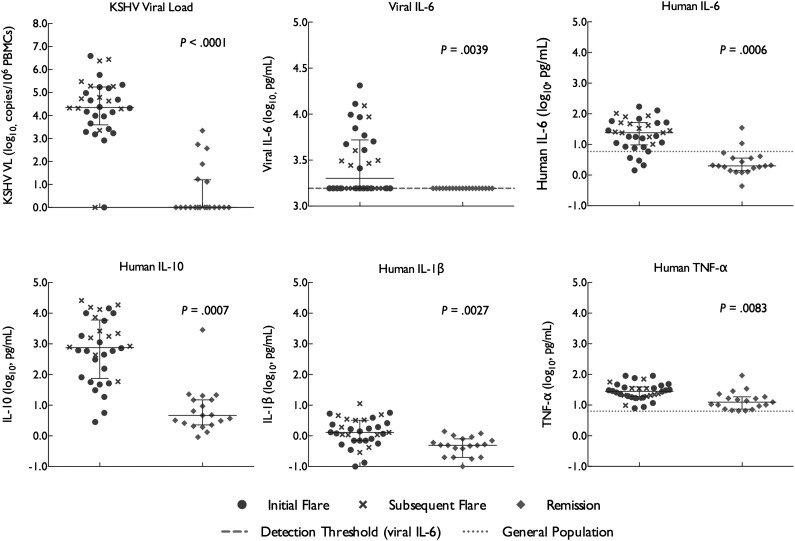
Figure 1.
KSHV VLs, vIL-6, and human cytokines in relation to KSHV-MCD disease activity. KSHV VL, vIL-6, and several human cytokines differ significantly between KSHV-MCD flare and remission. Statistical comparisons were made between initial flare and remission. Values are log transformed; units for all human cytokines and for vIL-6 are pg/mL and for KSHV VL is copies/106 PBMCs. For IL-6 and TNF-α, the midpoint of levels found in 9 to 10 normal donors using the same method is shown; other human cytokines illustrated were not detected in panels of 9 to 10 normal donors using this method.51 Note that vertical scales differ.
Table 2.
Cytokine levels and VLs at KSHV-MCD flare and remission
| Episodes (patients) | vIL-6 | hIL-6 | IL-1β | IL-5* | IL-8 | IL-10 | IL-12p70 | TNF-α | IFN-γ | KSHV VL | HIV VL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All flares | 34 (21) | 2068 (<1560-20 497) | 24.0 (1.4-171.5) | 1.3 (0.1-11.3) | 0.6 (0.1-15.4) | 36.6 (5.2-932.8) | 755 (2.8-85 857.8) | 6.8 (0.1-69.1) | 28.2 (7.9-90.8) | 6.7 (0.2-139.2) | 2 2391 (0-3 913 043) | <50 (< 50-64 100) |
| Initial flares | 21 (21) | <1560 (<1560-20 497) | 15.9 (1.4-171.5) | 1.2 (0.0-5.7) | 0.6 (0.1-15.4) | 36.2 (7.1-495.2) | 449.1 (2.8-85 857.8) | 5.3 (0.0-29.1) | 29.0 (7.9-90.8) | 4.1 (0.2-87.2) | 14 667 (1-3 913 943) | <50 (<50-64 100) |
| Subsequent flares | 13 (11) | 2 771 (<1560-12 335) | 33.2 (10.2-102.9) | 1.9 (0.3-11.3) | 0.7 (0.2-2.1) | 37.0 (5.2-932.8) | 2196.4 (58.9-33 712) | 11.2 (0.1-69.1) | 23.5 (9.8-76.2) | 12.1 (1.7-139.2) | 53 846 (0-2 777 778) | <50 (<50-139) |
| Remission | 20 (20) | <1560 (NA)† | 2.0 (0.4-34.8) | 0.5 (0.0-1.4) | 1.2 (0.2-4.2) | 35.5 (4.6-574.5) | 4.2 (0.9-2855.5) | 1.7 (0.3-40.4) | 11.5 (6.5-92.1) | 1.7 (0.3-40.4) | 1 (0-2 200) | <50 (<50-1150) |
| Change initial flare to remission | — | NA† | −80.8% (−99.3-+83.1) | −71.4% (−100.0-+248.9) | +100.0% (−91.5-+400.0) | +1.0% (−91.3-+156.6) | −98.0% (−100.0-+535.8) | −63.0% (−100.0-+348.3) | −39.9% (−89.7-+111.7) | −50.6% (−100.0-+210.6%) | −100.0% (100.0-+28.6%) | NA |
| Flare association | — | Yes | Yes | Yes | Trend (inverse) | No | Yes | No | Yes | No | Yes | NA |
| P = .0039† | P = .0006 | P = .0027 | P = .016 | P = 1.0 | P = .0007 | P = .066 | P = .0083 | P = .75 | P < .0001 |
For all cytokines, units are pg/mL. For KSHV VL, units are copies/106 PBMCs. For HIV VL, units are copies/mL. Statistical comparisons are made from initial flare to first remission.
IL-5 could not be assayed for 1 initial flare.
vIL-6 was <1560 pg/mL (the lower limit of detection) in all remissions and compared as a categorical variable using the McNemar test for paired categorical data.
Five cytokines measured were significantly elevated at initial flare when compared with remission (Figure 1 and Table 2): vIL-6 (median during all flares 2068 pg/mL, range<1560-20 500; P = .0039 for initial flare compared with remission); hIL-6 (median 24.0 pg/mL, range 1.4-171.5, P = .0006); IL-10 (median 755 pg/mL, range 2.8-26 000, P = .0007); IL-1L (median 1.3 pg/mL, range 0.1-11.3, P = .0027) and TNF-α (median 28.2 pg/mL, range 7.9-90.8, P = .0083). The most marked differences between flare and remission were observed in levels of vIL-6, hIL-6, and IL-10: vIL-6 was undetectable in all remissions, whereas hIL-6 and IL-10 were reduced by 80.8% and 98.0%, respectively, from initial flare to remission. Values of hIL-6 and IL-10 were also markedly elevated during flares in comparison with values in the general population (Figure 1).51 Elevations in IL-1-β and TNF-α were less marked in comparison with remission and general population values. By contrast, there was a trend toward decreased IL-5 levels at initial flare (median 0.6, range 0.1-15.4) in comparison with remission (median 2.0, range 0.4-34.8; P = .016), and no significant differences were observed in the levels of IL-12p70, IL-8, or IFN-γ between initial flare and remission (Table 2, supplemental Figure 1).
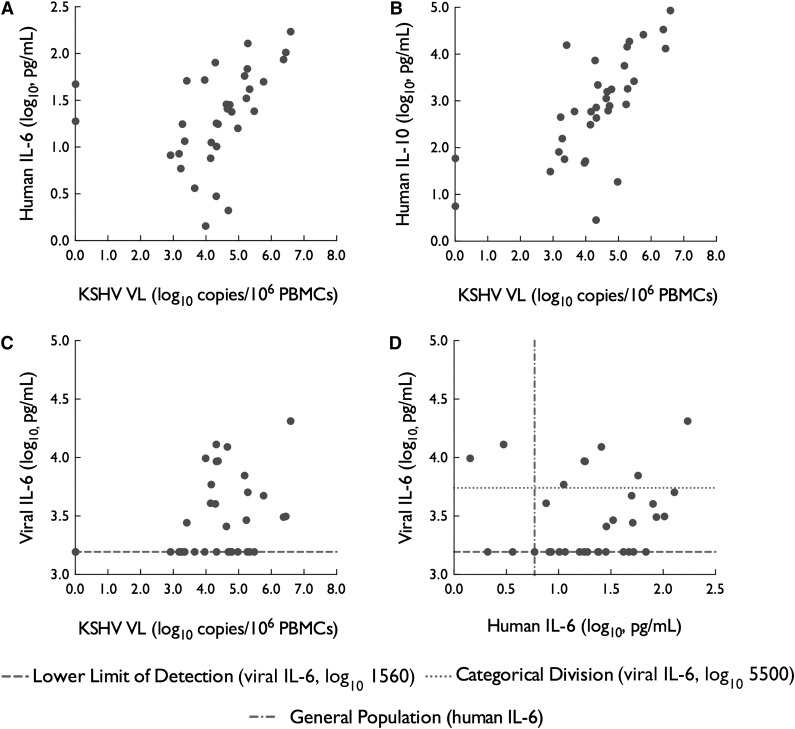
We next sought to analyze potential associations between KSHV VL and various flare-associated cytokines from all observed flares treated as independent events and using the first recorded values at each flare onset. KSHV VL was moderately correlated with serum hIL-6 (R = 0.56, P < .001) and strongly correlated with hIL-10 (R = 0.73, P < .001) during flares (Figure 2A-B, both by Spearman rank correlation). There was a strong association between KSHV VL and serum vIL-6 (P < .001 by Jonckheere-Terpstra test for trend) (Figure 2C). Correlations of KSHV VL with other flare-associated cytokines were weaker (for IL-1β, R = 0.21; for IL-5, R = −0.40; and for TNF-α R = −0.02).
Figure 2.
Relationship of KSHV VL and key cytokines during KSHV-MCD flares. (A-C) KSHV VL was associated with vIL-6, hIL-6, and IL-10 during KSHV-MCD flares. For vIL-6, P < .001 by Jonckheere-Terpstra test for trend; for hIL-6 R = 0.55 and P < .001, and for IL-10 R = 0.73 and P < .001. (D) In contrast, levels of hIL-6 were not associated with the 3 ordered categories (illustrated: undetectable; detectable below 5500 pg/mL; and detectable above 5500 pg/mL) of vIL-6 levels during KSHV-MCD flares (P = .27 by Jonckheere-Terpstra trend test). Correlations between KSHV VL and flare-associated cytokines not pictured were weaker: for IL-1β, R = 0.21; for IL-5 R = −0.40, and for TNF-α R = −0.02. For hIL-6, the midpoint of levels found in 9 to 10 normal donors using the same method is shown.
Cytokine profiles during flares
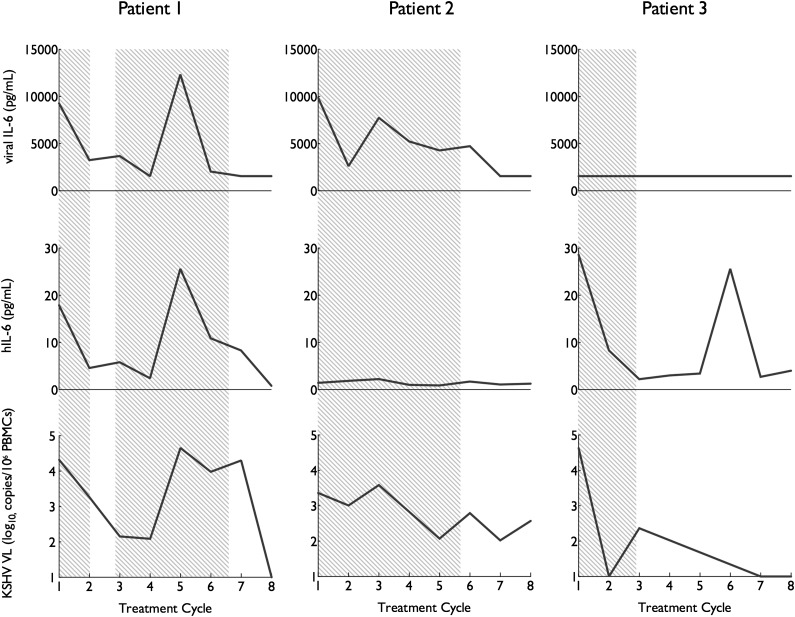
We then attempted to assess the respective contribution of vIL-6 and hIL-6 in the development of flares. Serum vIL-6 during flares was not associated with serum hIL-6 during the same flares (P = .27) (Figure 2D). Observed flares fell into 3 distinct IL-6 profiles (Figure 3): some were associated with elevations of vIL-6 only (2 flares, 6%), some were associated with hIL-6 elevations only (17 flares, 50%), and the remainder were associated with elevations in both vIL-6 and hIL-6 (13 flares, 38%; hereafter called h+vIL-6 flares). In 2 cases (6%), neither vIL-6 nor hIL-6 were elevated at flare onset, but in both cases hIL-6 (but not vIL-6) became elevated later during the flare.
Figure 3.
Distinct human and vIL-6 cytokine profiles during KSHV-MCD activity. Three representative patients illustrate the 3 h/vIL-6 flare patterns encountered (h+VIL-6, hIL-6 alone, and vIL-6 alone). Levels of vIL-6, hIL-6, and KSHV VL in are shown during their initial therapy. Periods where KSHV-MCD clinical manifestations were present are shaded gray. In all cases, KSHV VL is elevated during flares. Three distinct cytokine profiles are seen: vIL-6 and hIL-6 elevation (left); vIL-6 elevation without hIL-6 (center), and hIL-6 with undetectable vIL-6 (right).
In the 11 patients in whom more than 1 flare was observed on study, flare profile was conserved in 8 of the 13 subsequent flares (61.5%). This included 2 patients who had each experienced 3 flares of the same profile while on study (1 with hIL-6–only flares and 1 with h+vIL-6 flares). Neither of the patients with vIL-6–only flares experienced subsequent flares.
Cytokine profiles and clinical and laboratory disease manifestations
As vIL-6 can potentially bind to and activate a wider array of cell types than hIL-6, we explored the possibility that there were differences in the clinical and laboratory manifestations of the various flare profiles. The characteristics of all observed flares are shown in Table 3; because of the small number of vIL-6–only flares, statistical comparisons are made between hIL-6–only flares and h+vIL-6 flares. We observed no difference between flare types in symptomatic severity or symptomatic phenotype judged by the presence or absence of particular symptom groups or of KS. As treatments were not standardized across the cohort, our ability to assess for differences in therapeutic response or duration of remission was limited; nonetheless we observed no clear difference in these outcomes between flare types. However, we did observe significant differences in several laboratory, virological, and cytokine parameters, each in the direction of increased severity for h+vIL-6 flares compared with those with hIL-6 alone. In particular, CRP elevation and hyponatremia were both more pronounced in h+vIL-6 flares, and there was also more severe thrombocytopenia in h+vIL-6 flares. KSHV VL was likewise higher in h+vIL-6 flares compared with hIL-6–only flares. We also observed significant differences in the levels of 2 flare-associated cytokines, IL-10 and IL-5. No difference in TNF-α or the levels of non-flare-associated cytokines between differing h/vIL-6 flare profiles was seen (latter data not shown).
Table 3.
Characteristics of differing hIL-6 and vIL-6 flare profiles
| hIL-6 and vIL-6 | hIL-6 only | vIL-6 only | Neither* | P† | |
|---|---|---|---|---|---|
| Episodes | 13 | 17 | 2 | 2 | NA |
| Clinical manifestations | |||||
| Symptom severity‡ (median, Range) | 2 (1-3) | 2 (1-3) | 2 (1-2) | 2 (2-2) | NA |
| Respiratory symptoms§ | 8 (61.5%) | 10 (58.8%) | 1 (50%) | 2 (100%) | 1.0 |
| Gastrointestinal Symptoms§ | 8 (61.5%) | 12 (70.5%) | 2 (100%) | 1 (50%) | .71 |
| Active KS§ | 4 (30.8%) | 6 (35.2%) | 0 (0%) | 0 (0%) | 1.0 |
| Temperature (°C) (median, mange) | 38.4 (36.4-39.4) | 38.0 (36.2-40.5) | 37.6 (36.1-39.0) | 36.8 (36.6-36.9) | .26 |
| Laboratory manifestations (median, range) | |||||
| CRP (mg/L) | 140.5 (70.7-339.5) | 55.9 (6.3-157.2) | 68.2 (10.8-125.6) | 14.8 (14.6-14.9) | .0009 |
| Hb (g/dL) | 9.3 (6.8-14.4) | 10.3 (6.8-12.2) | 9.2 (7.4-10.9) | 9.3 (8.7-9.9) | .58 |
| Leukocytes (103 cells/L) | 4.7 (1.3-63.3) | 5.5 (1.8-15.7) | 6.9 (6.3-7.6) | 5.7 (1.0-10.3) | .22 |
| Platelets (103 cells/L) | 73 (6-377) | 132 (27.4-567) | 149 (36-262) | 118 (97-140) | .057 |
| Sodium (mEg/L) | 130 (127-143) | 135 (129-141) | 131 (129-134) | 133 (132-134) | .02 |
| Albumin (g/dL) | 2.7 (1.2-3.5) | 2.7 (1.7-3.3) | 2.8 (1.7-3.9) | 2.3 (2.0-2.6) | .55 |
| KSHV VL and flare-associated cytokine levels | |||||
| KSHV VL (copies/106 PBMCs) | 45 333 (2600-3 913 043) | 9167 (0-303 030) | 15 276 (9863-20 690) | 26 717 (4545-48 889) | .016 |
| vIL-6 (pg/mL) | 4725 (2575-20 497) | <1560 (NA) | 11 397 (9863-12 931) | <1560 (NA) | <.0001 |
| hIL-6 (pg/mL) | 50 (7.6-171.5) | 23.8 (5.9-68.6) | 2.2 (1.4-3.0) | 2.9 (2.1-3.6) | .06 |
| IL-1 (pg/mL) | 1.4 (0.29-11.3) | 1.66 (0.35-5.30) | 0.3 (0.0-0.5) | 0.4 (0.1-0.7) | .94 |
| IL-5 (pg/mL) | 0.4 (0.1-3.5) | 1.5 (0.1-15.4) | 0.3 (0.0-0.5) | 0.4 (0.1-0.7) | .009 |
| IL-10 (pg/mL) | 2 196 (309.9-85 858) | 156.3 (5.6-18 715) | 27.2 (2.8-51.6) | 604.4 (591.9-617.2) | .012 |
| TNF-α (pg/mL) | 34.5 (16.4-90.4) | 28.97 (9.78-90.8) | 8.3 (7.9-8.7) | 20.9 (19.7-22.1) | .51 |
hIL-6 but not vIL-6 became elevated after the onset of both of these flares.
Comparisons are made between the human plus vIL-6– and hIL-6–only groups, because of the small number of episodes in other groups.
Worst symptom attributable to KSHV-MCD present, including fever, scored using Common Toxicity Criteria for Adverse Events version 3.0.
Number and proportion of episodes where present.
Individual cytokines and clinical and laboratory disease manifestations
To assess further the contribution of individual cytokines to the clinical and laboratory manifestations of KSHV-MCD, we explored their relationship using multiple linear regression models. Given the relatively nonspecific nature of the symptoms observed, we grouped symptoms within organ systems (eg, gastrointestinal or respiratory) for this portion of the analysis. We selected 2 symptom groups (respiratory and gastrointestinal organ systems), each categorized as present or absent together with 5 quantitative parameters: temperature, hemoglobin, platelet count, sodium, and albumin. Backward selection logistic regression was performed to identify the cytokines most predictive of each individual manifestation, using all observed flares and remissions treated as independent events.
The primary predictors of each clinical and laboratory manifestation are shown in Table 4, together with the predictive model derived for each. For fever and respiratory and gastrointestinal symptom groups, the cytokine best predictive of the presence of symptoms was hIL-6. For laboratory manifestations, hIL-6 was again a key predictive element, whereas vIL-6 emerged as an independent predictor for hemoglobin, sodium, and albumin. hIL-6 was a predictor in all regression models except that for albumin, where vIL-6 and IL-1β were the key predictors. For each quantitative manifestation (laboratory manifestations and temperature), Table 4 presents the model derived to predict the manifestation from assayed cytokines, together with the adjusted R-squared value showing the effectiveness of the model prediction by correlating the predicted vs actual value for each flare. For the 2 nonquantitative symptom groups, the proportion of episodes in which the model correctly predicts the presence or absence of the symptom is shown with the model.
Table 4.
Relationship of individual cytokines with clinical and laboratory manifestations of KSHV-MCD
| Clinical manifestation | Human and viral cytokine prediction models | ||
|---|---|---|---|
| Predictor(s) | Model | Adjusted R2 for model prediction vs actual value | |
| Fever (temperature, °C) | hIL-6 | T = 36.9 + 0.019 (hIL-6) | 0.26 |
| Respiratory symptoms* | hIL-6 | Predicted to be present if hIL-6 ≥ 17.74 | NA (correctly predicted symptom presence or absence in 70.4% of cases: 25/33 [76%] without and 13/21 [62%] with) |
| Gastrointestinal symptoms* | hIL-6 | Predicted to be present if hIL-6 ≥ 19.55 | NA (correctly predicted symptoms presence or absence in 77.8% of cases: [77.8%] 27/31 [87%] without and 15/23 [65%] with) |
| Laboratory manifestation | |||
| Hemoglobin | hIL-6 with vIL-6† | Hb = 12.7 − 0.025(hIL-6) − 0.69(vIL-6)† | 0.30 |
| Platelets | hIL-6 | Plt = 202 − 1.35(hIL-6) | 0.15 |
| Sodium | hIL-6 with vIL-6 and IL-10† | Na = 139.9 − 0.0577(h-IL-6) + 0.0002(IL-10) − 2.77(vIL-6)† | 0.47 |
| Albumin | IL-1β with vIL-6† | Alb = 3.88 − 0.21(IL-1β) − 0.33(vIL-6)† | 0.33 |
Symptom groups categorized as present or absent.
Ordinal categorized vIL-6 was used for model development.
Discussion
This prospective analysis of patients studied during symptomatic flares and subsequent remissions provides new insights into the pathogenesis of KSHV-MCD, and it has implications for therapy development. By simultaneously examining KSHV VLs, the virally encoded cytokine vIL-6, and other proinflammatory human cytokines including hIL-6, we were able to shed light on their respective roles in the symptomatology of KSHV-MCD. With regard to the analysis of vIL-6 and hIL-6, the most striking observation was that either hIL-6 or vIL-6 appeared to be sufficient to induce flares: individual flares were associated with increases in vIL-6, hIL-6, or both. Notably, too, KSHV VL assessed in PBMCs was highly correlated with KSHV-MCD disease activity regardless of the cytokine profile and with implicated flare-associated cytokines.
Given preclinical evidence that vIL-6 can activate a wider range of cells than hIL-6, and our observations that some flares were uniquely associated with 1 or the other of these cytokines, we were interested in discovering whether differing cytokine profiles were associated with variations in disease manifestations. Overall, hIL-6 was most closely associated with several key manifestations, including fever, anemia, and hypoalbuminemia. These are consistent with the known downstream targets of IL-6, including albumin and hepcidin and its role as a central pyrogen.52,53 We could not discern a clear difference in clinical phenotype between the 3 v/hIL-6 profiles.27,28 Nonetheless, the data suggested that vIL-6 might be more strongly associated with the development of hypoalbuminemia than hIL-6 and also played a role in the development of anemia and hyponatremia.
Surprisingly, we did not detect a clinical correlate of IL-10 dysregulation when adjusted for v/hIL-6 in this analysis, despite the very high levels seen at flares. It is also notable that neither IFN-γ nor IL-12p70, both targets of IL-10, were elevated. It is possible that any clinical effects of IL-10 may be masked in our analysis by the close correlation of IL-10 with hIL-6. IL-10 may also be influencing other, unmeasured, clinical sequelae of active KSHV-MCD (perhaps including elevated risks of infection).54 Based on its known functions, it could also be that IL-10 is acting as facilitator of viral immune evasion. It is also known to have complex effects on developing B cells, and that these are acting to promote survival of KSHV-infected B cells. Similarly, the trend to depressed IL-5 may influence manifestations that were not assessed here, perhaps including allergic predisposition.
Our ability to evaluate the role of secreted vIL-6 in this study was limited by the relatively high cutoff of the assay, which almost certainly contributed to circulating vIL-6 being undetectable in some patients. It is likely that this contributed both to the number of flares we observed in which hIL-6 was elevated in the absence of vIL-6, and to the relatively poor predictive power of vIL-6 in linear regression models. Even with this limitation, it is noteworthy that the study identified a role for vIL-6. Unlike hIL-6, vIL-6 is poorly secreted and it has been postulated that its main role is in intracellular signaling.27,28 As such, it has been questioned as to whether secreted or systemic vIL-6 plays any role in KSHV-associated disease pathogenesis. The results here, and in particular the flares in which we observed elevated vIL-6 but not hIL-6, suggest that serum vIL-6 indeed plays an important role in KSHV-MCD pathogenesis and disease manifestations. Further analyses with more sensitive assays for vIL-6 will be needed to shed additional light on its role.
These findings may have therapeutic implications. The central role of KSHV lytic activation provides support for therapeutic strategies targeting lytically infected cells, as with high dose AZT in combination with valganciclovir.45 The findings here also provide support for targeting hIL-6, the IL6-R, or downstream effectors of gp130 such as the JAK/STAT pathway, which could block signaling of h and vIL-6. Given the high levels of hIL-6 seen in the majority of patients and their correlation with symptoms, it may be that hIL-6 blockade alone is sufficient to treat KSHV-MCD, both by ameliorating symptoms induced by IL-6 excess and perhaps also by interrupting paracrine feedback loops. Clinical studies of the anti-IL-6R antibody tocilizumab, alone and in combination with AZT and valganciclovir, are underway (NCT01441063) and will assist in elucidating these issues.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge participating patients and their families; Randy Stevens and Adam Rupert of the AIDS Monitoring Laboratory, Frederick National Cancer Laboratory; and the clinical staff of the HIV and AIDS Malignancy Branch, the Medical Oncology Branch, and the National Institutes of Health Clinical Center. M.N.P. gratefully acknowledges the mentorship of Dr Merrole Cole-Sinclair of St. Vincent’s Hospital, Melbourne, Australia.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.N.P. and R.Y. designed the study; M.N.P., T.S.U., K.A., K.M.W., D.O., R.F.L., and R.Y. cared for patients; M.N.P., T.S.U., K.A., K.M.W., D.O., S.P., R.F.L., and R.Y. collected data; V.W., G.T., and R.Y. developed and performed the vIL-6 assay; V.M. and D.W. developed and performed the KSHV VL assay; M.N.P., S.M.S., and R.Y. analyzed data; and M.N.P. and R.Y. wrote the manuscript.
Conflict-of-interest disclosure: G.T. is coinventor on a patent describing the measurement of KSHV vIL-6. This invention was made when G.T. was an employee of the US government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (PL 99-502). R.Y. is the spouse of G.T. The remaining authors declare no competing financial interests.
Correspondence: Robert Yarchoan, HIV and AIDS Malignancy Branch, Center for Cancer Research, National Cancer Institute, Room 6N106, Building 10, National Institutes of Health, Bethesda, MD 20892-1868; e-mail: robert.yarchoan@nih.gov; and Mark N. Polizzotto, HIV and AIDS Malignancy Branch, Center for Cancer Research, National Cancer Institute, Room 6N106, Building 10, National Institutes of Health, Bethesda, MD 20892-1868; e-mail: mark.polizzotto@nih.gov.
References
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670–683. doi: 10.1002/1097-0142(197203)29:3<670::aid-cncr2820290321>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Frizzera G, Peterson BA, Bayrd ED, Goldman A. A systemic lymphoproliferative disorder with morphologic features of Castleman’s disease: clinical findings and clinicopathologic correlations in 15 patients. J Clin Oncol. 1985;3(9):1202–1216. doi: 10.1200/JCO.1985.3.9.1202. [DOI] [PubMed] [Google Scholar]
- 4.Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360–1367. [PubMed] [Google Scholar]
- 5.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 6.Du MQ, Liu H, Diss TC, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97(7):2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 7.Oksenhendler E. HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS. 2009;4(1):16–21. doi: 10.1097/coh.0b013e328319bca9. [DOI] [PubMed] [Google Scholar]
- 8.Bower M, Newsom-Davis T, Naresh K, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. J Clin Oncol. 2011;29(18):2481–2486. doi: 10.1200/JCO.2010.34.1909. [DOI] [PubMed] [Google Scholar]
- 9.Parravicini C, Corbellino M, Paulli M, et al. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am J Pathol. 1997;151(6):1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 10.Teruya-Feldstein J, Zauber P, Setsuda JE, et al. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman’s disease and primary effusion lymphoma. Lab Invest. 1998;78(12):1637–1642. [PubMed] [Google Scholar]
- 11.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10(1):61–67. [PubMed] [Google Scholar]
- 12.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 14.Moore PS, Gao SJ, Dominguez G, et al. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae. J Virol. 1996;70(1):549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus-encoded oncogenes and oncogenesis. J Natl Cancer Inst Monogr. 1998;23:65–71. doi: 10.1093/oxfordjournals.jncimonographs.a024176. [DOI] [PubMed] [Google Scholar]
- 16.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol. 1999;73(5):4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R, Lin SF, Staskus K, et al. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol. 1999;73(3):2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenner RG, Albà MM, Boshoff C, Kellam P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol. 2001;75(2):891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulose-Murphy M, Ha NK, Xiang C, et al. Transcription program of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus). J Virol. 2001;75(10):4843–4853. doi: 10.1128/JVI.75.10.4843-4853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J Virol. 1998;72(2):1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402(6764):889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 22.Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278(5336):290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 23.Cesarman E, Nador RG, Bai F, et al. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70(11):8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 25.Neipel F, Albrecht JC, Ensser A, et al. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71(1):839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272(31):19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- 27.Hu F, Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J Virol. 2006;80(21):10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D, Sandford G, Nicholas J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J Virol. 2009;83(2):722–733. doi: 10.1128/JVI.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60(10):921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 30.Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal advance: Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2010;87(1):25–34. doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94(8):2871–2879. [PubMed] [Google Scholar]
- 32.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236–246. doi: 10.1097/PAP.0b013e3181a9d4d3. [DOI] [PubMed] [Google Scholar]
- 33.Chadburn A, Hyjek EM, Tam W, et al. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD). Histopathology. 2008;53(5):513–524. doi: 10.1111/j.1365-2559.2008.03144.x. [DOI] [PubMed] [Google Scholar]
- 34.Naresh KN, Trivedi P, Horncastle D, Bower M. CD20 expression in the HHV-8-infected lymphoid cells in multicentric Castleman disease. Histopathology. 2009;55(3):358–359. doi: 10.1111/j.1365-2559.2009.03344.x. [DOI] [PubMed] [Google Scholar]
- 35.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 36.Nishimoto N, Sasai M, Shima Y, et al. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95(1):56–61. [PubMed] [Google Scholar]
- 37.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest. 1990;86(2):592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoki Y, Jones KD, Tosato G. Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. J Hematother Stem Cell Res. 2000;9(2):137–145. doi: 10.1089/152581600319351. [DOI] [PubMed] [Google Scholar]
- 39.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001;97(8):2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- 40.Bower M, Veraitch O, Szydlo R, et al. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113(19):4521–4524. doi: 10.1182/blood-2008-12-197053. [DOI] [PubMed] [Google Scholar]
- 41.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood. 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- 42.Grandadam M, Dupin N, Calvez V, et al. Exacerbations of clinical symptoms in human immunodeficiency virus type 1-infected patients with multicentric Castleman’s disease are associated with a high increase in Kaposi’s sarcoma herpesvirus DNA load in peripheral blood mononuclear cells. J Infect Dis. 1997;175(5):1198–1201. doi: 10.1086/593567. [DOI] [PubMed] [Google Scholar]
- 43.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97(7):2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 44.Suthaus J, Stuhlmann-Laeisz C, Tompkins VS, et al. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood. 2012;119(22):5173–5181. doi: 10.1182/blood-2011-09-377705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood. 2011;117(26):6977–6986. doi: 10.1182/blood-2010-11-317610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415–4421. doi: 10.1182/blood-2010-07-290213. [DOI] [PubMed] [Google Scholar]
- 47.Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman’s disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350–3356. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 48.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836–839. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 49.van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28(23):3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Department of Health and Human Services. Common Toxicity Criteria For Adverse Events version 3.0. Bethesda, MD: DHHS; 2006. [Google Scholar]
- 51.Meso-Scale Discovery. MSD Multiarray Proinflammatory 7-plex Assay and MSD Multiarray Singleplex IL-5 assay Product Insert. Gaithersburg, MD: Meso-Scale Discovery; 2010. [Google Scholar]
- 52.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162(1):392–399. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.