Abstract
Chronic polymicrobial lung infections in adult cystic fibrosis patients are typically dominated by high levels of Pseudomonas aeruginosa. Determining the impact of P. aeruginosa growth on airway secretion composition is fundamental to understanding both the behaviour of this pathogen in vivo, and its relationship with other potential colonising species. We hypothesised that the marked differences in the phenotypes of clinical isolates would be reflected in the metabolite composition of spent culture media. 1H NMR spectroscopy was used to characterise the impact of P. aeruginosa growth on a synthetic medium as part of an in vitro CF lower airways model system. Comparisons of 15 CF clinical isolates were made and four distinct metabolomic clusters identified. Highly significant relationships between P. aeruginosa isolate cluster membership and both patient lung function (FEV1) and spent culture pH were identified. This link between clinical isolate growth behaviour and FEV1 indicates characterisation of P. aeruginosa growth may find application in predicting patient lung function while the significant divergence in metabolite production and consumption observed between CF clinical isolates suggests dominant isolate characteristics have the potential to play both a selective role in microbiota composition and influence pseudomonal behaviour in vivo.
Keywords: NMR, cystic fibrosis, Pseudomonal, lung function
1 Introduction
Chronic lung disease is the main determinant of morbidity and mortality in cystic fibrosis (CF) (Emerson et al., 2002, Rosenfeld et al., 2001), with bacterial infection considered a key driver in this process (Kosorok et al., 2001). Pseudomonas aeruginosa is a species that has long been regarded as a pathogen in the CF lung (CF Foundation, 2007) and whose presence is associated with reduced life expectancy (Lyczak et al., 2002). With the exception of end stage disease (Bjarnsholt et al., 2010), P. aeruginosa, though common, is only one of many species forming the bacterial microbiota associated with the CF lower airways by adulthood (Rogers et al., 2004, Armougom et al., 2009). Moreover, though the bacterial species reported in CF typically vary markedly between individuals (Stressmann et al., 2011), these colonising species are less phylogenetically diverse than the pool of bacterial species reported as passing transiently through the lower airways of healthy individuals (Rogers et al., 2006). Together, these factors suggest that the development of CF airway bacterial communities is a selective process, and that this selection differs between individuals. Exposure to an “infective dose” equivalence of a given species will be required for “infection” to occur. However, the existence of a group of core species, that is commonly but not universally reported (van der Gast et al., 2011), suggests that infection is not due to chance alone. The factors underpinning selection remain unclear; however the identification of key selective drivers offers the possibility of identifying those patients who are at greatest risk of developing a lower airway infection by a specific pathogen.
There are a number of factors that differ between CF patients that might contribute to such selection. For example, the severity of the underlying impairment of CFTR function (a trans-epithelial ion transport protein) (Dean and Santis, 1994), the degree to which oxygen tension in airway secretions is reduced as a result of neutrophilic influx (Kolpen et al., 2010), and antibiotic treatment history (Stressmann et al., 2011; Tunney et al., 2011). Here, we investigate the extent of a further potential selective force; the nutritional characteristics of secretions in the airways. Whilst differences in secretion rheology may arise as a result of the range of CFTR defect severities (Boucher, 2004), there is no evidence to suggest that the chemical composition of the secretions differs substantially between individuals at the point when they are produced. However, these secretions are typically colonised by high levels of P. aeruginosa (commonly 106-109 cfu/ml; Aaron et al., 2004; Stressmann et al., 2011). P. aeruginosa growth will, in turn, reduce the availability of certain carbon and nitrogen sources, and produce a wide range of metabolites. Such shifts in nutritional sources are known to influence bacterial community composition in other contexts (Resat et al., 2012, Dunaj et al., 2012). Together, these changes will result in an altered growth environment, potentially influencing the likelihood of successful colonisation by new bacterial species entering the lower airways, and the gene expression and growth strategies of P. aeruginosa itself (Bernier et al., 2011).
P. aeruginosa isolates from different CF patients are known to exhibit a broad range of phenotypic characteristics, employ a number of different growth strategies in vivo, including planktonic and biofilm modes (Ciofu et al., 2012), and exploit a wide range of carbon and nitrogen sources (Frimmersdorf et al., 2010). We therefore hypothesised that P. aeruginosa CF airway isolates differ in the manner in which they modify the composition of the airway secretions in which they grow, resulting in significant differences in the nutritional growth environment available to the CF airway bacterial community. Characterisation of such biochemical signatures requires the determination of changes in the levels of a large number of molecules. Consequently, we used 1H NMR spectroscopy to obtain an overview of the compositional changes that occur in a defined synthetic CF medium (SCFM) as a result of the growth of these isolates in an in vitro CF airway model. 1H NMR spectroscopy has been used previously to investigate the growth of P. aeruginosa type strain PA01 in Luria-Bertani broth, a standard laboratory medium (Gjersing et al., 2007). Here, we aimed to more closely replicate the physiochemical composition of CF airway secretions in a controlled manner and cultured clinical isolates in SCFM. Previous studies comparing P. aeruginosa gene expression in CF sputum with that in similar CF synthetic media have shown bacterial behaviour to be similar in the two contexts (Palmer et al., 2007, Fung et al., 2010). By combining 1H NMR spectroscopy with this CF airway growth model, we were therefore able to assess the degree to which the impact of P. aeruginosa growth differed between clinical isolates under conditions approximating those encountered in vivo. We report substantial variation in the observed spent media metabolomes and show that the variation between different clinical isolates is related to variation in clinical measures of respiratory disease.
2 Materials and Methods
Sputum samples were collected from 13 adult CF patients with ethical approval from Southampton and South West Hampshire Research Ethics Committee (06/Q1704/26). The collection of these samples has been described previously (van der Gast et al., 2011, Stressmann et al., 2011). P. aeruginosa isolates were recovered from these samples by inoculation on P. aeruginosa selective medium (CM0559 plus SR0103, Oxoid, Cambridge, UK). A representative colony of the numerically dominant morphotype was selected for each patient. In the case of Patients 12 and 13, two prevalent morphotypes were isolated concurrently, with both carried forward for analysis. Details of isolates, phenotypic characteristics, and the patients from which they were obtained, are presented in Table 1. Bacterial species diversity in these samples was previously determined by 16S rRNA gene clone sequencing (van der Gast et al., 2011). Spent culture pH was determined at 37 °C using an InLab Micro Pro pH electrode and Mi150 pH meter (Mettler Toledo, Leicester, UK). All P. aeruginosa isolates were screened for auxotrophy as described previously (Barth and Pitt, 1995). These data are also presented in Table 1.
Table 1. Information on isolates, the patients that they were obtained from and properties of the sputum sample.
“Diversity” indicates the number of bacterial species identified by 16S rRNA gene clone sequence analysis, as published previously (Stressmann et al., 2012). CFPE – “cystic fibrosis pulmonary exacerbation”. Genotype I was phe508del for all patients. “cfu/ml equiv.” refers to mean P. aeruginosa cells numbers per ml of spent medium, as determined through Q-PCR enumeration, with standard deviation values given in brackets.
| Isolate | Metabolomic cluster |
Mucoidy | Pigmentation | Auxotrophy | Age | Sex | Genotype II | BMI | Diabetes | mean FEV1 % pred. |
CFPE in 12 months |
Diversity | Culture pH |
cfu/ml equiv. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF1 | I | mucoid | yes | methionine | 30 | male | unknown | 29 | no | 54.9 | 3 | 28 | 5.32 | 1.17×109 (5.37×108) |
| CF2 | IIc | non-mucoid | no | no | 45 | female | unknown | 18.5 | yes | 40.2 | 4 | 2 | 6.54 | 8.69×108 (1.26×108) |
| CF3 | I | non-mucoid | no | proline | 47 | male | unknown | 20.7 | yes | 33.9 | 0 | 5 | 6.32 | 2.29×1010 (6.09×109) |
| CF4 | IIc | non-mucoid | no | no | 30 | female | 711+3A7G | 25 | no | 38.2 | 3 | 4 | 6.62 | 4.05×109 (9.12×108) |
| CF5 | IIc | non-mucoid | no | no | 22 | female | phe508del | 19 | no | 36.2 | 5 | 6 | 6.61 | 2.03×1010 (6.17×109) |
| CF6 | - | non-mucoid | yes | no | 55 | male | G85E | 24.5 | no | 52.2 | 2 | 24 | 6.26 | 1.34×108 (4.72×107) |
| CF7 | IIa | non-mucoid | yes | no | 21 | female | phe508del | 19 | no | 56.6 | 4 | 2 | 6.19 | 1.58×109 (9.34×108) |
| CF9 | IIb | non-mucoid | yes | no | 22 | male | phe508del | 17.9 | yes | 16.5 | 7 | 8 | 6.93 | 2.40×109 (5.31×108) |
| CF10 | I | non-mucoid | no | no | 18 | female | phe508del | 22.5 | no | 84 | 3 | 17 | 5.49 | 5.25×109 (1.15×109) |
| CF11 | IIa | non-mucoid | yes | no | 24 | female | G542X | 23.4 | no | 72.5 | 3 | 5 | 6.06 | 7.80×109 (2.08×109) |
| CF12 | IIb | mucoid | yes | no | 20 | male | phe508del | 30.4 | no | 26.8 | 4 | 14 | 6.53 | 5.25×109 (2.13×109) |
| CF12b | I | mucoid | no | no | 30.4 | no | 72.5 | 4 | 14 | 5.83 | 1.76×109 (5.64×108) | |||
| CF13 | IIa | mucoid | yes | no | 20 | male | phe508del | 21 | no | 54.4 | 4 | 6 | 6.65 | 1.81×109 (4.34×108) |
| CF13b | IIb | mucoid | yes | no | 21 | no | 26.8 | 4 | 14 | 6.87 | 8.55×109 (3.32×109) | |||
| CF14 | IIc | non-mucoid | yes | methionine | 23 | male | phe508del | 20.7 | yes | 53.6 | 4 | 42 | 6.58 | 2.18×109 (9.34×108) |
2.1 Bacterial growth conditions
P. aeruginosa in the CF lung grows in stagnant mucus, an environment that is characterised by microaerophilic and anaerobic conditions (Worlitzsch et al., 2002; Yoon et al., 2002). To reflect these in vivo sputum conditions, the following growth model was employed. A defined synthetic CF medium (SCFM) was used, based on a number of different CF synthetic media described previously (Sriramulu et al., 2004, Palmer et al., 2005, Palmer et al., 2007, Fung et al., 2010). The SCFM used contained 10 g/L BSA, 10 g/L porcine gastric mucin, 1.4 g/L herring sperm DNA, 10 mM MOPS, 5 g/L egg yolk emulsion, 3.6 μM FeSO4, 51.8 mM NaCl, 2.28 mM NH4Cl, 2.128 mM L-lysine · HCl, 14.9 mM KCl, 1.78 mM L-alanine, 1.754 mM CaCl2, 1.661 mM L-proline, 1.609 mM L-leucine, 1.549 mM L-glutamate · HCl, 1.446 mM L-serine, 1.3 mM NaH2PO4, 1.25 mM Na2HPO4, 1.203 mM L-glycine, 1.12 mM L-isoleucine, 1.117 mM L-valine, 1.072 mM L-threonine, 0.827 mM L-aspartate, 0.802 mM L-tyrosine, 0.676 mM L-ornithine · HCl, 0.633 mM L-methionine, 0.606 mM MgCl2, 0.53 mM L-phenylalanine, 0.519 mM L-histidine · HCl, 0.348 mM KNO3, 0.306 mM L-arginine · HCl, 0.16 mM L-cysteine · HCl, 0.119 mM diethylene triamine pentaacetic acid, 0.013 mM L-tryptophan. The pH of the medium was adjusted to 6.8. Media was sterilised by passage through a a 0.45-μm-pore-size syringe filter, except for porcine gastric mucin, which was sterilised separately by heating at 70°C for 24 h in 95% ethyl alcohol, as described previously (Mitsui et al., 1976).
Incubation was performed in 9 ml volumes of SCFM in 15 ml Falcon tubes (BD Biosciences, Oxford, UK) with tight lids, for 72 hours at 37 °C, with inversion every 12 hours, in order to to replicate reduced oxygen tensions and low relative physical disruption of the CF lower airways. Following incubation, bacterial cells were pelleted by centrifugation at 12,000 × g, 10 min at 4 °C, with the supernatant transferred to fresh NMR tubes with 10% v/v D2O added to provide a deuterium lock signal.
2.2 NMR
1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer equipped with a 5 mm QNP probe (Bruker UK Limited, Coventry, UK) with sample isolates tested in triplicate (three independent cultures from the same colony) and kept at room temperature. A zgesgp pulse sequence (Bruker) with excitation sculpting using gradients was used (Hwang and Shaka, 1995). The 1H 90 degree pulse was 9.75 μs. For each spectrum, 65,536 data points were acquired with 16 scans. To help in the assignment of the metabolite resonances, J-resolved 2D correlation with pre-saturation during relaxation delay using gradients (J-Res, Bruker) spectra were recorded for some of the samples, using default pulse sequences as provided by Bruker. The spectral width was 20 ppm. Free induction decays were multiplied with an exponential function corresponding to a line broadening of 0.3 Hz. The spectra were Fourier transformed and calibrated to a 2,2,3,3,-D4-3-(Trimethylsilyl) propionic acid sodium salt (TSP-2,2,3,3-D4) reference signal at 0 ppm. Phase correction was performed manually and automatic baseline correction was applied.
2.3 Bacterial quantification
P. aeruginosa density in samples at harvesting was determined by quantitative (Q) PCR enumeration of oprL gene copies in total DNA extracts, using a protocol described previously (Feizabadi et al., 2010). All Q-PCR reactions were carried out in a total volume of 25 μl using Taqman® Universal PCR Mastermix (Applied Biosystems, Warrington, UK). Quantitative PCR assays were carried out using the Rotorgene 6000 (Qiagen, Crawley, UK) with a temperature profile of 50 °C for 2 min, 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and 60 °C for 60 s. The cycling program was adjusted at 95 °C for 10 min and then 35 cycles of 10 s each at 95 °C (denaturation) followed by 35 s at 60 °C with fluorescent collection (annealing and extension). Analysis was performed in triplicate and the mean reported.
2.4 P. aeruginosa strain genotyping
Random Amplified Polymorphic DNA (RAPD) assays were performed for each P. aeruginosa isolate as described previously (Renders et al., 1996) using the primer ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′). In those isolates not distinguishable based on the resulting profile, a further RAPD profile using the primer ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) was performed. PCR reactions were performed in 25 μl volumes, containing REDTaq ReadyMix PCR Reaction Mix (Sigma-Aldrich, Dorset, United Kingdom) with the addition of MgCl2 to achieve a final concentration of 2.5 mM. Primers were used at a concentration of 0.5 μM and 50 ng of template DNA. Reactions were performed as follows: An initial denaturation step of 94°C for 2 min was followed by 32 cycles of denaturation at 94°C for 1 min, annealing at 25°C for 1 min, and extension at 72°C for 2 min, with a final extension step at 72°C for 10 min. Amplification was carried out by using a GeneAmp PCR System 2400 (Perkin-Elmer), and verified on Tris-acetate-EDTA–agarose gel electrophoresis and analysed using Phoretix 1D advanced software, version 5.0 (Nonlinear Dynamics, Newcastle upon Tyne, UK).
2.5 Multivariate analysis
Pre-processing and orthogonal projection to latent structures discriminant analysis (OPLS-DA) were carried out with software that was developed in our laboratory for a previous study (Vermeer et al., 2012) using the python programming language with numpy and scipy for calculations, and matplotlib for visualization. The nonlinear iterative partial least-squares (NIPALS) algorithm (Andersson, 2009) was used for OPLS-DA analysis.
Regions above 9.074 ppm and below 0.116 ppm were excluded because of noise content. The water peak, ethanol and TSP reference signal were also excluded. The spectra were bucketed using 0.02 ppm bin size with additional, manual bucketing applied to adjust for peak shifting as described below, leaving 336 data points per spectrum. Spectra were normalized using probabilistic quotient normalisation (Dieterle et al., 2006).
Principal component analysis (PCA) was used to identify clustering patterns from the major variations between the 49 NMR spectra. For this analysis, spectra were pareto-scaled after normalisation. In order to further investigate the compounds discriminating between clusters and provide a robust statistical analysis of putative cluster membership, each possible cluster was analysed against SCFM using orthogonal partial least-squares discriminant analysis (OPLS-DA). Here, spectra were auto-scaled (variance of every data point normalized to 1). Both normalisation and auto-scaling were included in the cross-validation procedure (Supplementary Table 1). Cross-validation was performed where 75% of the samples were used as a training set and the remaining 25% as a test set, ensuring that the number of samples in the test set was proportional to the total number of samples from each class, and that at least one sample from each class was present in the test set. To choose the number of components for the model, a leave-one-out cross-validation was carried out on the samples in the training set, and the F1 used to choose the number of components, with the additional constraint to use a maximum of 10 components. This double cross-validation was repeated 2000 times with randomly chosen samples in the training and test set to prevent bias due to the choice of training or test set. This leads to 4 × 2000 models (in the supplementary information, each of these models leads to a point on the scores plot, but loadings and weights are presented as averages over all these models). Finally, this procedure was repeated with randomly generated class assignments to provide a reference value for Q2. The chosen number of components minus one was then used as an OPLS filter and a PLS-DA analysis with two components was carried out on the filtered data to yield one predictive and one orthogonal component. Back-scaled loadings (Cloarec et al., 2005) were used to identify resonances with high variance and high weight, therefore the discriminating resonances, and verified against the peak intensity of the original spectra after PQN normalisation.
Peaks that allow the models to distinguish between classes were assigned by comparing chemical shift values and multiplicities from J-resolved NMR spectra to values from the BMRB (Ulrich et al., 2007) and HMDB (Wishart et al., 2009), analysis of published P. aeruginosa metabolic data (Son et al., 2007, Frimmersdorf et al., 2010) and NMR spectra generation from individual medium components was used to help in the assignment.
2.6 Relationships between PCA and clinical characteristics
One-way factorial ANOVA were performed to test for significant relationships between the P. aeruginosa strain cluster membership and sample characteristics, with a significance threshold of p < 0.05. Homogeneity of variance and normality of errors were assessed using the Fligner-Killeen and the Shapiro-Wilk tests respectively prior to the ANOVA. If a factor failed either test a non-parametric Kruskal-Wallis rank sum test was performed. Factors that were found to be significant using the ANOVA were further studied using Tukey’s honest significant difference (HSD) as a post-hoc test. Correlations between the sample characteristics were performed using Spearman’s rho correlations. Statistical analyses were performed using R (v.2.13.0, www.r-project.org).
3 Results
3.1 1H NMR spectroscopy of Pseudomonas CF isolates cultured in an airway model medium
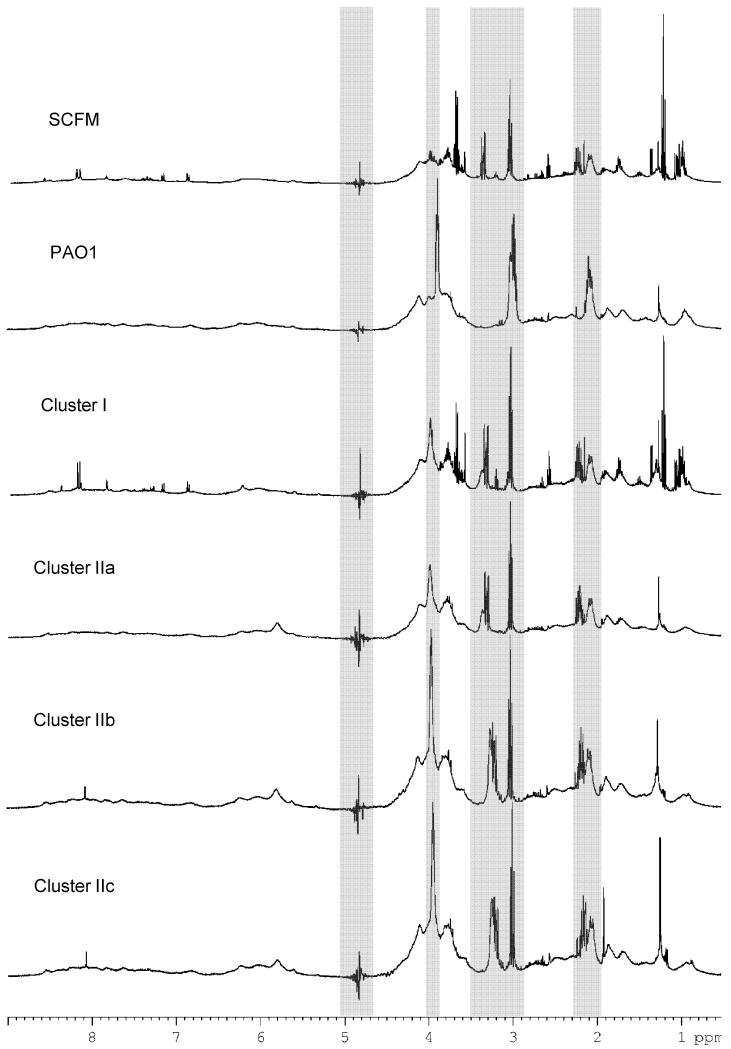
1H NMR spectroscopy has been used previously to investigate the growth of P. aeruginosa type strain PA01 in Luria-Bertani broth, a standard laboratory medium (Gjersing et al., 2007). Here, we aimed to more closely replicate the physiochemical composition of CF airway secretions in a controlled manner and cultured clinical isolates in SCFM. Representative 1D 1H NMR spectra are shown for each of the isolate clusters identified by the principal component analysis described below, revealing the effect of culturing either P. aeruginosa PA01 or CF clinical isolates (Fig. 1). Insufficient growth of isolate 6 occurred in SCFM medium and hence it was excluded from further analysis. Spectra generated from either sterile medium, or media inoculated by a particular strain showed a high degree of reproducibility in multiple independent replicates. Although we were successful in identifying metabolite changes that are linked to clinical measures, as described below, adherence to the well validated SCFM growth procedures presented some challenges for the NMR studies and subsequent multivariate analysis. In order to remain relevant to CF airway secretion composition, SCFM contains a number of components, such as mucin and BSA, which could result in peak broadening and loss of resolution due to high viscosity (Supp. Fig. 1). In addition, a number of the strains analysed were highly mucoid and were capable of producing large amounts of exopolysaccharide (EPS). Finally, although less noticeable in the SCFM spectrum, resonances attributable to the, presumably, non-metabolised MOPS buffer dominate the spectra derived from spent media. These factors led to a number of very broad resonances, particularly between 3.5-4.5 ppm, and substantial, pH dependent shifting of both broad and sharper resonances between 3.00 and 3.30 ppm and around 2.10 ppm, (Figure 1) as expected from the pH dependence of buffer resonance chemical shifts (Supp. Fig. 2). To remove the influence of MOPS buffer from the analysis, the following regions were excluded in addition to the water and ethanol peaks (5.02 - 4.65 ppm; 3.70 – 3.76 and 1.22 – 1.15 ppm): 4.02 – 3.87 ppm; 3.50 – 2.87 and 2.27 – 1.96 ppm. Ultimately, this has a number of implications for how the data may be treated and the degree of resonance assignment that is possible. Large scale shifting of buffer resonances resulted in ineffective peak alignment using correlation optimized warping (COW) [37] (Supp. Fig. 3). Although peak realignment could conceivably be achieved through pH adjustment of spent media as is done for e.g. urine samples (Beneduci et al., 2011), manual peak bucketing was able to account here for the observed peak shifts. The exclusion of large regions, considerable spectral overlap and the appearance of broad resonances following bacterial growth also precluded the use of statistical correlation spectroscopy (STOCSY) or other two-dimensional techniques to aid assignment for many resonances. For this reason our present efforts were largely restricted to using the 1H NMR technique and multivariate analysis to group the isolates according to apparent differences in growth strategies.
Figure 1. Representative 1H NMR spectra generated from non-inoculated SCFM, PA01 inoculated SCFM, and representative members of each of the four putative clinical isolate clusters.
Shaded regions indicate large regions of the 1H NMR spectra excluded on the basis of solvent or buffer peaks.
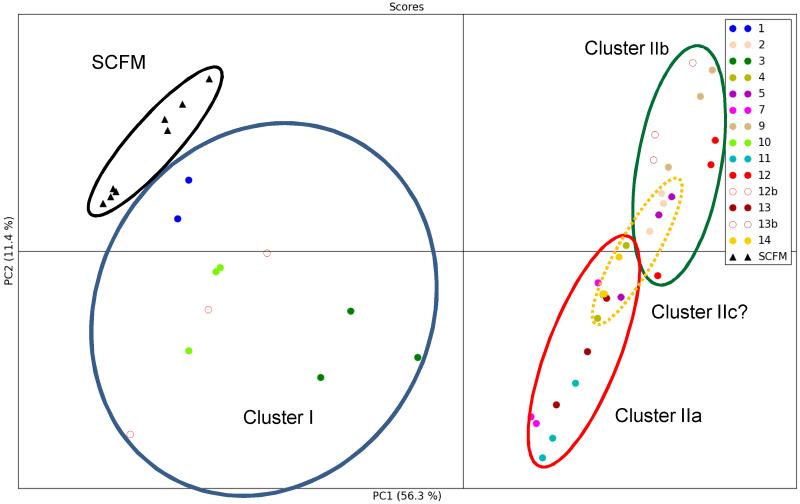
3.2 PCA identifies putative clusters based on isolate scores
Principal component analysis (PCA) was used to identify clustering patterns between spectra obtained for the clinical isolates (n = 41) and SCFM medium (n = 8). The representative 2D scores plots of component 1 (PC1) vs component 2 (PC2), which explain 67.7% of the variation in the spectra, reveal four putative separate clusters representing the different biochemical composition of the samples as detected by the NMR spectra (Fig. 2). In the PCA scores plot, each data point corresponds to one 1D 1H NMR spectrum, and the reproducibility of the method was supported by the close arrangement of data points corresponding to replicates from each isolate (Fig. 2).
Figure 2. Scores scatter plot resulting from applying PCA to the 1H NMR data by component 1(PC1) and component 2 (PC2).
The percentage of variance in the data explained by each component is indicated on the relevant axis. Strain identification numbers are shown. Ellipses are drawn to show putative clusters of spectra. SCFM – synthetic cystic fibrosis media. The corresponding loadings plot provided in the supplementary material identifies points in the NMR spectra that align with either PC1 or PC2.
NMR spectra obtained from media inoculated with each of the CF clinical isolates fell into two, readily identifiable, main clusters (I/II) that were distinguished on the basis their relative separation in PC1. The second of these clusters is possibly subdivided into two or three further putative clusters (IIa-c) since the isolates were further separated in PC2, with groups of isolates in separate quadrants of the PCA scores plot. The spectra from isolates in the three or four clusters were each well separated from those of the sterile synthetic media with the exception of isolate 1 which caused almost no change in the 1H NMR spectra of the spent media.
Cluster I was mostly separated from SCFM by PC2. Three of the four isolates found in Cluster I (1, 10, 12b) are notable in that they lead to a very acidic pH in the spent media (Table 1). This might cause the cluster members to be distinguished purely on the basis of pH dropping below pH 6.0 which causes MOPS resonances to shift even beyond the ranges excluded above (Supp. Fig. 2). A broad resonance at 4.04 ppm does appear for these isolates which can be assigned to the MOPS resonance expected in this region, however additional MOPS resonances expected between 3.50 and 3.87 ppm could not be discerned above the contribution from metabolite resonances.
Cluster II was further separated from SCFM by PC1 but subdivision of Cluster II was expected since isolates were also well distributed along PC2 and were separated into upper and lower quadrants. Initially a three cluster model was considered with Cluster IIa in the lower quadrant and Cluster IIb in the upper quadrant. The existence of a fourth putative Cluster IIc, with isolates located intermediate to Clusters IIa and IIb, was considered and tested by OPLS-DA below. Key resonances whose variation contributes to PC1 and PC2 are shown in the corresponding PC loadings plot (Supp. Fig. 4).
3.3 OPLS-DA supports clusters identification
Orthogonal projection to latent structures discriminant analysis (OPLS-DA) was then used to compare 1H NMR spectra data generated from sterile synthetic media with each of the four putative strain clusters and to test whether the clusters could indeed be considered separate. Cross-validation was performed on all models (Supp. Fig. 5-8). The resulting 2D scores plots show good separation between the three or four putative clusters and SCFM with Q2 values > 0.90, indicating a highly reliable model compared with an ideal score of 1 (Table 2). Initially, the PCA scores plot readily identified two separate isolate scores clusters (Cluster I/Cluster II) with Cluster I, comprising isolates 1, 3, 10 and 12b, well removed from the remaining isolates. The subdivision of Cluster II was tested; putative Clusters IIa and IIb, containing isolates 4, 7, 11, 13, 14 and 2, 5, 9, 12, 13b respectively, could be separated (Supp. Fig. 8; Q2 = 0.69) using OPLS-DA. However when a further putative cluster (IIc) was considered, comprising isolates 2, 4, 5 and 14 (PCA scores for these isolates show an intermediate distribution between the upper and lower quadrants due to PC2), the apparent separation as monitored by scores plots (Suppl. Fig. 6/7) and Q2 (Table 2) indicated that a four cluster model may be useful when greater numbers of patient isolates are available.
Table 2. Predictive Q2 values for all models.
Cluster IIa and IIb contain isolates 4, 7, 11, 13, 14 and 2, 5, 9, 12, 13b, respectively, in the 3 cluster model and lose isolates 2, 4, 5, 14 to Cluster IIc in the four cluster model. Q2 values for models run with permutated class assignments are given in parentheses.
| Test | 3 cluster model | 4 cluster model |
|---|---|---|
| SCFM vs Cluster I | 0.92 (−0.63) | 0.92 (−0.63) |
| SCFM vs Cluster IIa | 0.99 (−0.44) | 0.99 (−0.47) |
| SCFM vs Cluster IIb | 0.99 (−0.46) | 0.99 (−0.48) |
| SCFM vs Cluster IIc | - | 0.99 (−0.40) |
| Cluster IIa vs Cluster IIb | 0.71 (−0.46) | 0.91 (−0.50) |
| Cluster IIa vs Cluster IIc | - | 0.84 (−0.47) |
| Cluster IIb vs Cluster IIc | - | 0.92 (−0.44) |
3.4 Relationships between strain cluster membership and sample characteristics with 1D 1H NMR spectra
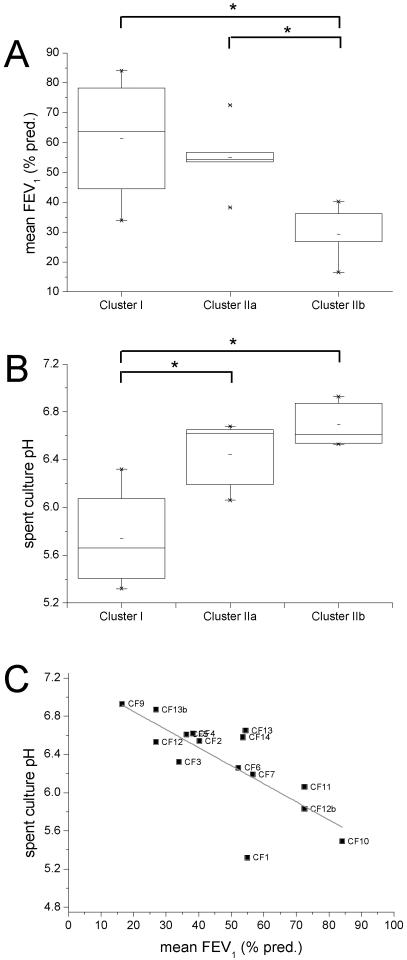
To determine whether differences in the nutritional modifications to airway secretion composition that result from the growth of P. aeruginosa may have clinical impacts, membership of CF sputum isolate clusters, as defined based on PCA and OPLS-DA, for both three and four cluster models, was compared with a number of potentially key strain or sputum sample characteristics. These factors were isolate auxotrophy, mucoidy, pigmentation, spent culture pH, sputum pH, species richness and relative phyla abundance as defined by 16S rRNA gene clone sequencing analysis, P. aeruginosa density as determined by quantitative PCR, patient age, sex, genotype, BMI, diabetic status, FEV1, and the number of respiratory exacerbations over the preceding 12 months. Of these, highly significant relationships were identified between both cluster membership and lung function (FEV1) (F(3,10) = 5.64, p = 0.0159) and cluster membership and spent culture pH (F(3,10) = 8.63, p = 0.004,) (Fig. 3). These significant relationships were tested using Tukey’s HSD to assess for significant differences between clusters. In the three cluster model, this analysis found significant differences between Clusters I and IIb (padj = 0.020) and between Clusters IIa and IIb (padj = 0.030) for lung function (FEV1) with patients in Cluster IIb having relatively poor lung function (Fig. 3A). In the four cluster model, Cluster I was shown to be significantly different from both clusters IIb (padj = 0.005) and IIc (padj = 0.010) (Supp. Fig. 9). A possible relationship was also observed between sputum pH and cluster membership (F(3,10) = 3.06, p = 0.078, pH ranged from 5.9 to 7.8). A strong negative correlation was found between FEV1 and spent culture pH (R = −0.76, p = 0.002) (Fig. 3C) and significant differences in spent culture pH were observed between Cluster I and both Clusters IIa and IIb in the three cluster model (Fig. 3B). No significant correlation was found however between lung function and sputum pH (R = 0.50, p = 0.067) or between sputum pH and spent culture pH (R = −0.37, p = 0.188) (further significant correlations are shown in Supplementary Information as are box plots for the four cluster model; Supp. Fig. 10). Therefore, although Cluster I and Cluster II are clearly separated in the PCA analysis (by PC1), the only significant differences that were found both in FEV1 and spent culture pH were between Cluster I and Cluster IIb with a significant difference in FEV1 also seen between Clusters IIa and IIb. These clusters are separated in the PCA analysis by PC2 and hence identification of resonances contributing to PC2, rather than PC1, or OPLS-DA analyses between these clusters should identify metabolomic changes that correlate with variance in FEV1 or spent culture pH.
Figure 3. Box plots comparing FEV1 (A) and spent culture pH (B) for each of the clusters in the three cluster model.
* p < 0.05. The relationship between mean FEV1 and spent culture pH is also shown for each of the isolates (C).
P. aeruginosa cell numbers in spent media were determined by Q-PCR enumeration, with mean values calculated from analysis of triplicate independent repeat cultures (Table 1). No significant difference between cultures of separate isolates, or independent repeat cultures of particular isolates, was observed, and no relationship was found between P. aeruginosa levels and cluster membership.
To determine whether the P. aeruginosa isolates belonging to separate clusters were genetically distinct, or represented the same strains growing differently, RAPD PCR analysis was performed. All 15 isolates studied here were found to represent distinct strain types (Supp. Fig. 11).
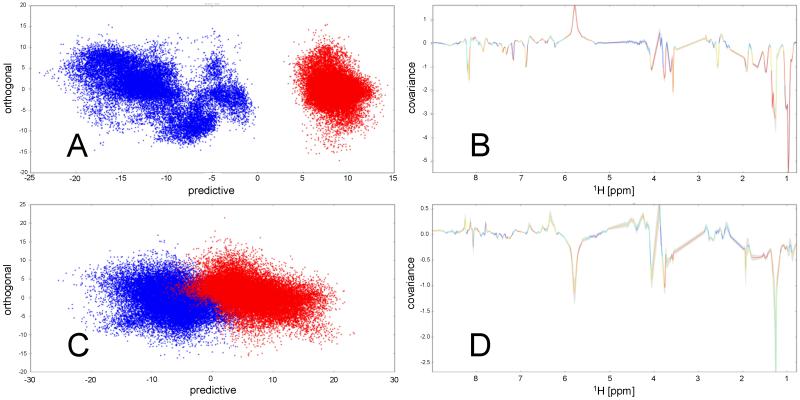
3.5 OPLS-DA identifies characteristic metabolite consumption and production
The OPLS-DA comparisons of Cluster IIb with Cluster I (Fig. 3A) and Cluster IIa (Fig. 3B) again support the clustering determined above and allow identification of resonances from metabolites that may be implicated in FEV1 and/or spent culture pH. Plotting normalised spectra from each of the four clusters, coloured according to cluster, highlights resonances whose intensity is consistently altered between clusters (Fig. 4). Comparing the back-scaled loadings plots for the comparisons between Cluster IIb and Cluster I and between Cluster IIb and Cluster IIa identifies metabolites whose differing intensities correlate with the significant differences identified above for lung function FEV1 and/or spent culture pH. Notably, levels of lysine or ornithine appear higher in the spent media of isolates in Cluster I or IIa when compared with those from Cluster IIb, where lung function was poorest, as evidenced by their characteristic resonances at 3.765 ppm (Fig. 4A) and 1.465 ppm (Fig. 4B). A resonance characteristic of leucine at 1.728 ppm (Fig. 4B) is also elevated in spectra of spent media of isolates in both Clusters I and IIa while a broad resonance that appears at 6.80 ppm (Fig. 4C), in many spectra from Cluster IIb isolates, is largely absent from either Clusters I or IIa. Spectra from isolates in Cluster IIc are intermediate between spectra from isolates in either Cluster IIa or IIb for these features. An additional broad resonance at 5.77 ppm is notable (Fig. 4C) but its intensity does not correlate with FEV1 and is one of the main resonances that contributes to the separation of the isolates by PC1 in the PCA analysis (Fig. 2B).
Figure 4. OPLS-DA scores plots (A, C) and back-scaled loadings plots (B, D) for the comparisons between Cluster IIb and Cluster I (A, B) and between Cluster IIb and IIa (C, D).
Complete Leave-one-out cross-validation (LOOCV) output files are provided in the Supplementary Material.
4 Discussion
The way in which chronic colonisation by high cell numbers of P. aeruginosa affects the composition of airway secretions in the CF lung is likely to be important in selecting co-infecting bacterial species, and in modifying the growth of P. aeruginosa itself, since nutritional sources are known to influence bacterial community composition in other contexts (Resat et al., 2012; Dunaj et al., 2012).
Bacterial growth results in both large- and fine-scale modifications to the composition of the growth medium, with the former primarily occurring through the utilisation of compounds as carbon and nitrogen sources and the production of major metabolites. Since the aim here was to obtain an indication of the degree to which P. aeruginosa isolate growth might have a differential impact on CF airway secretions, an approach that provides a comprehensive overview of major compositional changes, 1H NMR spectroscopy, was employed (Gjersing et al., 2007). In keeping with previous studies using 1H NMR spectroscopy, the data presented here show a high degree of independent-replicate reproducibility, whilst allowing differentiation of chemically divergent samples.
A defined synthetic growth medium designed to replicate the composition of CF lower airway secretions was used as part of an in vitro model of CF airway conditions. Previous studies comparing P. aeruginosa gene expression in CF sputum with that in similar CF synthetic media have shown bacterial behaviour to be similar in the two contexts (Palmer et al., 2007; Fung et al., 2010). By combining 1H NMR spectroscopy with this CF airway growth model, we were therefore able to assess the degree to which the impact of P. aeruginosa growth differed between clinical isolates under conditions approximating those encountered in vivo. The P. aeruginosa clinical isolates studied here showed substantial metabolomic differences, and were categorised into four separate clusters. These variations, particularly in levels of the amino acids, may reflect differences in energy strategies employed, and the ability of strains to adapt to the low oxygen tensions in muco-purulent CF sputum. Such differences may be important in vivo given that amino acid levels have been shown to influence the antibiotic susceptibility of P. aeruginosa (Nguyen et al., 2011) and the growth strategies that they adopt (Bernier et al., 2011; Shrout et al, 2006).
Cluster membership was found to be related to culture pH. Care was taken to exclude the contribution of pH induced peak shifting through the exclusion of resonances from MOPS buffer prior to multivariate analysis and manual bucketing of spectra following the failure of peak alignment using COW. Visual inspection of the spectra resulting from each cluster suggested that the observed isolate clustering is due to differences in levels of media constituents and metabolites produced/consumed rather than shifting peaks. Indeed, no relationship was found between cluster membership and P. aeruginosa cell numbers, further supporting divergence driven by growth strategy rather than cell density. The mechanism by which P. aeruginosa growth affects pH is not clear, and could occur either through a change in levels of non-pH neutral components of the growth media or through proton extrusion. However, the impact of P. aeruginosa growth on pH could have major clinical implications since it has been shown to influence both bacterial community composition (Romanowski et al. 2011; Duncan et al., 2009) and behaviour (Walker et al., 2005) in other clinical contexts, and alterations in airway secretion pH could affect a number of innate defence processes, such as ciliary function (Clary-Meinesz et al., 1998) and mucus viscosity (Inglis et al., 1998). Importantly, a highly significant relationship was found between the cluster membership of CF isolates analysed here and the lung function of the patient from which they were obtained (as measured by FEV1). This link between the nutritional modification of the environment by P. aeruginosa growth and patient lung function has clear implications for how our understanding of bacterial community composition in the CF airways needs to develop. A broad phylogenetic range of species have been reported in CF respiratory secretion (Rogers et al, 2004), with a common factor for their establishment in the airways being access to nutrients they require for growth. However, not all species common in associated areas, such as the oral cavity, are routinely reported in CF lower airway secretions. This may be influenced by modification of the nutritional context of the lower airway secretions by dominant bacterial species (Kloosterman et al, 2011; Rogosa & Bishop, 1964; Nakada & Itoh, 2003). Such nutritional interactions in the CF lower airways are poorly understood, and require further investigation.
5 Conclusions
In summary, the application of 1H NMR here to determining the impact of P. aeruginosa clinical isolate growth within a model CF system reveals substantial metabolomic differences between isolates. Membership of isolate clusters, defined through PCA, appears to be linked to divergence in metabolite production, with a significant correlation between cluster membership and spent culture pH. These findings suggest that P. aeruginosa isolates employ a range of growth strategies. Further, cluster membership was found to be significantly correlated with patient lung function, suggesting that there may be direct clinical implications for bacterial metabolic strategy in vivo. A more sophisticated characterisation of the metabolic and pH environment of the CF lower airway is now warranted, with the potential to inform our understanding of the chronic bacterial infections that typify cystic fibrosis airway disease.
Supplementary Material
Figure 5. Normalised 1H spectra (with excluded regions but otherwise untreated) of spent media coloured according to cluster membership.
(Cluster I – blue, Cluster IIa – red, Cluster IIb – green, Cluster IIc – yellow). Spectral regions between 3.5 and 3.85 ppm (A), 5.6 and 6.9 ppm (B) and 1.4 and 1.8 ppm (C) are shown.
Acknowledgement
This study was supported by the Anna Trust. JK is supported by a BBSRC Industrial CASE studentship.
References
- Aaron SD, Kottachchi D, Ferris WJ, Vandemheen KL, St Denis ML, Plouffe A, et al. Sputum versus bronchoscopy for diagnosis of Pseudomonas aeruginosa biofilms in cystic fibrosis. Eur. Respir. J. 2004;24:631–7. doi: 10.1183/09031936.04.00049104. [DOI] [PubMed] [Google Scholar]
- Andersson M. A comparison of nine PLS1 algorithms. J. Chemom. 2009;23:518–29. [Google Scholar]
- Armougom F, Bittar F, Stremler N, Rolain JM, Robert C, Dubus JC, et al. Microbial diversity in the sputum of a cystic fibrosis patient studied with 16S rDNA pyrosequencing. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:1151–4. doi: 10.1007/s10096-009-0749-x. [DOI] [PubMed] [Google Scholar]
- Barth AL, Pitt TL. Auxotrophy of Burkholderia (Pseudomonas) cepacia from cystic fibrosis patients. J Clin Microbiol. 1995;33:2192–4. doi: 10.1128/jcm.33.8.2192-2194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier SP, Ha DG, Khan W, Merritt JH, O’Toole GA. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res. Microbiol. 2011;162:680–8. doi: 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneduci A, Chidichimo G, Dardo G, Pontoni G. Highly rouotinely reproducible alignment of 1H NMR spectral peaks if metabolites in huge sets of urines. Analytica Chim. Acta. 2011;685:186–95. doi: 10.1016/j.aca.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, et al. Scandinavian Cystic Fibrosis Study Consortium Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One. 2010;5:e10115. doi: 10.1371/journal.pone.0010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004;23:146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- CF Foundation Patient registry annual data report 2007. http://cff.org/UploadedFiles/research/ClinicalResearch/2007-Patient-Registry-Report.pdf.
- Ciofu O, Mandsberg LF, Wang H, Høiby N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012;65:215–225. doi: 10.1111/j.1574-695X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur. Respir. J. 1998;11:330–333. doi: 10.1183/09031936.98.11020330. [DOI] [PubMed] [Google Scholar]
- Cloarec O, Dumas ME, Trygg J, Craig A, Barton RH, Lindon JC, et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal Chem. 2005;77:517–26. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
- Dean M, Santis G. Heterogeneity in the severity of cystic fibrosis and the role of CFTR gene mutations. Hum. Genet. 1994;93:364–8. doi: 10.1007/BF00201659. [DOI] [PubMed] [Google Scholar]
- Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalisation as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006;78:4281–90. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- Dunaj SJ, Vallino JJ, Hines ME, Gay M, Kobyljanec C, Rooney-Varga JN. Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012;46:1914–22. doi: 10.1021/es2032532. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009;11:2112–22. doi: 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- Feizabadi MM, Majnooni A, Nomanpour B, Fatolahzadeh B, Raji N, Delfani S, et al. Direct detection of Pseudomonas aeruginosa from patients with healthcare associated pneumonia by real time PCR. Infect. Genet. Evol. 2010;10:1247–51. doi: 10.1016/j.meegid.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Frimmersdorf E, Horatzek S, Pelnikevich A, Wiehlmann L, Schomburg D. How Pseudomonas aeruginosa adapts to various environments: a metabolomic approach. Environ. Microbiol. 2010;12:1734–47. doi: 10.1111/j.1462-2920.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- Fung C, Naughton S, Turnbull L, Tingpej P, Rose B, Arthur J, et al. Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J. Med. Microbiol. 2010;59:1089–100. doi: 10.1099/jmm.0.019984-0. [DOI] [PubMed] [Google Scholar]
- Gjersing EL, Herberg JL, Horn J, Schaldach CM, Maxwell RS. NMR metabolomics of planktonic and biofilm modes of growth in Pseudomonas aeruginosa. Anal. Chem. 2007;79:8037–8045. doi: 10.1021/ac070800t. [DOI] [PubMed] [Google Scholar]
- Hwang T-L, Shaka AJ. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. 1995;112:275–279. [Google Scholar]
- Inglis SK, Corboz MR, Ballard ST. Effect of anion secretion inhibitors on mucin content of airway submucosal gland ducts. Am. J. Physiol. 1998;274:L762–L766. doi: 10.1152/ajplung.1998.274.5.L762. [DOI] [PubMed] [Google Scholar]
- Kloosterman TG, Kuipers OP. Regulation of arginine acquisition and virulence gene expression in the human pathogen Streptococcus pneumoniae by transcription regulators ArgR1 and AhrC. J. Biol. Chem. 2011;286:44594–605. doi: 10.1074/jbc.M111.295832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpen M, Hansen CR, Bjarnsholt T, Moser C, Christensen LD, van Gennip M, et al. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65:57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr. Pulmonol. 2001;32:277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui Y, Matsumura K, Kondo C, Takashima R. The role of mucin on experimental Pseudomonas keratitis in rabbits. Invest. Ophthalmol. 1976;15:208–10. [PubMed] [Google Scholar]
- Nakada Y, Itoh Y. Identification of the putrescine biosynthetic genes in Pseudomonas aeruginosa and characterization of agmatine deiminase and N-carbamoylputrescine amidohydrolase of the arginine decarboxylase pathway. Microbiology. 2003;149:707–14. doi: 10.1099/mic.0.26009-0. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, et al. Active Starvation Responses Mediate Antibiotic Tolerance in Biofilms and Nutrient-Limited Bacteria. Science. 2011;334:982. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007;189:8079–87. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 2005;187:5267–77. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renders N, Römling Y, Verbrugh H, van Belkum A. Comparative typing of Pseudomonas aeruginosa by random amplification of polymorphic DNA or pulsed-field gel electrophoresis of DNA macrorestriction fragments. J Clin Microbiol. 1996;34:3190–3195. doi: 10.1128/jcm.34.12.3190-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resat H, Bailey V, McCue LA, Konopka A. Modeling Microbial Dynamics in Heterogeneous Environments: Growth on Soil Carbon Sources. Microb. Ecol. 2012;63:883–897. doi: 10.1007/s00248-011-9965-x. [DOI] [PubMed] [Google Scholar]
- Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Bruce KD. Characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16S ribosomal DNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 2004;42:5176–83. doi: 10.1128/JCM.42.11.5176-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Kehagia V, et al. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J. Clin. Microbiol. 2006;44:2601–4. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M, Bishop FS. The genus Veillonella. II. Nutritional studies. J Bacteriol. 1964;87:574–80. doi: 10.1128/jb.87.3.574-580.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski K, Zaborin A, Fernandez H, Poroyko V, Valuckaite V, Gerdes S, et al. Prevention of siderophore-mediated gut-derived sepsis due to P. aeruginosa can be achieved without iron provision by maintaining local phosphate abundance: role of pH. BMC Microbiol. 2011;11:212. doi: 10.1186/1471-2180-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. J. Pediatr. 2001;139:359–65. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–77. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- Son MS, Matthews WJ, Jr, Kang Y, Nguyen DT, Hoang TT. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:5313–24. doi: 10.1128/IAI.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramulu DD, Lünsdorf H, Lam JS, Römling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2004;54:667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- Stressmann FA, Rogers GB, Marsh P, Lilley AK, Daniels TW, Carroll MP, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J. Cyst. Fibros. 2011;10:357–65. doi: 10.1016/j.jcf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Stressmann FA, Rogers GB, van der Gast CJ, Marsh P, Vermeer LS, Carroll MP, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax. 2012;67:867–73. doi: 10.1136/thoraxjnl-2011-200932. [DOI] [PubMed] [Google Scholar]
- Tomasi G, van den Berg F, Andersson C. Correlation optimized warping and dynamic time warping as preprocessing methods for chromatographic data. J. Chemometrics. 2004;18:231–241. [Google Scholar]
- Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax. 2011;66:579–84. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, et al. BioMagResBank Nucleic Acids Research. 2007;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gast CJ, Walker AW, Stressmann FA, Rogers GB, Scott P, Daniels TW, et al. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 2011;5:780–91. doi: 10.1038/ismej.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer LS, Fruhwirth GO, Pandya P, Ng T, Mason AJ. NMR metabolomics of MTLn3E breast cancer cells identifies a role for CXCR4 in lipid and choline regulation. J. Proteome Res. 2012;11:2996–3003. doi: 10.1021/pr300111x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Duncan SH, McWilliam Leitch EC, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005;71:3692–700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009;37:D603–10. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms. Relationships to cystic fibrosis pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.