Abstract
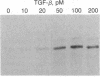
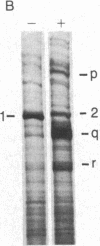
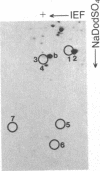
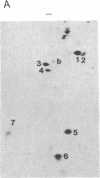
We have investigated the effect of type beta transforming growth factor (TGF-beta) on the differentiation of skeletal muscle myoblasts. TGF-beta potently (ID50 approximately 10 pM) prevents established cell lines and primary cultures of rat and chicken embryo myoblasts from fusing into multinucleated myotubes. Inhibition of morphological differentiation by TGF-beta correlates with inhibition of the expression of muscle-specific mRNAs and proteins, strong induction of extracellular matrix type I collagen and fibronectin, and a marked tendency of the treated myoblasts to aggregate into densely multilayered arrays or clusters. Myogenic differentiation can resume after removal of TGF-beta from the medium. Examination of the time of action of TGF-beta shows that myoblasts stochastically reach a point beyond which they become insensitive to the inhibitory action of TGF-beta. This resistance of committed myoblasts to the inhibitory action of TGF-beta is not associated with any measurable change in the number or affinity of TGF-beta receptors in those cells. The results indicate that TGF-beta is a potent inhibitor of myogenesis and may regulate muscle development in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Transient induction of poly(A)-short myosin heavy chain messenger RNA during terminal differentiation of L6E9 myoblasts. J Mol Biol. 1980 Jun 25;140(2):283–298. doi: 10.1016/0022-2836(80)90106-0. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Like B., Massagué J. Cellular distribution of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1986 Jul 25;261(21):9972–9978. [PubMed] [Google Scholar]
- Childs C. B., Proper J. A., Tucker R. F., Moses H. L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger-Hodges M. J., Ewton D. Z., Seifert S. C., Florini J. R. Inhibition of myoblast differentiation in vitro by a protein isolated from liver cell medium. J Cell Biol. 1982 May;93(2):395–401. doi: 10.1083/jcb.93.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel L. I., Periasamy M., Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982 Sep 25;257(18):11078–11086. [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Weseman J., Moran J. S., Lindstrom J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J Cell Biol. 1976 Aug;70(2 Pt 1):395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983 Nov 25;258(22):13606–13613. [PubMed] [Google Scholar]
- Massagué J., Kelly B., Mottola C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J Biol Chem. 1985 Apr 25;260(8):4551–4554. [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985 Mar 10;260(5):2636–2645. [PubMed] [Google Scholar]
- Massagué J. Subunit structure of a high-affinity receptor for type beta-transforming growth factor. Evidence for a disulfide-linked glycosylated receptor complex. J Biol Chem. 1985 Jun 10;260(11):7059–7066. [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Nusgens B., Delain D., Sénéchal H., Winand R., Lapierre C. M., Wahrmann J. P. Metabolic changes in the extracellular matrix during differentiation of myoblasts of the L6 line and of a Myo- non-fusing mutant. Exp Cell Res. 1986 Jan;162(1):51–62. doi: 10.1016/0014-4827(86)90425-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Schlessinger J., Yamada K. M. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979 Sep;122(2):317–326. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
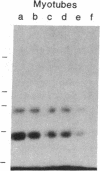
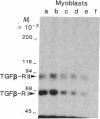
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]