Abstract
Skilled motor control is regulated by the convergence of somatic sensory and motor signals in brain and spinal motor circuits. Cervical deafferentation is known to diminish forelimb somatic sensory representations rapidly and to impair forelimb movements. Our focus was to determine what effect deafferentation has on the motor representations in motor cortex, knowledge of which could provide new insights into the locus of impairment following somatic sensory loss, such as after spinal cord injury or stroke. We hypothesized that somatic sensory information is important for cortical motor map topography. To investigate this we unilaterally transected the dorsal rootlets in adult rats from C4 to C8 and mapped the forelimb motor representations using intracortical microstimulation, immediately after rhizotomy and following a 2-week recovery period. Immediately after deafferentation we found that the size of the distal representation was reduced. However, despite this loss of input there were no changes in motor threshold. Two weeks after deafferentation, animals showed a further distal representation reduction, an expansion of the elbow representation, and a small elevation in distal movement threshold. These changes were specific to the forelimb map in the hemisphere contralateral to deafferentation; there were no changes in the hindlimb or intact-side forelimb representations. Degradation of the contralateral distal forelimb representation probably contributes to the motor control deficits after deafferentation. We propose that somatic sensory inputs are essential for the maintenance of the forelimb motor map in motor cortex and should be considered when rehabilitating patients with peripheral or spinal cord injuries or after stroke.
Introduction
The corticospinal system and limb proprioceptive afferents coordinate their limb movement control functions. The corticospinal tract (CST), which originates largely in primary motor cortex (M1), and proprioceptive afferents converge onto common sets of spinal interneurons as part of limb movement control circuits and reflex regulation (Drew et al., 2004). During both development and maturity, the CST and proprioceptive afferents interact to establish and maintain their spinal connections (Gibson et al., 2000; Clowry et al., 2004; Chakrabarty et al., 2009b; Chakrabarty & Martin, 2011; Tan et al., 2012). Signals from each of these inputs can significantly affect the other: the CST can modulate the central actions of proprioceptive afferents (Chakrabarty & Martin, 2010) and proprioceptive afferents can modulate CST spinal signals or influence CST spinal terminations (Martin et al., 2004; Jackson et al., 2006; Chakrabarty & Martin, 2010; Darian-Smith et al., 2013). From the perspective of corticospinal motor control, proprioceptive afferents may interact with the CST to regulate the efficacy of their descending signals, from M1 to muscle (Bretzner & Drew, 2005b). Proprioceptive afferents could play an important role in establishing the topographic organization of M1. Depending on whether afferents facilitate or suppress CST spinal signals, this could lead to a corresponding expansion or contraction of the motor representation and, in turn, promote or limit motor skill (Nudo, 2003; Monfils et al., 2005).
The overall focus of this study is the role of somatic sensory afferents in cortical motor control. While not limited to impairments in cortical motor control, in rats deafferentation produces manipulation impairments (Saling et al., 1992). Deafferentation in humans, produces severe, if not paralyzing, limb impairments (Marsden et al., 1984). Loss of control is more than loss of afferent feedback; there are also major defects in motor planning (Ghez et al., 1982). We examined changes in the M1 forelimb motor representation in rats produced by cervical deafferentation. The M1 motor map has a well-defined topographic organization, within which are located groups of neurons related to sets of muscles or movements about individual joints (Donoghue & Wise, 1982; Neafsey et al., 1986; Stout & Beloozerova, 2012). Whereas deafferentation has been an important tool in elucidating sensory representational changes in primary somatic sensory cortex (Wall, 1976; Franck, 1980; Darian-Smith & Brown, 2000), it is not known what effects deafferentation have on the M1 motor representation. Transcranial magnetic stimulation (TMS) can be used to probe the M1 motor map in humans, but to our knowledge it has never been used to examine M1 motor map changes in deafferented patients. Peripheral nerve lesions, which sever both sensory and motor axons, result in the loss of the denervated body part’s representation and expansion of the representation of adjacent non-affected parts (Sanes et al., 1990; Keller et al., 1996; Huntley, 1997; Sanes & Donoghue, 1997; Nudo, 2003). Importantly, motor nerve injury alone produces expansion of adjacent non-affected regions (Sanes et al., 1988; Donoghue et al., 1990; Sanes et al., 1990), implying a greater role for efferent than afferent regulation of the M1 motor map.
These studies show the modifiability of the M1 motor representation in response to peripheral nerve lesions, but none have examined the effect of selective somatic sensory loss. This is important both for understanding the somatic afferent regulation of cortical limb control and also because the loss of afferent input to the spinal cord occurs as part of spinal cord injury. Somatic sensory afferents are a major input to spinal circuits. Loss of afferent input would be expected to result in a substantial loss of motor circuit excitability.
To determine the somatic sensory regulation of the M1 motor representation, we transected unilaterally the cervical C4–C8 dorsal rootlets in adult rats. We used intracortical microstimulation (ICMS) to map M1 in each hemisphere; immediately after deafferentation and, to examine persistence, 14 days later. We also investigated how somatic sensory information regulates the capacity for M1 to produce particular limb movements by determining changes in the threshold to evoke forelimb movements after loss of this major spinal excitatory input. Our starting hypothesis was that partial forelimb deafferentation would both impair the forelimb motor map and substantially elevate forelimb movement thresholds. We found an immediate and persistent reduction in the distal forelimb representation, and a complementary expansion of the proximal representation, after C4–C8 deafferentation. Surprisingly, despite elimination of a major excitatory input to the spinal cord, deafferentation had a minimal effect on the M1 threshold to evoke movements. Our data suggest that somatic sensory input is critical for maintenance of the M1 distal forelimb representation, but not for regulating excitability of the corticospinal motor pathway.
Material and methods
Animals
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All animal protocols were approved by the City College of the City University of NY Institutional Animal Care and Use Committee. Adult male Sprague–Dawley rats (200–225g) were used for this study. Animals were pair-housed under a 12-h light–dark cycle in a pathogen-free area with water and food provided ad libitum. All surgeries were performed under general anesthesia (80 mg/kg ketamine, 10 mg/kg xylazine, via intraperitoneal injection) and aseptic conditions. Anesthesia was maintained using intraperitoneal ketamine injections to render the animal unresponsive to paw pinch. In all surgeries, the animals’ body temperature was maintained at 39°C by a heating pad.
Dorsal rootlet rhizotomy (DRR; C4–C8)
Animals were fixed in a stereotaxic frame under general anesthesia, as described above. Using the vertebral spiny protuberance on T2 as a landmark, the left C3 to T1 cervical spinal cord segments were exposed by hemi-laminectomy, and the dura was cut. The left C4–C8 dorsal rootlets were clearly identified and carefully cut to avoid any damage to spinal cord (termed DRR). We verified that each rootlet was cut by gently separating the distal and proximal stumps. In addition, in a separate set of animals we traced muscle afferents bilaterally to verify the deafferentation procedure (see next section and Results). We examined DRR animals either immediately after surgery (termed immediate) or 2 weeks later. For 2-week animals, the overlying tissues were then sutured in layers. Sham surgeries were performed in which the dorsal rootlets were isolated as indicated above but without transection. Postoperative treatment included subcutaneous injection of 0.9% saline solution for rehydration and 5 mg/ml Carprofen (Rimadyl) for analgesia. None of the animals showed any signs of self-inflicted lesions.
Muscle afferent tracing
As a way to assess completeness of deafferentation, 7 days after DRR, we made bilateral injections of the transganglionic tracer cholera toxin B subunit (CTB; List Biological Laboratories, 1% in distilled water) into the surgically exposed extensor carpi radialis (ECR) or biceps brachii muscles to label muscle afferents. A volume of 10 μl was slowly injected into multiple intramuscular locations using a Hamilton syringe (24-gauge). We have optimized the CTB injections to maximally label the motoneurons of injected muscles, taking advantage of recent findings that many forelimb motor pools have an extended rostrocaudal distribution (Tosolini et al., 2013).We inserted the Hamilton syringe into the muscle, advanced it along the long axis of the muscle, and injected tracer as the needle was withdrawn. To maximally label the muscle, we re-advanced the needle and injected more tracer. We verified that there was no leakage of CTB. The solution was tinted with the dye Evans blue and, after removal of the needle, any residual tracer was blotted with a cotton swab and washed with saline. Further, the needle was held within the muscle for 2 min after the last injection (Tan et al., 2012).
Spinal tissue preparation and CTB staining
Fourteen days after DRR, animals were deeply anesthetized and perfused with saline followed by 4% paraformaldehyde. The spinal cords were removed and postfixed in 4% paraformaldehyde at room temperature for 2–3 hours, and then transferred to a solution of 20% sucrose in 0.1 M PBS and stored at 4° C. Frozen tissues were cut transversely at 40 µm thickness using a sliding microtome. All staining was conducted on free-floating section, and sections were mounted on gelatin-coated slides. Sections of C3 to T1 spinal cord were processed for CTB labeling.
Immunofluorescence staining of CTB has been described previously (Tan et al., 2012). Briefly, free-floating sections were incubated at room temperature (RT) in PBS containing goat anti-CTB primary antibody (1: 1000; List Biologicals) in blocking buffer (3% donkey serum in 1 · PBS with 0.2% Tween, pH 7.4). After rinsing, sections were incubated at RT in blocking buffer containing donkey anti-goat secondary antibody conjugated to FITC (1:500; at RT for 2 h), sections were mounted on gelatin-coated slides, air-dried and coverslipped with Vectashield (Vector Laboratories). Finally, sections were visualized and digitally imaged using a Nikon eclipse 80i fluorescence microscope equipped with an HQ Coolsnap camera (Roper Scientific).
ICMS
ICMS mapping of the forelimb representation in both hemispheres was performed as in our previous study (Brus-Ramer et al., 2009). Anesthesia was induced with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and maintained using intraperitoneal ketamine injections to render the animal unresponsive to paw pinch. Ketamine anesthesia maintains muscle tone and therefore is well suited for motor mapping studies. Animals were placed in a stereotaxic frame. A craniotomy was made to expose the forelimb [both rostral forelimb area (RFA) and caudal forelimb area (CFA)] and hindlimb areas of motor cortex of both hemispheres. We used low-impedance tungsten microelectrodes (Microprobe, Inc.; 0.1 MOhm impedance; 0.081 mm shaft diameter, 1–2 µm tip diameter). Electrode penetrations were made perpendicular to the pial surface and spaced 0.5 mm apart. We mapped motor cortex in both hemispheres.
Stimuli (45 ms duration train, 330 Hz, 0.2 ms biphasic; every 2 s) were delivered using a constant-current stimulator (A-M Systems). We noted the motor effects produced by ICMS at threshold. The threshold is defined as the lowest current that produced a motor effect on 50% of trials. Typically the evoked movements at threshold were at the same depths at which we recorded multiunit activity with the largest amplitude spikes (typically 1.1–1.6 mm below the pial surface). For a given site, we started at a low current and increased the current until a contralateral response was evoked. We then increased the current to a suprathreshold level and lowered the current until the response was lost. As we obtained the motor map within motor cortex, we randomized placement of the electrode to prevent biasing our results by anesthesia level or other state-dependent changes between the two hemispheres. A maximal current of 100 µA was used. Forelimb posture during stimulation was the same for all experiments, with the shoulder and elbow extended and the wrist flexed in the prone position. We conducted experiments on four groups of animals: (i) immediately after DRR (surgery completed within 2–6 hours after C4–C8 DRR; n=4); (ii) immediately after sham surgery (2–6 h after surgery to expose the rootlets; n=4); (iii) 2 weeks after DRR (n=5); and (iv) 2 weeks after sham surgery (n=2).
Statistics
Statistical analyses were performed using Prism 5 (Graph Pad) for the Macintosh computer and Excel (Microsoft). The difference of representation area between two hemispheres within individual groups was tested with paired Student’s t-tests. The differences in area and threshold between groups were tested with one-way or two-way ANOVA and post hoc analyses.
Results
We chose to section the C4–C8 dorsal rootlets (discussed further in next section) because these are the principal segments containing motoneurons innervating shoulder, elbow and wrist muscles in rats (McKenna et al., 2000; Tosolini & Morris, 2012). Motoneurons innervating axial muscles (e.g., acromiotrapezius) and wrist muscles (e.g., ECR brevis) are located rostral and caudal to these levels, respectively. After C4–C8 unilateral DRR, all animals were able to move the deafferented limb as soon as they recovered from anesthesia. During locomotion or while maintaining a prone posture, they often plantarflexed the deafferented paw and wrist; the digits were never clenched. Although neither systematically studied nor quantified, we found that forelimb control in these animals was severely impaired during exploratory reaching movements and horizontal ladder walking, similar to prior studies (Saling et al., 1992; Wu et al., 2009). Overground locomotion was less impaired.
C4–C8 unilateral DRR largely eliminated forelimb muscle afferent projections into the affected cervical dorsal horn
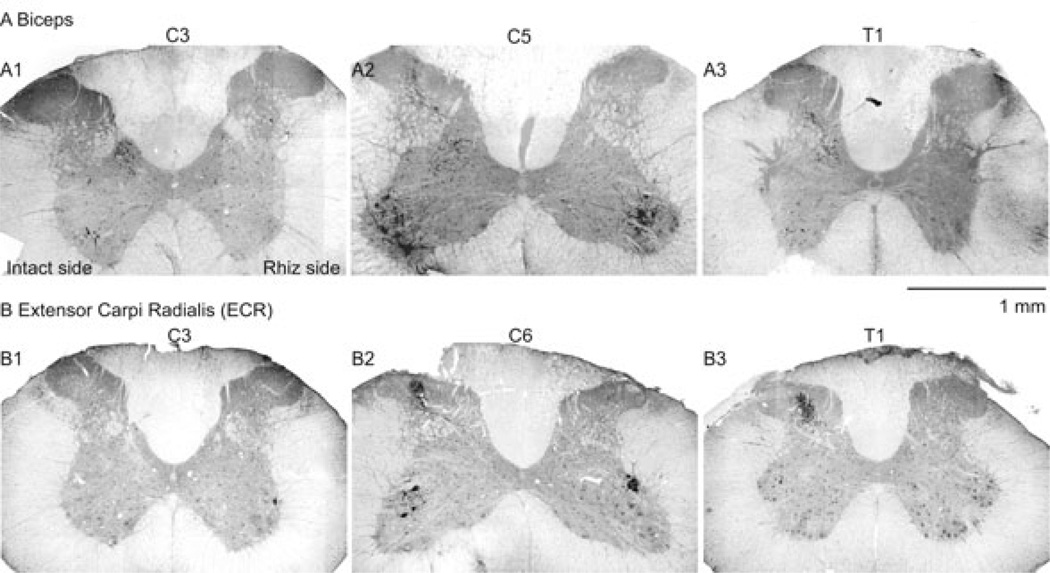
We monitored the spinal terminations of selected forelimb muscles as an indication of the loss of forelimb afferent input after the C4–C8 deafferentation. Intramuscular injection of CTB labels proprioceptive afferents transganglionically and motor neurons retrogradely. We made bilateral injections into biceps and ECR after dorsal rhizotomy to verify the loss of forelimb afferents (n=4 rats for ECR; n=4 rats for biceps). The rostrocaudal and dorsoventral distributions of motor neuron columns on both the intact and rhizotomy sides were similar to those reported by others in the rat (McKenna et al., 2000; Tosolini & Morris, 2012), with biceps motor neurons located in the ventral motor pool from C4 to C5 (McKenna et al., 2000), and ECR motor neurons in the dorsolateral pool from C4 to C6 (McKenna et al., 2000; Tosolini & Morris, 2012). CTB labeling of proprioceptive afferents from both biceps and ECR extended from C3 to T1 on the intact side (contralateral to rhizotomy; Fig. 1A and B, left). Representative images of proprioceptive afferent labeling in C5–C6 spinal cord are shown in Fig. 1, A2 (biceps) and B2 (ECR), showing complete elimination of biceps and ECR proprioceptive afferents on the lesioned (right) side of spinal dorsal horn 14 days after C4–C8 DRR. The proprioceptive afferent labeling in C3 (Fig. 1, A1 and B1) and T1 (Fig. 1, A3 and B3), which are, respectively, rostral and caudal to the rhizotomy segments, was largely eliminated. The substantial decrease in CTB labeling of biceps and ECR proprioceptive afferents within the levels of rhizotomy, as well as rostral and caudal, confirms what we determined during surgery, that the C4–C8 dorsal rootlets were fully sectioned. Further, motoneurons of both groups (biceps in Figure 1, A2, ECR in Figure 1, B2) showed symmetrical labeling in both sides of the spinal cord at C6 segment, showing that there was no damage to the motor roots or motoneurons during the rhizotomy surgery. Thus, using our highly sensitive transganglionic labeling method, we showed substantial loss of proprioceptive afferents within and beyond the region of rhizotomy. This validates the deafferentation surgery.
Figure 1.
CTB labeling of (A) biceps and (B) ECR muscles indicate substantial loss of proprioceptive afferents in the spinal dorsal horn after C4-C8 DRR. Fluorescence micrographs are shown; CTB immunoreactivity is black. The right side of the sections in this figure corresponds to the side of the rhizotomy. There was complete loss of labeling at C5 (compare intermediate zones on intact and deafferented sides). Note also that C4-C8 rootlet section eliminated (e.g., A3) or largely eliminated labeling at levels rostral and caudal to dorsal rootlet section. A1 and B1 are sections through the third cervical (C3) segment; A2 and B2, through the fifth cervical segment (C5); A3 and B3 are sections through the first thoracic segment (T1). Scale bar, 1 mm.
Rhizotomy reduced but did not change the location or total area of forelimb motor representations in contralateral M1
ICMS motor mapping was applied to M1 to determine the extent of forelimb and hindlimb representations and movement thresholds in sham, immediate DRR and 2-week DRR rats. We mapped M1 between 3.0 mm caudal to and 5.5 mm rostral to bregma, and 0.5 and 5.0 mm lateral to midline. This area includes both RFA and CFA, and also hindlimb areas as control representations (Kartje-Tillotson et al., 1986). Although we did not map the vibrissae and face areas systematically, we used non-forelimb areas to define the boundaries of forelimb and hindlimb areas.
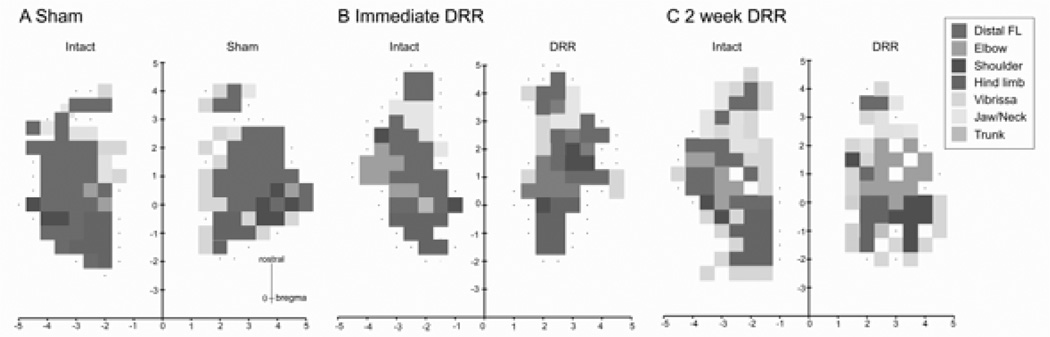
Figure 2 shows representative motor maps obtained from animals with sham surgery (A), immediately after DRR (B) and 2 weeks after DRR (C). For both the sham and deafferented animals, we examined the contralateral motor effects produced by ICMS of M1 in both hemispheres. Ipsilateral effects were present at higher stimulation currents (Brus-Ramer et al., 2009), but were not systematically examined. The vast majority of forelimb sites in the three groups were located within an area from about bregma to close to the frontal pole. This region included both the large CFA and the small RFA. CFA and RFA are typically separated by non-responsive sites or sites where neck, jaw and/or vibrissa movements are evoked (Neafsey et al., 1986). For all animals, and for each side, medially and laterally, the forelimb area is bordered by either vibrissa or non-responsive sites. The hindlimb area is just medial and caudal to CFA. For the sham groups, the size and topography of the motor maps on the two sides were similar. For the DRR rats, the distal forelimb representation was smaller and the proximal representation was correspondingly larger on the rhizotomy side compared with the intact side. Note that there was no change in the sizes of the overall forelimb and hindlimb representations. The total area of the CFA, RFA and total forelimb and hindlimb representations in both hemispheres remained the same after either immediate DRR or 2 weeks after DRR compared to the sham group (Table 1; ANOVA, CFA: F4,19 = 0.3173, P = 0.8629; RFA: F4,19 = 2.209, P = 0.1067; total forelimb: F4,19 = 0.6024, P = 0.6656; hindlimb: F4,19 = 0.4882, P = 0.7443).
Figure 2.
Representative motor maps obtained using intracortical microstimulation of the motor cortex in each hemisphere of individual (A) sham, (B) immediate and (C) 2-week rhizotomy rats. Sites that evoked different joint movements are shown in different colors (see legend). No response is indicated by dots. Note that in the sham animal distal forelimb sites are dominant in the forelimb area compared with fewer numbers of proximal response sites. By contrast, immediately and 2 weeks after unilateral DRR there was a reduction in the number of distal sites and an increase in the number of proximal sites.
Table 1.
Summary of motor map size.
| Group | RFA | CFA | Total FA | HA |
|---|---|---|---|---|
| Sham | 0.67 ± 0.14 | 5.58 ± 0.69 | 6.25 ± 0.75 | 2.04 ± 0.36 |
| Imimed DRR - intact | 1.19 ± 0.21 | 5.75 ± 0.76 | 7.00 ± 0.67 | 2.44 ± 0.43 |
| Immed DRR - rhiz | 0.88 ± 0.16 | 4.50 ± 0.81 | 5.37 ± 0.94 | 2.37 ± 0.39 |
| 2 week DRR - intact | 1.80 ± 0.60 | 5.25 ± 0.94 | 7.05 ± 0.77 | 2.00 ± 0.24 |
| 2 week DRR - rhiz | 0.70 ± 0.22 | 5.45 ± 0.79 | 6.15 ± 0.99 | 1.90 ± 0.20 |
RFA = rostral forelimb area; CFA = caudal forelimb area; FA = forelimb area; HA = hindlimb area.
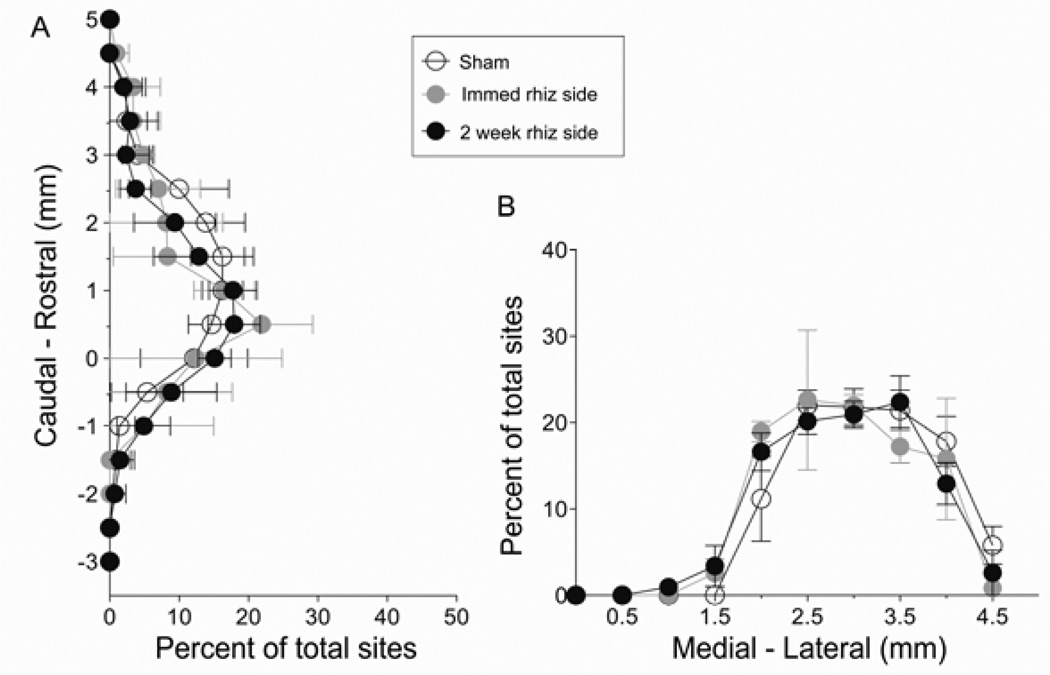
Figure 3 summarizes the distribution of the forelimb sites, aligned according to bregma and the midline. Sites where forelimb movements were evoked were centered at ~ 1 mm rostral to bregma (Fig 3A; rostrocaudal range, –1 to ~ +4 mm). Mediolaterally, the forelimb area range was centered at ~ 3 mm lateral to midline (Fig 3B; mediolateral range, +1.5 to +4.5 mm). We found that the distributions of forelimb sites on the affected side in the sham, immediate and 2-week rhizotomy groups overlapped. Statistical analyses revealed that the distributions of forelimb motor maps after DRR were not different (Fig 3A and B; mediolateral distribution: sham and immediate rhizotomy side, F1,180 = 0.8144, P = 0.60; sham and 2-week rhizotomy side, F1,190 = 0.9908, P = 0.45; rostrocaudal distribution: sham and immediate rhizotomy side F1,136 = 1.201, P = 0.27; sham and DRR rhizotomy side, F1,153 = 1.5, P = 0.106). These results suggest that loss of somatic sensory input from the forelimb has no effect on either the overall size or location of the forelimb motor representation. Further, there was no effect on the size of the adjacent hindlimb representation.
Figure 3.
The location of forelimb area in motor cortex did not change immediately or 2 weeks after deafferentation. (A) Mean percentage of total sites at a given rostral-caudal coordinate; (B) sites along the medial-lateral axis. There were no significant differences between groups.
Rhizotomy rapidly diminished the area of the distal forelimb representation
The most common distal forelimb movement evoked by ICMS is wrist extension, rarely digit flexion; the most common proximal responses are elbow flexion and shoulder flexion, similar to other studies in the rat (Sanes et al., 1992; Brus-Ramer et al., 2009). Normally, there were more sites where distal forelimb movements were evoked (green) than proximal movements (elbow, light blue; shoulder, dark blue; sham; Fig. 2), and they appear to be distributed randomly within the CSA. After DRR, whereas the overall size of the forelimb motor representation did not change, there were fewer distal sites and concomitantly more proximal sites in the hemisphere contralateral to rhizotomy (rhizotomy side) both in the animals with immediate and 2-week DRR, as shown in the representative maps (Fig. 2B and C).
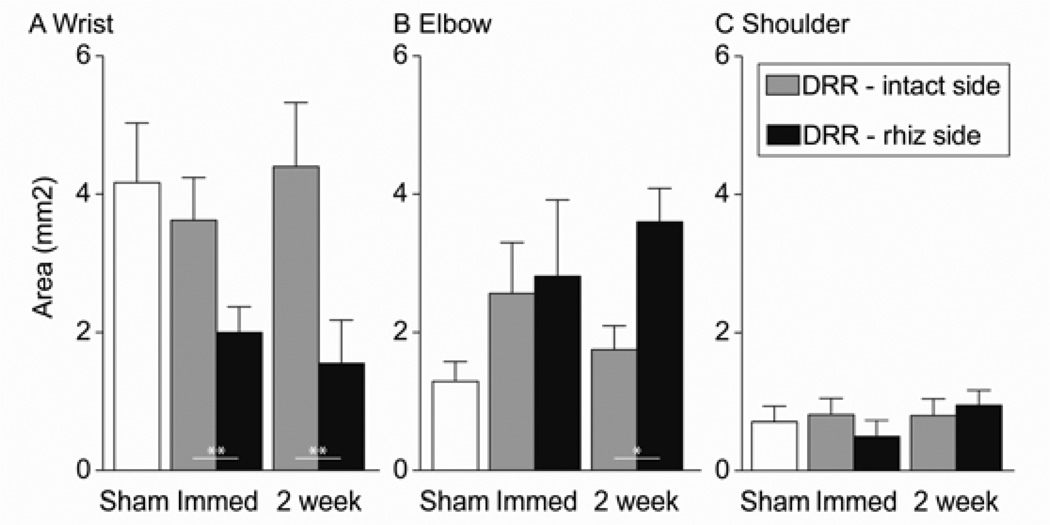
We next compared the size of individual forelimb joint representations between groups. As summarized in Figure 4, the area of distal and proximal joints showed different patterns of reorganization. The wrist area in the contralateral hemisphere was significantly decreased immediately after DRR (44.8% reduction; paired Student’s t-test, P = 0.008 between two hemispheres) and 2 weeks later (64.8% reduction; paired Student’s t-test, P = 0.004 between two hemispheres). The elbow area showed a significant increase in size in the 2-week DRR animals (105.7% increase, paired Student’s t-test, P = 0.05 between two hemispheres), but not in immediate DRR animals (paired Student’s t-test, P = 0.572 between two hemispheres). The lack of a corresponding absolute increase in the elbow representation in immediate rhizotomy animals may be because this group showed a small (albeit not significant) decrease in total forelimb area (5.2±0.9 mm2 for the DRR side versus 7.0±0.7 mm2 for the intact side). The shoulder area, which is the smallest representation among the three forelimb joints, remained unchanged after injury (comparisons between the two hemispheres; paired Student’s t-test, P = 0.517 for the immediate DRR group, 0.710 for the 2-week group).
Figure 4.
Changes in the representational area of individual forelimb joints in contralateral motor cortex in response to immediate DRR and 2 weeks after DRR. Significant decreases in wrist area were found immediately after DRR (paired t-test, **P = 0.008 between intact and rhizotomy sides) and after 2 weeks (paired t-test, **P = 0.004 between intact and rhizotomy sides). A significant increase in elbow area was found 2 weeks after 2-week DRR (paired t-test, *P = 0.05 between intact and rhizotomy sides). The shoulder area did not change.
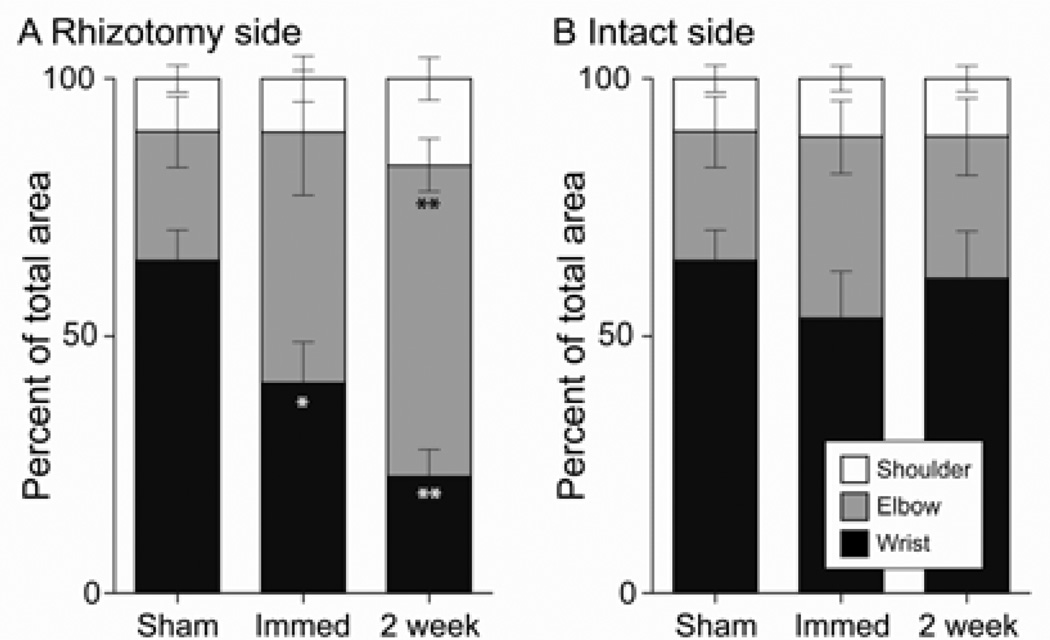
As there was no significant change in overall forelimb representation size, the reduction in the size of the distal representation and associated expansion of proximal representation is more clearly shown as changes in the percentage of the total size of the forelimb representation (Fig. 5). In the hemisphere contralateral to deafferentation (rhizotomy side; Fig. 5A), the percent of the forelimb motor map representing the wrist dropped from 53.35 ± 9.18% on the intact side to 40.85 ± 8.04 % on the rhizotomy side in immediate DRR rats (paired t-test, P = 0.03 between intact and rhizotomy sides), and from 61.10 ± 9.12% on the intact side to 22.73 ± 5.26 % on the rhizotomy side in the 2-week DRR rats (paired t-test, P = 0.003 between the two hemispheres). The elbow representation increased modestly, but not significantly, from 35.28 ± 7.09 % on the intact side to 48.64 ± 12.09 % on the rhizotomy side after immediate DRR. However, in the 2-week DRR group it increased significantly from 27.57 ± 7.52 % on the intact side to 60.40 ± 5.05 % on the rhizotomy side (paired t-test, P = 0. 09 between sham and immediate DRR rhizotomy side, P = 0.001 between sham and 2-week DRR rhizotomy side). The size of the shoulder area did not change (11.37 ± 2.51 % and 10.51 ± 4.46 % for the intact and rhizotomy sides in immediate DRR rats; and 11.33 ± 2.54 % and 16.86 ± 4.17 % for the intact and rhizotomy sides in the 2-week DRR group; paired t-test, P = 0.90 and 0.37 between the two hemispheres in immediate and 2-week DRR groups, respectively). These findings suggest that forelimb somatic sensory input is critical for the maintenance of the somatotopy of the contralateral forelimb motor representation, especially that of distal forelimb joints.
Figure 5.
Percentages of individual joint representations indicate a switch from wrist to elbow on the rhizotomy side with no change on the intact side. Stacked histograms show progressive reduction of the wrist (black) and concomitant expansion of the elbow (gray) representations on (A) the rhizotomy side compared with (B) the intact side. (Paired t-test between the rhizotomy and intact sides, *P = 0.03 for wrist in immediate DRR rats; **P = 0.003 for wrist in 2-week DRR rats; ***P = 0.001 for elbow in 2-week DRR rats).
Unilateral cervical DRR increased distal movement thresholds only in animals 2 weeks after DRR
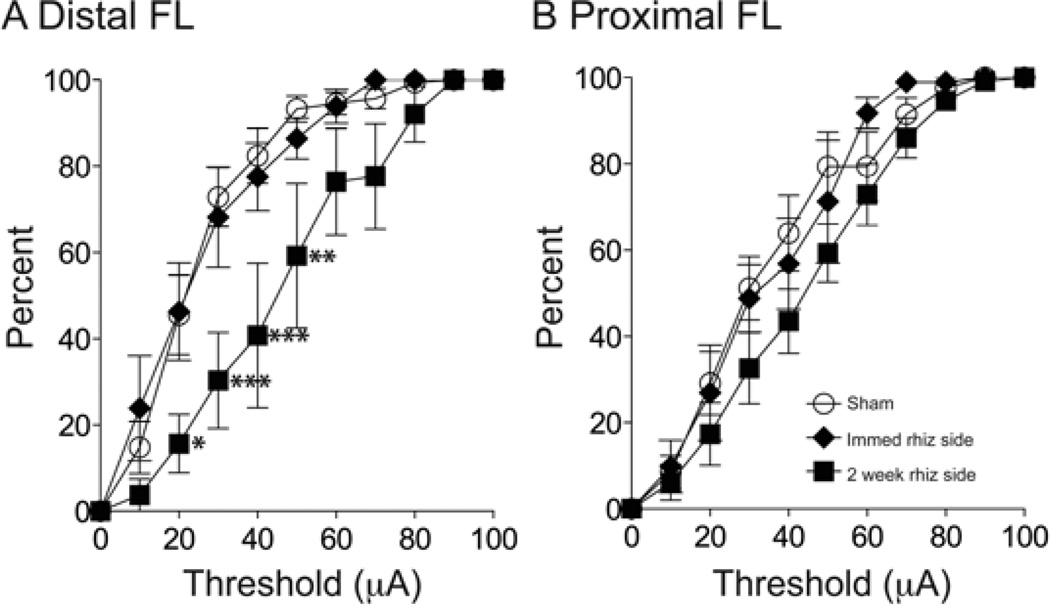
The dorsal roots are a major source of excitatory drive to the spinal and ascending sensory–motor circuits. Changes in proprioceptive inputs by postural adjustment influence the synaptic efficacy, or excitability, of sensory–motor circuits, resulting in changes in evoked muscle activity (Gellhorn & Hyde, 1953; Sanes et al., 1992). With the loss of afferent input after DRR, the overall level of excitability of the sensory–motor systems, especially that of the spinal cord, would be expected to become reduced. To determine whether excitability changes in spinal, and possibly ascending, sensory–motor circuits, affected the capacity to evoke motor responses from M1, we compared separately the threshold to evoke distal and proximal movements (Fig. 6). We binned data (10 µA) and then constructed cumulative distribution histograms of the percentage of effective sites (sites where stimulation evoked a response at threshold ≤100 µA) for each animal group. The cumulative distributions reveal the stimulus amplitude–motor response relationship and is a sensitive measure of changes in the capacity of M1 to evoke motor responses (Chakrabarty et al., 2009a). The stimulus–response relations for distal responses in the contralateral hemispheres of the sham and immediate DRR groups overlapped, indicating no change in M1 capacity to evoke distal movements. The stimulus– response relation for the contralateral hemisphere of the 2-week experimental animals shifted rightward, indicating a greater number of high-threshold sites for distal movements 2 weeks after DRR (Fig. 6A). This threshold increase was significantly different from sham (two way ANOVA, F1,99 = 2.538, P = 0.009), whereas the immediate DRR threshold distribution was not (two way ANOVA, F1,88 = 0.3146, P = 0.976). Figure 6B plots data for proximal responses. We combined elbow and shoulder data, rather than plot each joint separately, because the number of elbow and shoulder sites before DRR, and shoulder sites after DRR, was sparse in some animals. In contrast to changes in distal movement thresholds, there were no group differences for the proximal joint thresholds (two way ANOVA, F1,88 = 0.4195, P = 0.934 between sham and immediate DRR groups; F1,99 = 1.079, P = 0.386 between sham and 2-week DRR groups). The thresholds for evoking hindlimb movements were also not different between groups (sham, 49.42 ± 4.51 µA; immediate DRR-ipsilateral, 50.71 ± 5.02 µA; immediate DRR-contra, 50.02 ± 4.24 µA; 2-week DRR-ipsi, 48.96 ± 3.54 µA; 2-week DRR-contra, 49.34 ± 5.02 µA. One-way ANOVA, F4,19 = 0.49, P = 0.74). Our findings in animals with immediate DRR show that the capacity for activation of M1 to evoke motor responses does not depend on primary afferent input; threshold became elevated only after 2 weeks.
Figure 6.
The threshold for evoking distal forelimb movements increased 2 weeks after rhizotomy. (A) Cumulative distribution curves of distal forelimb thresholds, as a percentage of total number of effective sites. There was a rightward shift after 2 weeks but not immediately after deafferentation (two-way ANOVA, P = 0.976 between sham and immediate DRR groups, P = 0.009 between sham and 2-week DRR groups), significant difference were found at 20 µA (*P < 0.05), 30 µA (***P < 0.001), 40 µA (***P < 0.001) and 50 µA (**P < 0.01). (B) Cumulative distribution curves of proximal forelimb threshold. There were no differences in thresholds for proximal movements immediately or 2 weeks after deafferentation (two-way ANOVA, P = 0.934 between sham and immediate DRR groups, P = 0.386 between sham and 2-week DRR groups).
Discussion
The M1 motor representation provides a substrate for motor skills (Monfils et al., 2005). Its direct spinal output pathway, the corticospinal tract, together with somatic sensory information, is crucial for skilled movement control. Here we show that selective ablation of forelimb sensory inputs induced a rapid and persistent reorganization of the forelimb M1 representation. Surprisingly, despite the loss of a major excitatory input to the spinal cord, deafferentation produced no immediate change in M1-evoked motor threshold; only a modest elevation in the threshold for evoking distal forelimb movements was observed 2 weeks after deafferentation. An absence of threshold change is all the more unexpected as peripheral nerve block in humans, which interrupts both sensory and motor signaling, leads to an increase in TMS-evoked motor responses (Brasil-Neto et al., 1992; Kaelin-Lang et al., 2002). Our data provide, for the first time, evidence for a selective role of forelimb somatic sensory input in shaping and maintaining the M1 forelimb motor representation.
Deafferentation leads to impairments in limb use during reaching and ladder walking in rats, and in a variety of skilled, especially those involving the distal limb, motor tasks in humans (Marsden et al., 1984; Saling et al., 1992; McKenna & Whishaw, 1999; Wu et al., 2009). For example, Saling and colleagues examined reaching behavior after bilateral C5 – T2 dorsal rhizotomy in rats. Even after 2 weeks of training after the injury, the rat continued to display sever deficits in manipulation, which involves mostly distal movements, but not deficits in the protraction and retraction phases of reaching (Saling et al., 1992). Those authors also reported that the initial inability to initiate a reach, reflecting both proximal and distal impairments, abated after 14 days, but manipulation (i.e., distal) defects persisted. In another study, reaching was examined after unilateral C5–C8 dorsal root crush (Wu et al., 2009). Even after 7 weeks, animals had severe distal impairments reflected in an inability to grasp the food pellets. This finding is similar to the distal defect at 2 weeks reported by Saling and colleagues. Taken together, our ICMS findings showing that an impairment in the distal forelimb representation match well the distal impairments observed in these behavior studies.
The changes we observed in motor cortex help to explain these findings. Two weeks after deafferentation (i.e., when motor testing occurs) there is a 64.5% reduction in the distal forelimb representation and a 44% increase in threshold. Based on prior studies that have shown associations between a reduction in motor cortex forelimb representation size (Nudo, 2003; Kleim et al., 2004; Monfils et al., 2005) and elevated thresholds (Chakrabarty et al., 2009a) with impaired performance, we propose that the changes we observed after deafferentation could explain much of the motor deficits. Further, distal motor impairments persist after partial cervical deafferentation (tested up to 7 weeks; Wu et al., 2009). Although we only examined the motor map up to 2 weeks after DRR, the persistent behavioral findings suggest that there is an enduring distal representation impairment.
Spared afferents to the cervical spinal cord
To model the effects of deafferentation on cortical forelimb control, we severed the dorsal rootlets from C4 to C8. These segments contain the majority of distal and proximal forelimb motoneurons, as defined by the locations of retrogradely labeled motoneurons (McKenna et al., 2000). As the projections of proprioceptive afferents to motor neurons are mostly homonymous (Hultborn, 2006), it is expected that this deafferentation substantially eliminates limb afferent input to most of the key segmental levels for distal and proximal forelimb control. Our findings cannot be explained by spared afferent inputs because the extent of deafferentation was similar for wrist and elbow but the effects on their motor representations were different. CTB labeling from elbow or wrist (biceps and ECR, respectively) showed a similar substantial loss in spinal labeling over the rostrocaudal extent of their motor pools, and beyond, but opposite M1 representational changes. Thus, the extensive loss of afferent input should lead to a substantial loss of afferent feedback from both distal (wrist) and more proximal (elbow) forelimb muscles. Although both joints also showed some residual labeling at the rostral–caudal limits, immediately after dorsal rootlet section there was a large reduction in the absolute size of the wrist representation only. The elbow showed only a percentage increase immediately after DRR, not an absolute size increase. There was no change in the shoulder representation. This differential pattern of representational changes strongly suggests a selective dependence on afferent input for the M1 distal representation, which would be important for distal motor control. The immediate changes to the forelimb map following deafferentation were not transient. They persisted in the 2-week animals, where there were further reduction in the distal representation, as well as absolute and relative increases in the elbow representation. We propose that the increase in the size of the elbow representation is due largely to a reciprocal relationship between the representations of the elbow and distal joints; as the distal representation decreased, the elbow representation expanded. It is unlikely that expansion of the elbow representation after deafferentation reflects greater proximal limb use, to compensate for the M1 distal limb deficit, because there was no change in the shoulder representation.
Plasticity in the ascending somatic sensory systems could contribute to the afferent regulation of the M1 motor representation
For the ascending somatic sensory pathways and somatic sensory cortex, sensory loss, including partial limb deafferentation, produces a reduction in or loss of the representations of the affected body part and expansion of adjacent parts into the regions of the deafferented representations in S1 (Dostrovsky et al., 1976; Franck, 1980; Pettit & Schwark, 1993; Jones & Pons, 1998; Darian-Smith & Brown, 2000; Darian-Smith & Ciferri, 2006; Hooks et al., 2013). A basic reciprocal inhibitory relationship may underlie the representational expansion, which could be mediated by local intracortical mechanisms (Jacobs & Donoghue, 1991). These findings show that there is synaptic reorganization throughout the ascending pathways after sensory loss. The motor cortex receives somatic sensory input indirectly from the spinal cord, primarily via the dorsal column and relayed in the dorsal column nuclei and thalamus, as well as from the somatic sensory cortex (Asanuma, 1981). Reorganization of sensory representations that are presynaptic to M1 could change the local balance of afferent input within M1 and contribute to the distal motor representational change following deafferentation. Different somatic sensory input pathways may be utilized in unique ways by M1 depending on the motor control demands. Transection of the dorsal column abolished stimulus-evoked potentials in motor cortex, which suggests that ascending pathways provide moment-to-moment limb somatic sensory information to motor cortex (Asanuma et al., 1979). By contrast, ablation of somatic sensory cortex does not abolish the peripheral receptive fields of neurons in the motor cortex (Asanuma et al., 1979; Asanuma et al., 1980), suggesting less of a role for S1 in transmitting afferent input to motor cortex. Rather, corticocortical transmission from S1 appears to be more important in learning of new motor skills (Favorov et al., 1988). According to the classical model of Asanuma (Rosen & Asanuma, 1972), loss of afferent input to M1 distal control zones could result in reduced excitatory drive. This could result in a concomitant reduction in M1 distal representational space.
Spinal motor circuits may be an important locus for corticospinal system and afferent fiber interactions for regulating the M1 motor representation
As discussed above, representational changes in M1 could be imposed by changes in ascending and corticocortical afferent projections. However, a mechanism may exist at the level of spinal motor circuits, comprised of descending motor pathway terminations, somatic sensory afferents, interneurons and motoneurons, where important integration between afferent input and descending control signals occurs (Matsuyama et al., 2004; Rossignol et al., 2006). The spinal circuits integrate CST and proprioceptive signals to regulate motoneuron activity (Schouenborg & Weng, 1994; Clarac et al., 2004; Chakrabarty & Martin, 2011). Corticospinal and afferent terminations are dynamically modified during development and after injury to either of these inputs (Gibson et al., 2000; Tan et al., 2012). Selective loss of hindlimb cutaneous input results initially in foot drag, but recovery occurs over several weeks (Bretzner & Drew, 2005b). This was accompanied by a long-term adaptive increase in M1-evoked hindlimb motor responses, which reflected increased efficacy of the corticospinal tract, presumably at the spinal level. Interestingly, compensation was mediated by proximal muscles (e.g., sartorious and semitendinosus).
Early studies have shown that the receptive fields of interneurons in the dorsal horn reorganize within a few days after peripheral nerve transection (combined afferent and motor axons; Devor & Wall, 1981; Koerber & Mirnics, 1996; Wilson & Kitchener, 1996). Darian-Smith and colleagues also demonstrated important changes in the organization of spinal sensory–motor circuits. They found that dorsal rhizotomy in monkey not only results in an expansion of corticospinal tract from motor cortex into more dorsal (sensory) laminae of the spinal cord (Darian-Smith et al., 2013), but also induces neurogenesis in the dorsal horn and intermediate zone (Vessal et al., 2007). Dorsal rhizotomy also increases the number of GABAergic synapses (Darian-Smith et al., 2010), which could affect the balance of excitatory and inhibitory actions of spared sensory information. These findings point to reorganization of spinal motor circuits in response to spinal deafferentation, and this could influence how joint-specific CST actions (Stout & Beloozerova, 2012) affect motoneurons. One should note that although spinal circuit reorganization to somatic sensory loss could certainly alter cortical motor control and help restore excitability of spinal motor circuits, such changes would be expected to occur over the long term, as many are dependent on structural changes (e.g., sprouting; neurogenesis). Thus, we propose that plasticity in spinal circuits would more likely contribute to the later change in the motor map after deafferentation, including expansion of the elbow representation and persistent reduction of the distal representation, than the immediate and selective reduction in distal effects.
Rehabilitation potential of afferent stimulation after corticospinal system injury
Our finding that afferent input is important in the maintenance of the M1 distal representation may help inform the role of afferent stimulation in motor impairment and rehabilitation after a brain or spinal cord injury that damages the corticospinal system. In addition to the loss of descending pathways after SCI, there is to a variable extent combined loss of segmental afferent input and ascending sensory information. This somatic sensory loss would be expected to impair the cortical motor representations, as suggested by our results, exacerbating the movement impairment produced by the loss of descending spinal control through, for example, the loss of M1 feedback and feed-forward regulation of movement. Activation of spared limb afferent input, for example through the use of functional electrical stimulation, could help substitute for this sensory loss (Doucet et al., 2012). The immediate and substantial loss of the distal representation in anesthetized animals implies that this is more of a loss of constitutive regulation by afferent input than the loss of time-varying signals that occur during movements or an adaptive effect (Bretzner & Drew, 2005a). If so, then sensory activation might be effective in stabilizing the M1 motor representation after injury by preventing maladaptive representational plasticity. Early intervention with peripheral stimulation combined with motor training or motor cortex electrical stimulation (Carmel et al., 2010) could be beneficial in maintaining the M1 motor representation and sparing motor control following interruption of sensory inflow to motor cortex, such as after spinal cord injury or stroke.
Acknowledgments
We thank Harry Acosta and Dr Sulli Popilskis for animal care, and Xiuli Wu for histology and immunohistochemistry. Supported by NIH Grant R01NS64004 (J.H.M).
Abbreviations
- CFA
caudal forelimb area
- CST
corticospinal tract
- CTB
cholera toxin B subunit
- DRR
dorsal rootlet rhizotomy
- ECR
extensor carpi radialis
- ICMS
intracortical microstimulation
- M1
primary motor cortex
- RFA
rostral forelimb area
- TMS
transcranial magnetic stimulation
References
- Asanuma H. Functional role of sensory inputs to the motor cortex. Progress in neurobiology. 1981;16:241–262. doi: 10.1016/0301-0082(81)90015-0. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Larsen K, Yumiya H. Peripheral input pathways to the monkey motor cortex. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1980;38:349–355. doi: 10.1007/BF00236655. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Larsen KD, Zarzecki P. Peripheral input pathways projecting to the motor cortex in the cat. Brain research. 1979;172:197–208. doi: 10.1016/0006-8993(79)90532-8. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. Journal of neurophysiology. 2005a;94:657–672. doi: 10.1152/jn.01245.2004. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. Journal of neurophysiology. 2005b;94:673–687. doi: 10.1152/jn.01247.2004. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Berol l, Brus-Ramer M, Martin JH. Chronic Electrical Stimulation of the Intact Corticospinal System After Unilateral Injury Restores Skilled Locomotor Control and Promotes Spinal Axon Outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Friel KM, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. Journal of neurophysiology. 2009a;101:1283–1293. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Postnatal development of a segmental switch enables corticospinal tract transmission to spinal forelimb motor circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2277–2288. doi: 10.1523/JNEUROSCI.5286-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Co-development of proprioceptive afferents and the corticospinal tract within the cervical spinal cord. The European journal of neuroscience. 2011;34:682–694. doi: 10.1111/j.1460-9568.2011.07798.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:8816–8827. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Progress in brain research. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. [DOI] [PubMed] [Google Scholar]
- Clowry GJ, Davies BM, Upile NS, Gibson CL, Bradley PM. Spinal cord plasticity in response to unilateral inhibition of the rat motor cortex during development: changes to gene expression, muscle afferents and the ipsilateral corticospinal projection. The European journal of neuroscience. 2004;20:2555–2566. doi: 10.1111/j.1460-9568.2004.03713.x. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Brown S. Functional changes at periphery and cortex following dorsal root lesions in adult monkeys. Nature neuroscience. 2000;3:476–481. doi: 10.1038/74852. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Ciferri M. Cuneate nucleus reorganization following cervical dorsal rhizotomy in the macaque monkey: its role in the recovery of manual dexterity. The Journal of comparative neurology. 2006;498:552–565. doi: 10.1002/cne.21088. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Hopkins S, Ralston HJ., 3rd Changes in synaptic populations in the spinal dorsal horn following a dorsal rhizotomy in the monkey. The Journal of comparative neurology. 2010;518:103–117. doi: 10.1002/cne.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Lilak A, Alarcon C. Corticospinal sprouting occurs selectively following dorsal rhizotomy in the macaque monkey. The Journal of comparative neurology. 2013;521:2359–2372. doi: 10.1002/cne.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Wall PD. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1981;1:679–684. doi: 10.1523/JNEUROSCI.01-07-00679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. The Journal of comparative neurology. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Millar J, Wall PD. The immediate shift of afferent drive to dorsal column nucleus cells following deafferentation: a comparison of acute and chronic deafferentation in gracile nucleus and spinal cord. Experimental neurology. 1976;52:480–495. doi: 10.1016/0014-4886(76)90219-3. [DOI] [PubMed] [Google Scholar]
- Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. The Yale journal of biology and medicine. 2012;85:201–215. [PMC free article] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Progress in brain research. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Favorov O, Sakamoto T, Asanuma H. Functional role of corticoperipheral loop circuits during voluntary movements in the monkey: a preferential bias theory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:3266–3277. doi: 10.1523/JNEUROSCI.08-09-03266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JI. Functional reorganization of cat somatic sensory–motor cortex (Sml) after selective dorsal root rhizotomies. Brain research. 1980;186:458–462. doi: 10.1016/0006-8993(80)90991-9. [DOI] [PubMed] [Google Scholar]
- Gellhorn E, Hyde J. Influence of proprioception on map of cortical responses. The Journal of physiology. 1953;122:371–385. doi: 10.1113/jphysiol.1953.sp005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Vicario D, Martin JH, Yumiya H. Role of the motor cortex in the initiation of voluntary motor responses in the cat. Electroencephalography and clinical neurophysiology. 1982;36:409–414. Supplement. [PubMed] [Google Scholar]
- Gibson CL, Arnott GA, Clowry GJ. Plasticity in the rat spinal cord seen in response to lesions to the motor cortex during development but not to lesions in maturity. Experimental neurology. 2000;166:422–434. doi: 10.1006/exnr.2000.7511. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Progress in neurobiology. 2006;78:215–232. doi: 10.1016/j.pneurobio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Huntley GW. Differential effects of abnormal tactile experience on shaping representation patterns in developing and adult motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:9220–9232. doi: 10.1523/JNEUROSCI.17-23-09220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Baker SN, Fetz EE. Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. The Journal of physiology. 2006;573:107–120. doi: 10.1113/jphysiol.2005.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. The Journal of physiology. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartje-Tillotson G, Neafsey EJ, Castro AJ. Topography of corticopontine remodelling after cortical lesions in newborn rats. The Journal of comparative neurology. 1986;250:206–214. doi: 10.1002/cne.902500207. [DOI] [PubMed] [Google Scholar]
- Keller A, Weintraub ND, Miyashita E. Tactile experience determines the organization of movement representations in rat motor cortex. Neuroreport. 1996;7:2373–2378. doi: 10.1097/00001756-199610020-00019. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K. Plasticity of dorsal horn cell receptive fields after peripheral nerve regeneration. Journal of neurophysiology. 1996;75:2255–2267. doi: 10.1152/jn.1996.75.6.2255. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. The Use of Peripheral Feedback in the Control of Movement. Trends Neurosci. 1984;7:253–257. [Google Scholar]
- Martin JH, Choy M, Pullman S, Meng Z. Corticospinal system development depends on motor experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2122–2132. doi: 10.1523/JNEUROSCI.4616-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Progress in brain research. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. The Journal of comparative neurology. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain research. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2003:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- Pettit MJ, Schwark HD. Receptive field reorganization in dorsal column nuclei during temporary denervation. Science. 1993;262:2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- Rosen I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1972;14:257–273. doi: 10.1007/BF00816162. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiological reviews. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Saling M, Sitarova T, Vejsada R, Hnik P. Reaching behavior in the rat: absence of forelimb peripheral input. Physiology & behavior. 1992;51:1151–1156. doi: 10.1016/0031-9384(92)90301-h. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Static and dynamic organization of motor cortex. Advances in neurology. 1997;73:277–296. [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Wang J, Donoghue JP. Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cereb Cortex. 1992;2:141–152. doi: 10.1093/cercor/2.2.141. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Weng HR. Sensorimotor transformation in a spinal motor system. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1994;100:170–174. doi: 10.1007/BF00227291. [DOI] [PubMed] [Google Scholar]
- Stout EE, Beloozerova IN. Pyramidal tract neurons receptive to different forelimb joints act differently during locomotion. Journal of neurophysiology. 2012;107:1890–1903. doi: 10.1152/jn.00650.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12896–12908. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Tosolini AP, Mohan R, Morris R. Targeting the full length of the motor end plate regions in the mouse forelimb increases the uptake of fluoro-gold into corresponding spinal cord motor neurons. Front Neurol. 2013;4:58. doi: 10.3389/fneur.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M, Aycock A, Garton MT, Ciferri M, Darian-Smith C. Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. The European journal of neuroscience. 2007;26:2777–2794. doi: 10.1111/j.1460-9568.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Wall PD. Plasticity in the adult mammalian central nervous system. Progress in brain research. 1976;45:359–379. doi: 10.1016/S0079-6123(08)60998-X. [DOI] [PubMed] [Google Scholar]
- Wilson P, Kitchener PD. Plasticity of cutaneous primary afferent projections to the spinal dorsal horn. Progress in neurobiology. 1996;48:105–129. doi: 10.1016/0301-0082(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Wu A, Lauschke JL, Morris R, Waite PM. Characterization of rat forepaw function in two models of cervical dorsal root injury. Journal of neurotrauma. 2009;26:17–29. doi: 10.1089/neu.2008.0675. [DOI] [PubMed] [Google Scholar]