Abstract
First-generation adenovirus vectors (FG AdVs) expressing short-hairpin RNA (shRNA) effectively downregulate the expressions of target genes. However, this vector, in fact, expresses not only the transgene product, but also virus-associated RNAs (VA RNAs) that disturb cellular RNAi machinery. We have established a production method for VA-deleted AdVs lacking expression of VA RNAs. Here, we showed that the highest shRNA activity was obtained when the shRNA was inserted not at the popularly used E1 site, but at the E4 site. We then compared the activities of shRNAs against hepatitis C virus (HCV) expressed from VA-deleted AdVs or conventional AdVs. The VA-deleted AdVs inhibited HCV production much more efficiently. Therefore, VA-deleted AdVs were more effective than the currently used AdVs for shRNA downregulation, probably because of the lack of competition between VA RNAs and the shRNAs. These VA-deleted AdVs might enable more effective gene therapies for chronic hepatitis C.
RNA interference (RNAi) technology is a versatile tool for analyzing the function of genes in vitro and in vivo in various research fields. It also presents a therapeutic approach for the treatment of human diseases and for the selection of effective drugs. Two types of small non-coding RNAs function as RNAi, small interfering RNAs (siRNAs) and microRNAs (miRNAs). Short-hairpin RNA (shRNA) is artificially produced RNAi that downregulates the expression of the target gene.
First-generation adenovirus vectors (FG AdVs), which lack the E1 and E3 regions, have been widely used not only for basic studies of various gene functions in vitro and in vivo, but also for preclinical and clinical gene therapy. The transduction efficiency of this vector is very high, and viral stocks with a high titer are easily obtained. FG AdVs are often used to deliver shRNA or miRNA expression cassettes into target cells in vitro1,2. They are also used in in vivo studies, particularly in gene therapy fields3,4,5. FG AdVs are usually considered not to express any viral gene products because they lack the E1A gene, which is essential for the expression of all viral genes driven by polymerase II. In fact, however, FG AdVs express viral-associated RNAs (VA RNAs), which are vector-encoded small RNAs that are always expressed together with the transgene product both in vitro and in vivo since they are transcribed by polymerase III.
The VA RNAs, known as VAI and VAII, consist of 157–160 nucleotides (nt) and are encoded at about 30 map units on the genome of adenoviruses. In the normal life cycle of adenoviruses possessing the E1 genes, these VA RNAs are abundantly present during the late phase of infection and inhibit cellular RNAi pathways by saturating Exportin 5, RISC, and Dicer6. They are also processed and generate miRNAs7,8, known as mivaRNAI and mivaRNAII, that disturb the expression of many cellular genes, with the probable result of blocking cellular antiviral machinery. VA RNAs are also expressed during the early phase of viral infection, though their functions during this phase remain unknown and the target genes of mivaRNAs have not been adequately studied. VA RNAs are expressed in AdV-transduced target cells at a level similar to that during the early phase and are considered to be a cause of severe immune responses9,10, which are a major drawback of this vector. Therefore, AdVs lacking the expression of VA RNAs (VA-deleted AdVs) are desired for both basic and clinical studies and may enable safer gene therapy.
Because VA RNAs are processed using the same pathway as shRNAs, a question arises as to whether VA RNAs influence the RNAi strategy when this vector is used. However, this possibility has not been previously tested because AdVs lacking the expression of VA RNAs have been extremely difficult to develop11, though low titers of VA-deleted, E1-containing adenoviruses have been obtained. Recently, however, we have established a method for the very efficient production of VA-deleted AdVs12 that is sufficient for practical use in vitro and in vivo. The titers of the VA-deleted AdVs are comparable to those of the currently used FG AdVs. Also, we established 293 cell lines that constitutively express VA RNAs and support the growth of shRNA-expressing VA-deleted AdVs (unpublished data). These progresses have enabled us to examine whether VA RNAs actually influence the shRNA strategy.
Hepatitis C virus (HCV) infects 2%–3% of the world's population and is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma13,14,15. The most abundant genotype of HCV worldwide is genotype 1, which has two prevalent subtypes (1a and 1b). Genotype 2 is the second most common genotype. In addition to standard care combined with interferon (IFN) and rivavirin, the emergence of direct-acting antivirals, such as HCV protease inhibitors, has enabled advances in treatment. However, considering the development of drug-resistant viruses and side effects induced by drug–drug interactions, additional options for anti-HCV therapy are inevitably needed. HCV is considered to be an attractive target for shRNA-based therapy16,17,18,19 because the viral genome is a single-stranded RNA of positive polarity and all the steps of viral replication occur in the cytoplasm.
shRNA-expressing cassettes are usually inserted at the E1 cloning site. However, the suitability of this site has not been examined. Here, we report that the shRNA activity was influenced by the position and orientation of the shRNA in the vector genome, and the most effective position/orientation the E4 position. We also show that shRNAs expressed by VA-deleted AdVs inhibited HCV replication more efficiently than those expressed by FG AdVs. The present report is the first to demonstrate that VA RNAs expressed from FG AdVs do indeed reduce the shRNA activity and that VA-deleted AdVs are useful for shRNA strategies.
Results
shRNA-expressing unit inserted at the E4 position worked more efficiently than that at the E1 position in the AdV genome
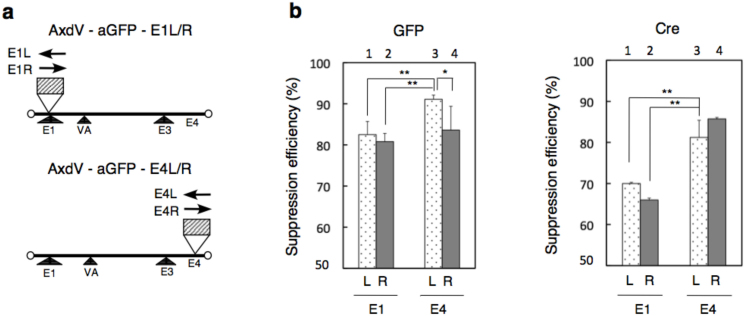
We constructed four VA-deleted AdVs containing shRNA that suppresses GFP expression (Fig. 1a). Cassettes containing anti-GFP shRNA under the control of the human U6 promoter were inserted at the E1 and E4 insertion positions in left (L) and right (R) orientations (AxdV-aGFP-E1L, -E1R, -E4L, and -E4R, respectively). To assay shRNA suppression we used FC-18 cells that constitutively express GFP under the control of the EF1α promoter from its gene integrated in the chromosome20. The cells were infected with these AdVs at a multiplicity of infection (MOI) of 50, and the copy numbers of the GFP mRNA present in the cells were measured using quantitative PCR (qPCR). The suppression efficiency of the E4L vector (Fig. 1b, left, bar 3) was significantly higher than those of the E1L and E1R vectors (bars 1and 2). Therefore, the E4L position/orientation was more effective than the E1L or E1R position/orientation. This finding is notable because the E1 position is popularly used for the insertion of shRNA cassettes. The present results were confirmed using a FACS analysis (Supplementary Fig. S1). We also examined the suppression efficiency of mRNA transiently expressed from a transgene located on the AdV genome: a Cre-expressing AdV was co-infected together with each of the four VA-deleted AdVs expressing anti-Cre shRNA instead of anti-GFP shRNA (AxdV-aCre-E1L, -E1R, -E4L, and -E4R, respectively). The suppression efficiencies of the E4L and E4R anti-Cre vectors were higher than those of the E1L and E1R vectors at MOI 50 (Fig. 1b, right; compare bars 3 and 4 with bars 1 and 2, respectively) and at MOI 200 (Supplementary Fig. S2), while the efficiencies of the E4L and E4R anti-Cre vectors were not statistically different. Based on these data, the E4L position/orientation was adopted for further experiments examining HCV replication.
Figure 1. Structures of vectors containing shRNA cassettes and suppression efficiencies.
(a) Structures of AdV-aGFP vectors. The arrow shows the orientation of transcription. Hatched box, shRNA cassette including the human U6 promoter. (b) (Left) Suppression efficiency of GFP RNA expressed from the cell line using anti-GFP vectors. FC-18 cells that constitutively express GFP RNA were infected with the vectors at MOI 50. (Right) Suppression efficiency of Cre RNA expressed from the AdV genome using anti-Cre vectors. FC-18 cells were doubly infected with AdV expressing Cre under the control of the CAG promoter at MOI 10 and anti-Cre vectors at MOI 50. Three days after infection, the amount of cytoplasmic RNA of GFP and Cre were measured using qPCR. The suppression efficiency for vector-infected FC-18 cells was calculated using copy numbers per cell, where uninfected FC-18 cells were denoted as 0% suppression of GFP RNA, while that of CV1 cells, the parent cells of FC-18 that do not contain the GFP gene, is denoted as the control of 100% suppression. Copy number, n = 6. *P < 0.05, **P < 0.01 compared with the E4L vector (unpaired Student's t-test).
Anti-HCV shRNA activity in cells replicating HCV subgenome RNA was enhanced using VA-deleted AdV
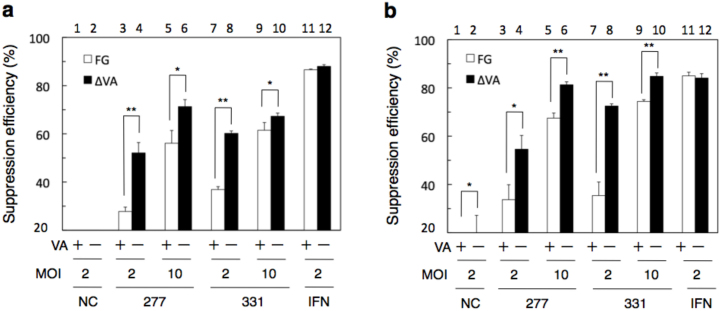
The anti-HCV activity of AdVs carrying shRNA was assessed in Huh-7-derived cells carrying the viral subgenomic replicon RNA (viral structural genes are replaced by either the neomycin-resistant [neo] gene or the luciferase-neo fusion gene, and all the nonstructural genes sufficient for replication of the viral genome are retained; Fig. 2). The HCV RNA is constitutively maintained in these cells. Two kinds of shRNAs were used (Fig. 2, arrows). The shRNA331 (called sh331 in this paper) targeting nt 322–342 at the 5′ end of the HCV genome has been reported to inhibit HCV replication efficiently16. The shRNA277 (called sh277 in this paper) targeting nt 279–297 is well conserved among genotypes and was newly constructed in this study. HuH 5–15 cells, which are genotype 1b-replicon cells21, were infected with FG AdVs expressing either sh277 or sh331 or VA-deleted AdVs (VA-del AdVs in this figure) that were identical to AxdV-aGFP-E4L but used sh277 and sh331 as the shRNA (Fig. 3a). These AdVs contained cassettes expressing shRNAs under the control of the human U6 promoter at the E4L position/orientation. HCV RNA copies in the cells were measured using qPCR, and the suppression efficiency was calculated. VA-deleted AdVs expressing sh277 suppressed the viral RNA replication more efficiently than the corresponding FG AdV at MOI 2 (Fig. 3a, bars 3 and 4). A higher anti-HCV activity was also observed for the VA-deleted AdV compared with the FG AdV at MOI 10 (bars 5 and 6). Similarly, VA-deleted AdV expressing sh331 suppressed HCV RNA replication more efficiently than the corresponding FG AdV at both MOI 2 and MOI 10 (bars 7 to 10). Among these settings, VA-deleted AdV expressing sh277 at MOI 10 yielded the highest suppression efficiency (bar 6, 71%).
Figure 2. Schematic representation of the HCV replicons.
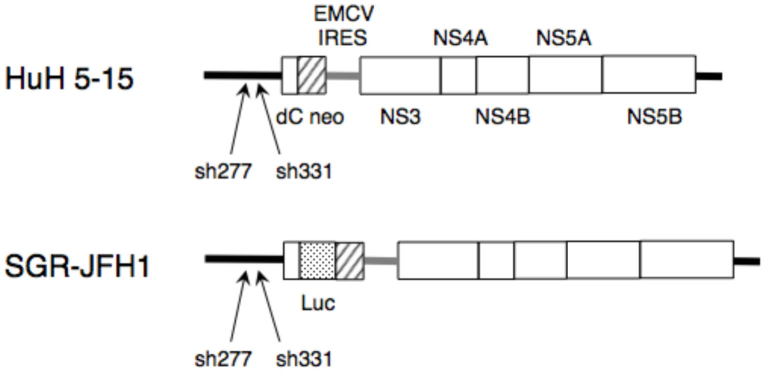
The coding regions in the HCV polyproteins are indicated by the open boxes. The bold lines indicate the HCV 5′-untranslated region (UTR), which is the target of sh277 and sh331 (arrows), and the 3′-UTR. Gray bars, EMCV internal ribosome entry site; dC, 5′-region of Core gene; neo, neomycin-resistance gene; Luc-neo, firefly luciferase gene fused with neo gene.
Figure 3. Suppression of HCV RC RNA by the AdVs expressing sh277 and sh331.
Effects on HCV RNA replication in HuH 5–15 cells (a) and SGR-JFH1 cells (b). The cells were infected with FG AdVs (FG, white bars) and VA-deleted AdVs (VA-del, black bars). NC, AdVs expressing negative-control shRNA. The copy numbers of intracellular HCV RNA were measured at 72 h after infection. The suppression efficiency was calculated relative to the copy numbers in uninfected cells as 0%; the copy numbers of HCV RNA in the control, uninfected cells were 4.0 × 104 copies/cell and 1.1 × 104 copies/cell in HuH 5–15 cells (a) and SGR-JFH1 cells (b), respectively. The suppression efficiencies of NC FG AdV and NC VA-deleted AdV were (a) 3.8 (±6.7) and 15.1 (±4.4), while those were (b) −4.1 (±3.4) and 18.2 (±9.0), respectively. Each data point represents an average of n = 3, mean ± S.D. (error bars). *P < 0.05, **P < 0.01.
SGR-JFH1/LucNeo cells (called SGR-JFH1), which harbor a genotype 2a subgenomic replicon carrying a luciferase-neo fusion gene (Fig. 2, lower), were infected with a series of AdVs carrying shRNAs as described above and the numbers of HCV RNA copies in the cells were determined. We obtained results that were very similar to those obtained using HuH 5–15: the viral RNA level in cells infected with VA-deleted AdVs expressing sh277 or sh331 at MOI 2 or MOI 10 was significantly lower than that in cells infected with the corresponding FG AdVs, respectively (Fig. 3b, bars 3 to 10). Thus, these results, together with those obtained in HuH5–15 cells, demonstrated that VA-deleted AdV expressing shRNA was more effective than FG AdV for the suppression of HCV-RNA replication.
To evaluate whether VA-deleted AdV is advantageous to the anti-HCV effect of IFN, VA-deleted AdV and FG AdV expressing human α2-IFN were also constructed. As shown in Fig. 3, both the VA-deleted AdVs and the FG AdVs enabled the efficient suppression of HCV RNA replication in HuH 5–15 and SGR-JFH1 cells (bars 11 and 12). However, in contrast to the results obtained with shRNAs, no significant difference in the suppression efficiency was observed between these two AdVs, supporting the notion that the antiviral mechanisms of IFN are distinct from those of shRNAs.
Coinfection of a VA-deleted AdV expressing sh277 and a VA-deleted AdV expressing sh331 enabled more efficient suppression than single infection alone
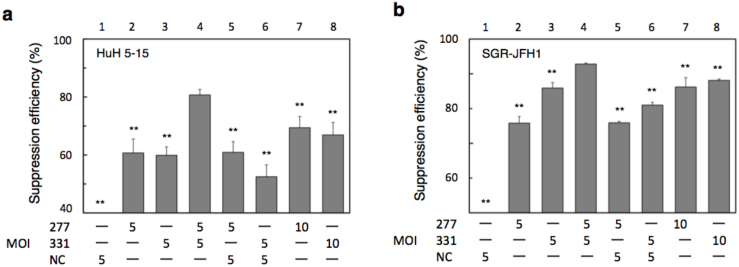
Because the sequences of sh277 and sh331 do not overlap, they may work independently and suppress more efficiently when doubly introduced to cells. Therefore, HuH 5–15 cells were coinfected with a VA-deleted AdV expressing sh277 and a VA-deleted AdV expressing sh331, and the resulting suppression efficiency was compared with that in cells infected with either the sh277 AdV or the sh331 AdV alone (Fig. 4a). As a result, the suppression efficiency of the coinfected AdVs was higher than those of the singly infected AdVs at MOI 5 (compare bar 4 with bars 2, 3, 5 and 6) and at MOI 10 (compare bar 4 with bars 7 and 8). These results were confirmed using SGR-JFH1 (Fig. 4b; compare bar 4 with bars 7 and 8). Interestingly, the coinfection of AdV expressing commercially available control shRNA appeared to decrease the activity of sh331 (Fig. 4a and 4b, bars 3 and 6) but not that of sh277 (Figs. 4a and 4b, bars 2 and 5) in both cell lines. These results might suggest that the control shRNA might compete with sh331, but not with sh277, at some step in siRNA processing.
Figure 4. Suppression of HCV RC RNA by double infection of the shRNA-expressing AdVs.
HuH 5–15 cells (a) and SGR-JFH1 cells (b) were infected with the VA-deleted AdVs expressing shRNAs and, three days later, the intracellular RNA levels of HCV RC were measured. The copy numbers of HCV RC were 5.7 × 104 copies/cell and 1.4 × 104 copies/cell in the HuH 5–15 cells (a) and the SGR-JFH1 cells (b), respectively. The suppression efficiencies of NC FG AdV and NC VA-deleted AdV were (a) 8.7 (±13.6) and (b) 18.7 (±2.0), respectively. **P < 0.01 against the value of the coinfection (bar 6) (a), (b). The other presentations are the same as in Fig. 3.
VA-deleted AdVs expressing shRNA or IFN efficiently suppressed HCV replication in HCV-infected cells
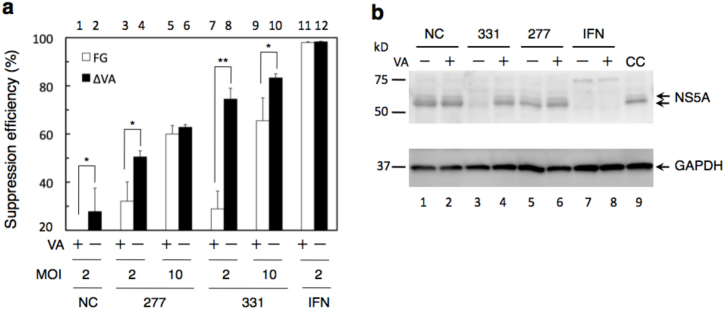
The anti-HCV activity of AdVs expressing shRNA or IFN was further investigated in cells infected with the HCV JFH-1 strain22. The cells were infected with VA-deleted AdVs and FG AdVs expressing sh331, sh277, or IFN. Three days later, the HCV RNA copy number was measured (Fig. 5a). The VA-deleted AdVs expressing sh277 or sh331 exhibited higher anti-HCV activities than the FG AdVs with only one exception: for sh277-expressing AdVs at MOI 10, suppression efficiencies were similar between VA-deleted AdVs and FG AdVs (bars 5 and 6), though at MOI 2 VA-deleted AdV was significantly more efficient than that of FG AdV (bars 3 and 4). The results might be explained that the effect of sh277 may be saturated at MOI 10, because the copy numbers of HCV genome were very low (figure legends of Fig. 5).
Figure 5. Suppression of replicating HCV genome and the expressed proteins in the HCV-infected HuH cells.
(a) Suppression levels of HCV genomes. HCV-infected HuH-7 cells were infected with shRNA-expressing AdVs and interferon-expressing AdVs at MOI 2 (bars 1 to 4, 7, 8, 11 and 12) or at MOI 10 (bars 5, 6, 9 and 10). The copy numbers of HCV RNA were 1.0 × 103 copies/cell. The suppression efficiencies of NC FG AdV and NC VA-deleted AdV were 3.7 (±5.5) and 27.8 (±9.7), respectively. The other presentations are the same as in Fig. 3. (b) Western blot analysis of HCV NS5A protein expressed in the viral-infected cells. Three days after the AdV infection at MOI 10, HCV NS5A and GAPDH proteins were detected using specific antibodies. CC, Cells infected with HCV but not with AdVs expressing the shRNAs. Arrows for NS5A indicate its hyper- (upper) and hypo- (lower) phosphorylated forms.
The expression of the HCV protein NS5A in the cells was determined using immunoblotting (Fig. 5b). The level of NS5A protein in the cells infected with the VA-deleted AdV expressing sh331 was nearly undetectable and was comparable with those for the cells infected with FG AdV and VA-deleted AdV expressing IFN (lanes 3, 7 and 8). Meanwhile, as for sh277 NS5A protein was significantly less when using VA-deleted AdV than that using FG AdV (lanes 5 and 6). However, the difference was not evident compared with sh331 correlating with the suppression efficiency of sh277 at MOI 2 and MOI 10 (Fig. 5a, bars 3 to 6). Altogether, these results demonstrated that, although a limited anti-HCV activity was obtained using the FG AdV, a more efficient shRNA-mediated inhibition of HCV replication was achieved using the VA-deleted AdV.
Discussion
We showed here that the highest shRNA activity was obtained when the shRNA-expressing cassette was inserted at the E4-insertion site in the leftward orientation. This site is located upstream of the E4 promoter and is very close to the right terminal of the vector genome23. The above results appear to be true for the FG AdVs that were used in our preliminary experiments. Although the E4 site offered the best shRNA activity, the E1 insertion site is commonly used for the insertion of shRNA cassettes. In contrast to the above-mentioned findings for shRNAs, the expressions of transgenes under the control of the CMV, CAG, and EF1α promoters at the E4 site were lower than those of transgenes under the control of these promoters at the E1 site (MS and YK, manuscript in preparation). In the present study, we used a polymerase III promoter of the human U6 promoter. A specific sequence that enhances this particular promoter might exist near the E4 site. Alternatively, differences in the position effect between polymerases II and III might explain this difference, at least in part. In either case, the above information is valuable for designing more effective shRNA strategies using AdVs both in vitro and in vivo, including strategies for gene therapy. Notably, the present results suggest that, when one wants to express a transgene product and an shRNA simultaneously, the best AdV is likely to contain the transgene at the E1 position and an shRNA cassette at the E4L position/orientation.
We showed that VA RNAs expressed from currently used FG AdVs decreased the activity of shRNA in the assay system for HCV replication. Because VA RNAs are processed and produce miRNAs, this result can be explained by competition with the shRNAs that are processed using the same pathway. The results also indicated that VA-deleted AdVs are a more efficient vehicle for shRNA strategies than FG AdVs, at least for the suppression of HCV replication. The advantage of using VA-deleted AdVs may not be restricted to the HCV system. Our preliminary experiment showed that, when using a commercially available anti-GFP shRNA, the suppression efficiency of the VA-deleted AdV was slightly higher than that of the FG AdV (85% ± 1.1% and 77% ± 4.1%, respectively) at MOI 200. This level was similar when using SGR-JFH1cells and shRNAs of sh277 and sh331 at MOI 10 (for example, Figs. 3b and 5a, bars 5, 6, 9 and 10). The difference in the suppression efficiency was much more evident when measuring HCV subgenomic and genomic RNAs at MOI 2 (Figs. 3 and 5a, bars 3, 4, 7 and 8). The reason for this finding may be that HCV RNAs are self-replicating and possibly amplified differences in the suppression efficiency, especially in the early step of replication. Alternatively, VA RNAs might increase HCV replication by interacting with some regulation factors, such as mir122. In any case, we demonstrated, for the first time, that VA RNAs expressed in FG AdV did reduce the shRNA activity, at least for HCV RNA.
VA-deleted AdVs are useful not only for shRNA strategies, but also for other purposes, since VA RNAs disturb the RNAi machinery. VA-deleted AdVs with a high titer nearly comparable to FG AdVs can now be obtained using the production method reported by Maekawa et al.12. This production efficiency is sufficient for practical use, even for large-scale preparations, and the production protocol is almost the same as that currently used for FG AdVs. FLP-expressing 293 cells (293hde12) are used in the last two passages to remove the VA RNA genes on the “pre-vector” genome. We expect that VA-deleted AdVs may become as prevalent as FG AdVs. In fact, we no longer use FG AdVs in our laboratory, having replaced them with VA-deleted AdVs instead.
The efficiency at which the HCV RNA genome is inhibited using sh277 and sh331 is almost comparable with that in cells infected with AxEF-IFN, an AdV expressing interferon under the control of the potent EF1α promoter (AM and YK, unpublished results). Because the inhibition mechanisms of shRNA and interferon differ, they are likely to work independently. Therefore, as noted above, VA-deleted AdVs containing both an IFN-expressing unit located at the E1 position and an anti-HCV shRNA at the E4L position/orientation might serve as powerful vectors for gene therapy for hepatitis C. Although high immune responses are a major concern with AdVs, we have reported that this problem can be overcome, at least in part, by avoiding the aberrant expression of the viral pIX gene arising from the use of the CAG or CMV promoter. We have called this type of AdV a “low inflammatory AdV”24. Thus, the combination of these improvements, i.e. VA-deleted, low-inflammatory AdVs expressing IFN and shRNA at the position/orientation described above, may yield an effective vector for gene therapy for chronic hepatitis C. The examination of this possibility is underway.
Methods
Cells and vector titration
FG AdVs were prepared using 293 cells25, which are derived from human embryonic kidney and constitutively express adenoviral E1 genes and support the replication of E1-substituted AdVs. VA-deleted AdVs were prepared according to the method using 293hde12 cells described by Maekawa et al.12 or using 293U6VA-1 cells that constitutively express both VAI and VAII. The VA-deleted AdVs in this study grew efficiently in this cell line. To establish 293U6VA-51 cell line we constructed a plasmid pU6VA-SVPur. This plasmid contains VAI and VAII genes located downstream of human U6 promoter and puromycin gene under the control of SV40 early promoter. 293 cells were transfected with pU6VA-SVPur and puromycin-resistant cell clones were isolated. Among these clones 293U6VA-51 expressed highest level of VA RNAs measured using qPCR primers described in Maekawa et al.12 FC-18 cells20 are derived from the monkey kidney cell line CV1 and conditionally express GFP: GFP expression can be turned off by infection with AxCAhFLPe, which expresses a codon-humanized FLPe.26.
The human hepatocarcinoma cell line HuH-7 and its derived-HCV replicon cells were cultured in DMEM supplemented with nonessential amino acids and 10% fetal calf serum. HuH 5–15 cells support the replication of the subgenomic HCV replicon derived from the genotype 1b, Con 1 strain in HuH-7 cells21. pSGR-LucNeo-JFH1 plasmid was constructed by inserting the firefly luciferase gene into the 5′ end of the neomycin phosphotransferase gene of pSGR-JFH1 carrying subgenomic RNA from the genotype 2a, JFH-1 strain27 and was introduced into HuH-7 cells. SGR-JFH1/LucNeo cells (simply called SGR-JFH1 in this paper) were developed after 1 month of culture with 0.5 mg/mL of G418-containing medium. The VA-deleted AdVs and FG AdVs were titrated using the methods described by Pei et al.28. Briefly, the copy numbers of a viral genome that was successfully transduced into infected target cells, (HeLa cells in this study) were measured using real-time PCR (relative virus titer: rVT). The titer of the standard virus was determined using the copy number of serially diluted plasmid DNA. When FG AdVs are used, the rVT (copies/mL) normally corresponds to about one fifth of the TCID50 titer, when the gene product is not deleterious; the reason for this difference is probably that the transduction efficiency of 293 cells is exceptionally higher than that of the other cells.
Quantitative real-time PCR
The copy numbers of the expressed Cre RNA were quantified using the primers and the probe: forward primer, 5′-ATCCAGCAACATTTGGGCC-3′; probe, 5′-CGACAAGCAGAAGAACGGCATCAAGG-3′; reverse primer 5′-ACGACCAAGTGACAGC-3′. HCV RNA copies in the replicon cells and HCV-infected cells were determined using the primers and the probe: forward primer, 5′-GAGTGTCGTGCAGCCTCCA -3′; probe, 5′-CTGCTAGCCGAGTAGTGTTGG -3′; reverse primer 5′-CACTCGCAAGCACCCTATCA -3′. The sequences of the primers and the probe for quantifying GFP RNA have been reported12. The total RNA of the infected cells was extracted, and the amounts of the expressed target RNA and 18S-rRNA (correction standard) were quantified using reverse-transcription and real-time PCR (Applied Biosystems Prism 7000), and the ratio of the target RNA to 18S-rRNA was then calculated. The qPCR reaction was performed according to the manufacturer's protocol: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min (Applied BioSystems).
Immunoblotting
Cells were lysed with the lysis buffer (1% Triton X-100, 25 mM Tris [pH7.5], 150 mM NaCl, 1 mM EDTA, protease inhibitor cocktail and phosphatase inhibitor cocktail [Roche, Basel, Switzerland]) and were diluted 1:2 (v/v) with 3 × sampling buffer after removing the cell debris by centrifugation. The cell lysates were separated using SDS-PAGE and were transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore, Bedford, MA, USA). After blocking in 4% BlockAce (DS Pharma Biomedical, Osaka, Japan), the blots were incubated with the respective primary antibodies, followed by the secondary antibody in TBST (25 mM Tris, [pH7.5], 150 mM NaCl, and 0.1% Tween 20). The primary antibodies that were used were anti-NS5A antibody29 and anti-GAPDH antibody (clone 6C5; Santa Cruz, CA, USA). Anti-rabbit IgG, HRP-linked antibody and anti-mouse IgG, HRP-linked antibody (Cell Signaling Technology, Danvers, MA, USA) were used as a secondary antibodies. Finally, the proteins were visualized using an enhanced chemiluminescence (ECL) reagent (ECL Select Western Blotting Detection Reagent;, GE Healthcare, Little Chalfont, UK).
Construction of vector cosmids
All the AdVs described here lacked the expression of VAI and VAII and were constructed using a cosmid cassette, pAxdVw4c, derived from pAxcw containing the full-length AdV genome30 except for the VAI and VAII genes, which were disrupted because of 15-nt and 17-nt deletions in their B-box sequences, respectively12. This cassette contains cloning sites for SwaI in the E1 region and for ClaI upstream of the E4 region23. A cassette expressing shRNA under the control of the human U6 promoter was purchased from Takara Bio. The AdV genome was excised with PacI and was transfected into 293U6VA-51 cells. The cassette cosmids for construction of VA-deleted AdVs are described in Maekawa et al12.
Author Contributions
Z.P. performed most of the experiments. G.S. and M.I. designed the experiments for evaluation of anti-HCV activity of AdV. S.K. discussed the experimental data and helped with troubleshooting. A.M. and M.S. constructed AdV-generating cosmids and continuously encouraged Z.P. I.S. and T.S. advised Z.P., G.S. and M.I. and wrote the manuscript. Y.K. organized this study and performed virological work. All authors read and approved the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We thank K. Nakashima for technical support and helpful discussions, and Ms. T. Shiino for excellent secretarial work. This work was supported in part by grants-in-aids from the Ministry of Health, Labor and Welfare for I.S. and T.S. and from the Ministry of Science, Education, Sports and Culture to Y.K.
References
- Scherr M. & Eder M. Gene silencing by small regulatory RNAs in mammalian cells. Cell Cycle 6, 444–449 (2007). [DOI] [PubMed] [Google Scholar]
- Amarzguioui M., Rossi J. J. & Kim D. Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS. Lett. 579, 5974–5981 (2005). [DOI] [PubMed] [Google Scholar]
- Liu Y. P. & Berkhout B. miRNA cassettes in viral vectors: problems and solutions. Biochim. Biophys. Acta. 1809, 732–745 (2011). [DOI] [PubMed] [Google Scholar]
- Mowa M. B., Crowther C. & Arbuthnot P. Therapeutic potential of adenoviral vectors for delivery of expressed RNAi activators. Expert Opin. Drug Deliv. 7, 1373–1385 (2010). [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Induction of stable RNA interference in mammalian cells. Gene Ther. 13, 503–508 (2006). [DOI] [PubMed] [Google Scholar]
- Lu S. & Cullen B. R. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J. Virol. 78, 12868–12876 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero E., Sutherland J. D. & Fortes P. Adenovirus and miRNAs. Biochim. Biophys. Acta. 1809, 660–667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio O. et al. Adenovirus VA RNA-derived miRNAs target cellular genes involved in cell growth, gene expression and DNA repair. Nucleic Acids Res. 38, 750–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. et al. Induction of type I interferon by adenovirus-encoded small RNAs. Proc. Natl. Acad. Sci. USA 107, 17286–17291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamitani T., Iwakiri D. & Takada K. Adenovirus virus-associated RNAs induce type I interferon expression through a RIG-I-mediated pathway. J. Virol. 85, 4035–4040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machitani M. et al. Development of an adenovirus vector lacking the expression of virus-associated RNAs. J. Control Release 154, 285–289 (2011). [DOI] [PubMed] [Google Scholar]
- Maekawa A. et al. Efficient production of adenovirus vector lacking genes of virus-associated RNAs that disturb cellular RNAi machinery. Sci. Rep. 3, 1136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R. & Sparacio S. Hepatitis C virus molecular clones and their replication capacity in vivo and in cell culture. Virus Res. 127, 195–207 (2007). [DOI] [PubMed] [Google Scholar]
- Pezacki J. P., Singaravelu R. & Lyn R. K. Host-virus interactions during hepatitis C virus infection: a complex and dynamic molecular biosystem. Mol. Biosyst. 6, 1131–1142 (2010). [DOI] [PubMed] [Google Scholar]
- Suzuki T., Ishii K., Aizaki H. & Wakita T. Hepatitis C viral life cycle. Adv. Drug Deliv. Rev. 59, 1200–1212 (2007). [DOI] [PubMed] [Google Scholar]
- Yokota T. et al. Inhibition of intracellular hepatitis C virus replication by synthetic and vector-derived small interfering RNAs. EMBO Rep. 4, 602–608 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N. et al. Inhibition of hepatitis C virus infection and expression in vitro and in vivo by recombinant adenovirus expressing short hairpin RNA. J. Gastroenterol Hepatol. 23, 1437–1447 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D. & Kay M. A. Therapeutic short hairpin RNA expression in the liver: viral targets and vectors. Gene Ther. 13, 563–575 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Down-regulation of viral replication by adenoviral-mediated expression of siRNA against cellular cofactors for hepatitis C virus. Virology 320, 135–143 (2004). [DOI] [PubMed] [Google Scholar]
- Kondo S. et al. Efficient sequential gene regulation via FLP-and Cre-recombinase using adenovirus vector in mammalian cells including mouse ES cells. Microbiol. Immunol. 50, 831–843 (2006). [DOI] [PubMed] [Google Scholar]
- Lohmann V. et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285, 110–113 (1999). [DOI] [PubMed] [Google Scholar]
- Wakita T. et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11, 791–796 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegae Y. et al. High-level expression by tissue/cancer-specific promoter with strict specificity using a single-adenoviral vector. Nucleic Acids Res. 39, e7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M. et al. Expression of pIX gene induced by transgene promoter: possible cause of host immune response in first-generation adenoviral vectors. Hum. Gene. Ther. 18, 925–936 (2007). [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C. & Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59–74 (1977). [DOI] [PubMed] [Google Scholar]
- Kondo S., Takata Y., Nakano M., Saito I. & Kanegae Y. Activities of various FLP recombinases expressed by adenovirus vectors in mammalian cells. J. Mol. Biol. 390, 221–230 (2009). [DOI] [PubMed] [Google Scholar]
- Kato T. et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology 125, 1808–1817 (2003). [DOI] [PubMed] [Google Scholar]
- Pei Z., Kondo S., Kanegae Y. & Saito I. Copy number of adenoviral vector genome transduced into target cells can be measured using quantitative PCR: application to vector titration. Biochem. Biophys. Res. Commun. 417, 945–950 (2012). [DOI] [PubMed] [Google Scholar]
- Murakami K. et al. Virological characterization of the hepatitis C virus JFH-1 strain in lymphocytic cell lines. J. Gen. Virol. 89, 1587–1592 (2008). [DOI] [PubMed] [Google Scholar]
- Fukuda H., Terashima M., Koshikawa M., Kanegae Y. & Saito I. Possible mechanism of adenovirus generation from a cloned viral genome tagged with nucleotides at its ends. Microbiol. Immunol. 50, 643–654 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information