Abstract
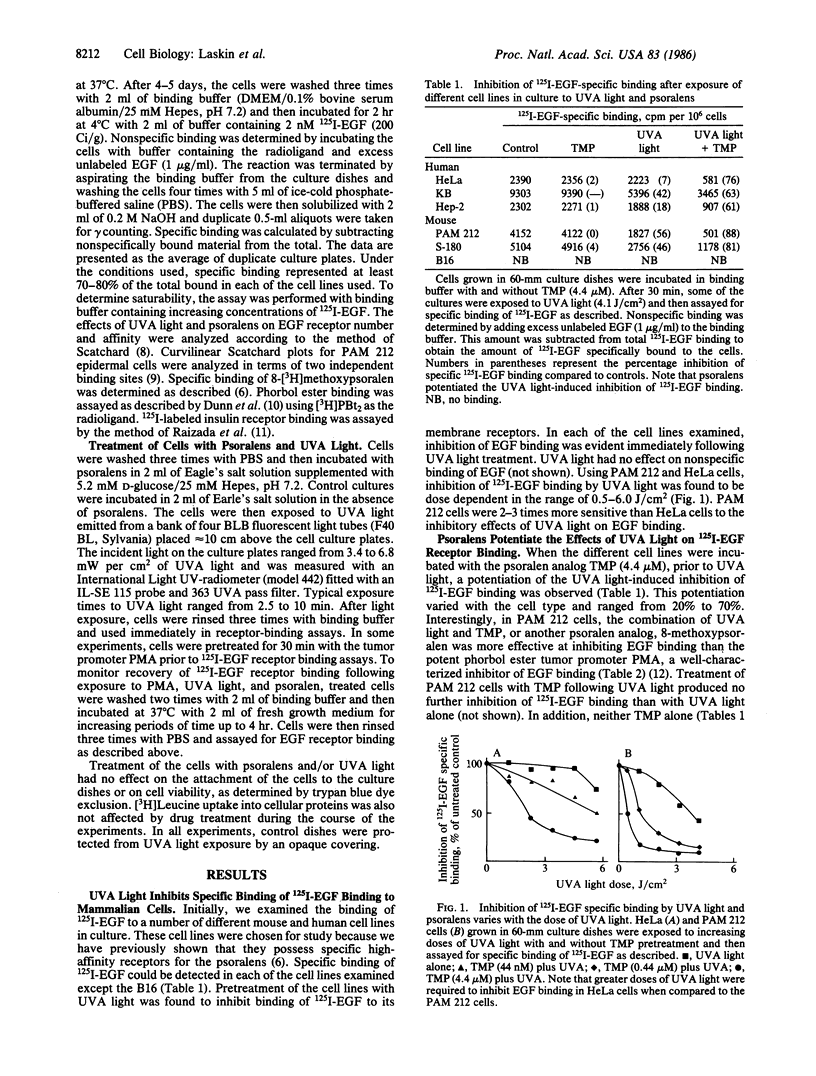
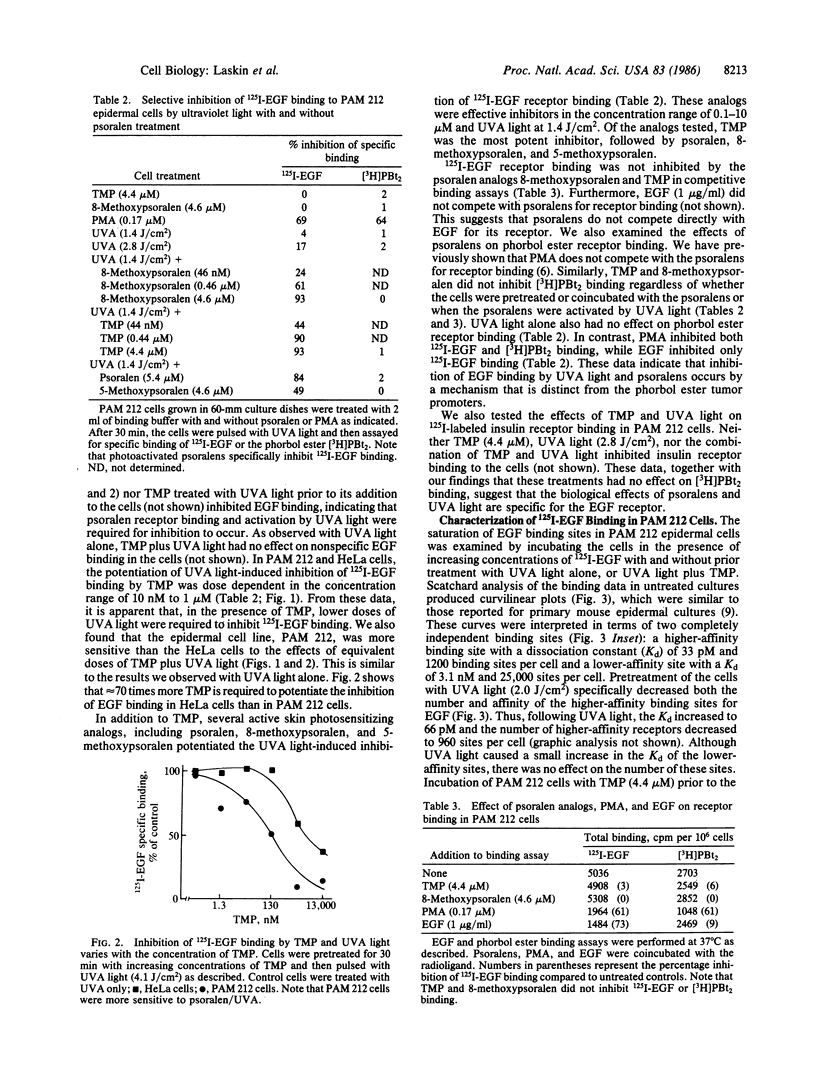
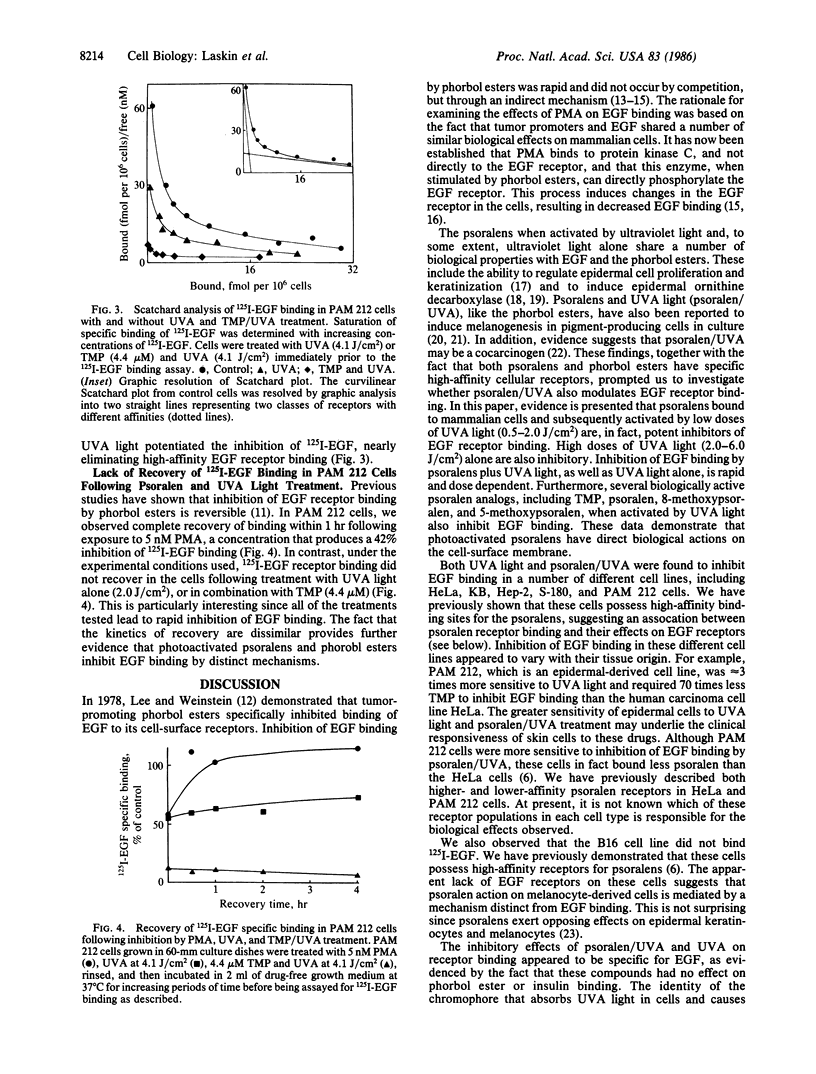
The psoralens, when activated by ultraviolet light of 320-400 nm (UVA light), are potent modulators of epidermal cell growth and differentiation. Previously, we reported that, in mammalian cells, these compounds bind to specific saturable high-affinity cellular receptor sites. In the present studies, we demonstrate that binding of psoralens to their receptors followed by UVA light activation is associated with inhibition of epidermal growth factor (EGF) receptor binding. Inhibition of EGF binding, which required UVA light, was rapid and dependent on the dose of UVA light (0.5-2.0 J/cm2), as well as the concentration of psoralens (10 nM to 1 microM). Higher doses of UVA light (2.0-6.0 J/cm2) by themselves were also inhibitory, indicating that psoralens potentiate the UVA-induced inhibition of EGF binding. A number of biologically active analogs of psoralen, including 8-methoxypsoralen, 5-methoxypsoralen, and 4,5',8-trimethylpsoralen, when activated by UVA light, were found to be inhibitors of binding. Inhibition of EGF binding by psoralens was observed in a variety of human and mouse cell culture lines known to possess psoralen receptors. In the epidermal-derived line PAM 212, at least two populations of receptors with different affinities for EGF were found. Psoralens and UVA light selectively inhibited binding to the higher-affinity EGF receptors, an effect analogous to that of the phorbol ester tumor promoters. As observed with phorbol esters, photoactivated psoralens appeared to inhibit EGF binding by an indirect mechanism. These data demonstrate that the psoralens and UVA light have direct biological effects on cell-surface membranes. Since EGF is a growth-regulatory peptide, the ability of psoralens and UVA light to inhibit EGF binding may underlie the biologic effects of these agents in the skin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Properties of the receptor for epidermal growth factor. Cell. 1984 Jun;37(2):357–358. doi: 10.1016/0092-8674(84)90365-9. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Dunn J. A., Jeng A. Y., Yuspa S. H., Blumberg P. M. Heterogeneity of [3H]phorbol 12,13-dibutyrate binding in primary mouse keratinocytes at different stages of maturation. Cancer Res. 1985 Nov;45(11 Pt 1):5540–5546. [PubMed] [Google Scholar]
- Fitzpatrick T. B., Pathak M. A. Research and development of oral psoralen and longwave radiation photochemotherapy: 2000 B.C.-1982 A.D. Natl Cancer Inst Monogr. 1984 Dec;66:3–11. [PubMed] [Google Scholar]
- Gange R. W., Parrish J. A. Cutaneous phototoxicity due to psoralens. Natl Cancer Inst Monogr. 1984 Dec;66:117–126. [PubMed] [Google Scholar]
- Grossweiner L. I. Mechanisms of photosensitization by furocoumarins. Natl Cancer Inst Monogr. 1984 Dec;66:47–54. [PubMed] [Google Scholar]
- Harrist T. J., Gonzalez E. Histologic alterations of psoralen- and longwave ultraviolet radiation-exposed skin. Natl Cancer Inst Monogr. 1984 Dec;66:197–204. [PubMed] [Google Scholar]
- Huberman E., Heckman C., Langenbach R. Stimulation of differentiated functions in human melanoma cells by tumor-promoting agents and dimethyl sulfoxide. Cancer Res. 1979 Jul;39(7 Pt 1):2618–2624. [PubMed] [Google Scholar]
- Laskin J. D., Lee E., Yurkow E. J., Laskin D. L., Gallo M. A. A possible mechanism of psoralen phototoxicity not involving direct interaction with DNA. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6158–6162. doi: 10.1073/pnas.82.18.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Mechanism of tumor promoter inhibition of cellular binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5168–5172. doi: 10.1073/pnas.76.10.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Lockyer J. M., Bowden G. T., Matrisian L. M., Magun B. E. Tumor promoter-induced inhibition of epidermal growth factor binding to cultured mouse primary epidermal cells. Cancer Res. 1981 Jun;41(6):2308–2314. [PubMed] [Google Scholar]
- Lord J. T., Ziboh V. A. Specific binding of prostaglandin E2 to membrane preparations from human skin: receptor modulation by UVB-irradiation and chemical agents. J Invest Dermatol. 1979 Nov;73(5):373–377. doi: 10.1111/1523-1747.ep12550453. [DOI] [PubMed] [Google Scholar]
- Lowe N. J., Connor M. J., Cheong E. S., Akopiantz P., Breeding J. H. Psoralen and ultraviolet A effects on epidermal ornithine decarboxylase induction and DNA synthesis in the hairless mouse. Natl Cancer Inst Monogr. 1984 Dec;66:73–76. [PubMed] [Google Scholar]
- Magun B. E., Matrisian L. M., Bowden G. T. Epidermal growth factor. Ability of tumor promoter to alter its degradation, receptor affinity and receptor number. J Biol Chem. 1980 Jul 10;255(13):6373–6381. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Lerner A. B. Vitiligo. It is important. Arch Dermatol. 1982 Jan;118(1):5–8. doi: 10.1001/archderm.118.1.5. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975 Jul;35(7):1662–1670. [PubMed] [Google Scholar]
- Pathak M. A. Mechanisms of psoralen photosensitization reactions. Natl Cancer Inst Monogr. 1984 Dec;66:41–46. [PubMed] [Google Scholar]
- Raizada M. K., Tan G., Fellows R. E. Fibroblastic cultures from the diabetic db/db mouse. Demonstration of decreased insulin receptors and impaired responses to insulin. J Biol Chem. 1980 Oct 10;255(19):9149–9155. [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Stern R. S., Thibodeau L. A., Kleinerman R. A., Parrish J. A., Fitzpatrick T. B. Risk of cutaneous carcinoma in patients treated with oral methoxsalen photochemotherapy for psoriasis. N Engl J Med. 1979 Apr 12;300(15):809–813. doi: 10.1056/NEJM197904123001501. [DOI] [PubMed] [Google Scholar]



