Abstract
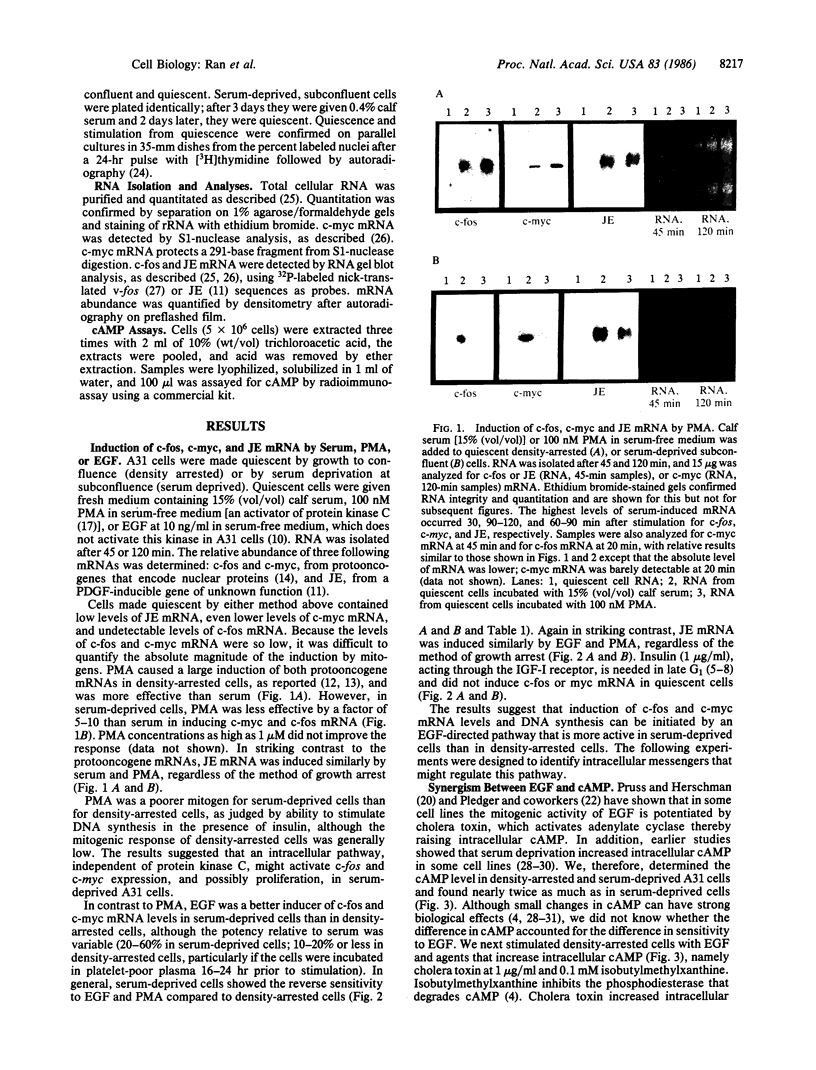
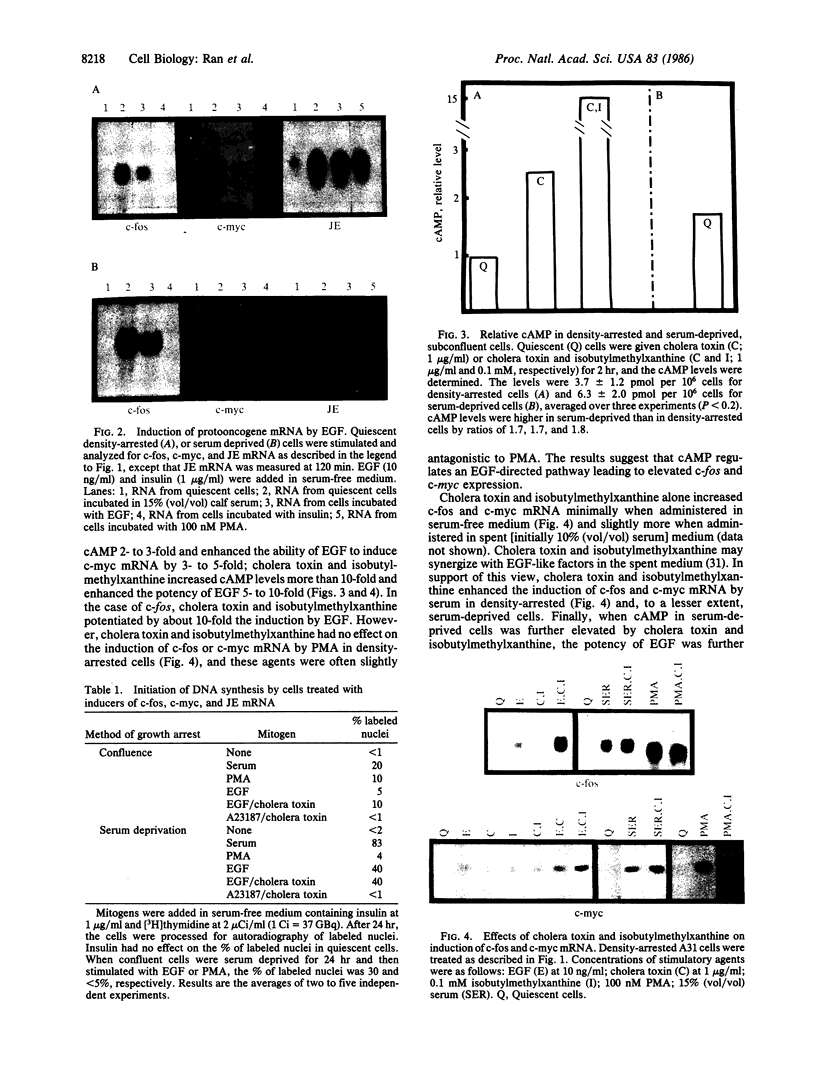
Phorbol esters activate protein kinase C and induce expression of the c-fos and c-myc protooncogenes in density-arrested BALB/c 3T3 (A31) cells; in contrast, epidermal growth factor (EGF) does not activate protein kinase C and is a poor inducer of c-fos and c-myc in these confluent cells. We show that, when A31 cells were subconfluent and made quiescent by serum deprivation, the phorbol ester phorbol 12-myristate 13-acetate induced c-fos and c-myc mRNA poorly, whereas EGF was a better inducer. Another platelet-derived growth factor-inducible gene, JE, did not show this differential regulation by phorbol 12-myristate 13-acetate and EGF. The ability of EGF to induce protooncogene mRNA was associated with elevated levels of intracellular cAMP. First, serum-deprived cells maintained cAMP at about 2-fold higher level than density-arrested cells. Second, induction was greatly enhanced by cholera toxin and 3-isobutyl-1-methylxanthine, which increased intracellular cAMP 3- to 10-fold. The calcium ionophore A23187 mimicked EGF in that it elevated c-fos and c-myc mRNA when administered with cholera toxin and isobutylmethylxanthine. Neither cholera toxin and isobutyl-methylxanthine nor A23187 appreciably induced these mRNAs when used alone. Our results suggest that c-fos and c-myc expression can be regulated by an EGF-directed pathway that utilizes calcium and cAMP as cooperating cytoplasmic messengers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bottenstein J., Hayashi I., Hutchings S., Masui H., Mather J., McClure D. B., Ohasa S., Rizzino A., Sato G., Serrero G. The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 1979;58:94–109. doi: 10.1016/s0076-6879(79)58127-0. [DOI] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E., Morreo G., Pardee A. B. Restriction point control of cell growth by a labile protein: evidence for increased stability in transformed cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):436–440. doi: 10.1073/pnas.79.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. H., Hoban C. J., Owen A. J., Geyer R. P. Platelet-derived growth factor stimulates rapid polyphosphoinositide breakdown in fetal human fibroblasts. J Cell Physiol. 1985 Sep;124(3):391–396. doi: 10.1002/jcp.1041240306. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Campisi J. c-myc regulation during retinoic acid-induced differentiation of F9 cells is posttranscriptional and associated with growth arrest. Mol Cell Biol. 1986 Feb;6(2):518–524. doi: 10.1128/mcb.6.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox M. K., Magun B. E., Russell D. H. Differential expression of type I and type II cyclic AMP-dependent protein kinases during cell cycle and cyclic AMP-induced growth arrest. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3445–3449. doi: 10.1073/pnas.77.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Van Wyk J. J., O'Keefe E. J., Pledger W. J. Epidermal growth factor (EGF) is required only during the traverse of early G1 in PDGF stimulated density-arrested BALB/c-3T3 cells. Exp Cell Res. 1983 Aug;147(1):202–208. doi: 10.1016/0014-4827(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., O'Keefe E., Pledger W. J. Elevated intracellular concentrations of cyclic AMP inhibited serum-stimulated, density-arrested BALB/c-3T3 cells in mid G1. J Cell Biochem. 1982;19(1):93–103. doi: 10.1002/jcb.240190108. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- Moens W., Vokaer A., Kram R. Cyclic AMP and cyclic GMP concentrations in serum- and density-restricted fibroblast cultures. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1063–1067. doi: 10.1073/pnas.72.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Oey J., Vogel A., Pollack R. Intracellular cyclic AMP concentration responds specifically to growth regulation by serum. Proc Natl Acad Sci U S A. 1974 Mar;71(3):694–698. doi: 10.1073/pnas.71.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olashaw N. E., Leof E. B., O'Keefe E. J., Pledger W. J. Differential sensitivity of fibroblasts to epidermal growth factor is related to cyclic AMP concentration. J Cell Physiol. 1984 Mar;118(3):291–297. doi: 10.1002/jcp.1041180312. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F. Growth-dependent synthesis of c-myc-encoded proteins: early stimulation by serum factors in synchronized mouse 3T3 cells. Mol Cell Biol. 1985 Nov;5(11):2903–2912. doi: 10.1128/mcb.5.11.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. B. Cholera toxin stimulates division of 3T3 cells. J Cell Physiol. 1979 Mar;98(3):469–474. doi: 10.1002/jcp.1040980305. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Wang T., Sheppard J. R., Foker J. E. Rise and fall of cyclic AMP required for onset of lymphocyte DNA synthesis. Science. 1978 Jul 14;201(4351):155–157. doi: 10.1126/science.208147. [DOI] [PubMed] [Google Scholar]
- Wharton W., Leof E. B., Olashaw N., Earp H. S., Pledger W. J. Increases in cyclic AMP potentiate competence formation in BALB/c-3T3 cells. J Cell Physiol. 1982 May;111(2):201–206. doi: 10.1002/jcp.1041110212. [DOI] [PubMed] [Google Scholar]











