Abstract
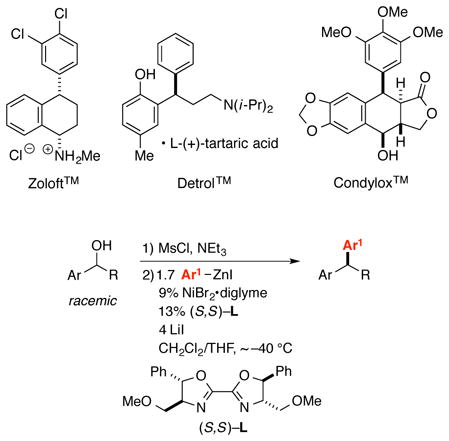
A tertiary stereogenic center that bears two different aryl substituents is found in a variety of bioactive compounds, including medicines such as Zoloft™ and Detrol™. We have developed an efficient method for the synthesis of enantioenriched 1,1-diarylalkanes from readily available racemic benzylic alcohols. Formation of a benzylic mesylate (which is not isolated), followed by treatment with an arylzinc reagent, LiI, and a chiral nickel/bis(oxazoline) catalyst, furnishes the Negishi cross-coupling product in high ee and good yield. A wide array of functional groups (e.g., an aryl iodide, a thiophene, and an N-Boc-indole) are compatible with the mild reaction conditions. This method has been applied to a gram-scale synthesis of a precursor to Zoloft™.
Because an array of bioactive compounds bear a tertiary stereocenter with two aryl substituents, including medicines such as Zoloft™, Detrol™, and Condylox™,1 the development of methods for the enantioselective synthesis of 1,1-diarylalkanes has been the focus of substantial effort. A variety of strategies that exploit asymmetric catalysis have been pursued, such as the hydrogenation of 1,1-diarylalkenes2 and the 1,4-addition of aryl nucleophiles to α,β-unsaturated carbonyl compounds.3 An alternative approach, the enantioconvergent cross-coupling of a racemic benzylic electrophile with an arylmetal reagent, has not yet been accomplished, although impressive progress has been recorded in the stereoselective coupling of enantioenriched benzylic electrophiles with aryl nucleophiles using an achiral catalyst.4,5 In this report, we describe nickel-catalyzed asymmetric Negishi reactions of racemic benzylic electrophiles with arylzinc reagents that generate a broad spectrum of 1,1-diarylalkanes in good ee (eq 1).
 |
(1) |
We have recently pursued the development of an array of enantioconvergent cross-couplings of racemic alkyl electrophiles.6,7 Most relevant to the objective described herein, we have reported two nickel-catalyzed methods for asymmetric Negishi reactions of benzylic halides with alkylzinc reagents.6a,8 Unfortunately, neither of these procedures proved effective when an arylzinc was employed as the nucleophile.9
An ongoing challenge in the field of stereoconvergent cross-coupling is the use of alkyl electrophiles that bear oxygen, rather than halogen, leaving groups. The significance of this objective arises in part from the fact that the conversion of alcohols to more-reactive oxygen-based leaving groups is generally accomplished under Brønsted-basic conditions, whereas their conversion to alkyl halides is often achieved under Brønsted-acidic conditions. In 2012, we described a nickel/pybox-catalyzed enantioconvergent Negishi arylation of propargylic carbonates.6d However, this method was not effective for a racemic benzylic carbonate.
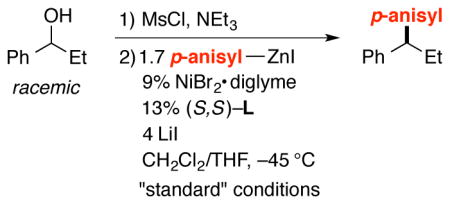
We next turned our attention to a different family of oxygen leaving groups: readily accessible mesylates.10 Because benzylic mesylates are highly reactive electrophiles,11 we chose to employ them without isolation. As depicted in entry 1 of Table 1, under the appropriate conditions, a nickel/bis(oxazoline) catalyst can achieve the net arylation of a benzylic alcohol to generate a 1,1-diarylalkane with high enantioselectivity and in good yield.
Table 1.
Influence of Reaction Parameters on the Catalytic Asymmetric Synthesis of a 1,1-Diarylalkanea
| |||
|---|---|---|---|
| entry | variation from the “standard” conditions | ee (%) | yield (%)b |
| 1 | none | 94 | 93 |
| 2 | no NiBr2• diglyme | – | <2 |
| 3 | no L | – | 11 |
| 4 | 1 instead of L | – | <2 |
| 5 | 2 instead of L | – | <2 |
| 6 | 3 instead of L | 82 | 94 |
| 7 | no LiI | 66 | 22 |
| 8 | LiBr instead of LiI | 92 | 36 |
| 9 | 4.5% NiBr2• glyme, 6.5% L | 94 | 51 |
| 10 | 1.2 p-anisyl–ZnI | 93 | 51 |
| 11 | 0 °C | 87 | 61 |
| 12 | r.t. | 79 | 33 |
All data are the average of two experiments.
The yields were determined through GC analysis with the aid of a calibrated internal standard.
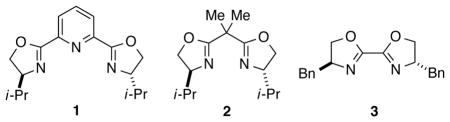
Essentially no carbon–carbon bond formation occurred in the absence of NiBr2•diglyme (Table 1, entry 2), whereas a small amount of the cross-coupling product was formed in the absence of bis(oxazoline) ligand L (entry 3). Two families of ligands that are useful for other asymmetric Negishi arylation reactions (1 and 2)12 were not useful under these conditions (<2% yield; entries 4 and 5), and a commercially available 2,2′-linked bis(oxazoline) (3) that is related to ligand L furnished somewhat lower ee, but comparable yield (entry 6). The omission of LiI led to a loss in enantioselectivity and yield (entry 7), whereas use of LiBr instead provided very good ee, but only a modest quantity of the desired cross-coupling product (entry 8). When the asymmetric Negishi arylation was conducted with less catalyst, less nucleophile, or at a higher temperature, inferior results were obtained relative to the best conditions (entries 9–12 vs. entry 1).
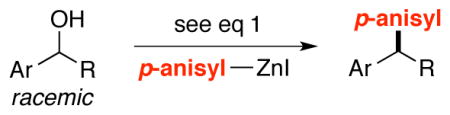
With an effective method for the catalytic asymmetric synthesis of a model 1,1-diarylalkane in hand, we examined the scope with respect to the electrophile (Table 2). For alkyl substituents (R) that range in size from methyl to cyclobutyl, the Negishi arylations proceeded with consistently good enantioselectivities (entries 1–6).13 Furthermore, asymmetric cross-couplings could be achieved in the case of aryl substituents (Ar) that are either electron-rich14 or electron-poor (e.g., entries 15–24).15 A wide array of functional groups are compatible with the cross-coupling conditions, including an olefin (entry 9), a silyl ether (entry 10), an acetal (entry 11), an alkyl ester (entry 12), a ketone (entry 13), a nitrile (entry 14), an aryl fluoride (entries 15 and 24), an aryl chloride (entry 16), an aryl bromide (entry 17), an aryl ether (entries 19 and 21), an aryl ester (entry 20), an aryl amine (entry 23), a furan (entry 26), and a thiophene (entries 27 and 28). On a gram scale (1.40 g of product), the enantioconvergent Negishi arylation illustrated in entry 2 proceeded in 95% ee and 89% yield.
Table 2.
Catalytic Asymmetric Synthesis of 1,1-Diarylalkanes from Racemic Benzylic Alcohols: Scope with Respect to the Alcohola
| ||||
|---|---|---|---|---|
| entry | benzylic alcohol | ee (%) | yield (%)b | |
| 1 |
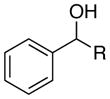
|
R =Me | 88 | 88 |
| 2 | Et | 94 | 92 | |
| 3 | n-Pr | 94 | 90 | |
| 4 | n-Bu | 95 | 83 | |
| 5 | i-Bu | 95 | 77 | |
| 6 | cyclobutyl | 94 | 51 | |
| 7c |
|
95 | 80 | |
| 8 |
|
88 | 90 | |
| 9 |
|
86 | 59 | |
| 10d |
|
94 | 77 | |
| 11 |
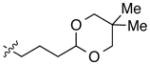
|
92 | 84 | |
| 12 |
|
94 | 84 | |
| 13 |
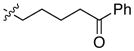
|
95 | 88 | |
| 14 |
|
94 | 75 | |
|
|
||||
| 15 |
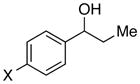
|
X = F | 93 | 87 |
| 16 | Cl | 90 | 88 | |
| 17 | Br | 91 | 79 | |
| 18 | Ph | 92 | 78 | |
| 19e |
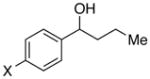
|
X = OMe | 95 | 94 |
| 20c,e | OPiv | 84 | 84 | |
|
|
||||
| 21e |
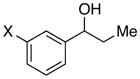
|
X = OMe | 94 | 94 |
| 22c | CF3 | 89 | 63 | |
| 23e |
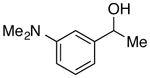
|
92 | 88 | |
|
|
||||
| 24 |
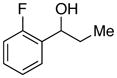
|
81 | 66 | |
| 25 |
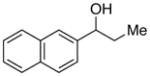
|
91 | 79 | |
| 26 |
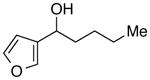
|
93 | 88 | |
| 27 |
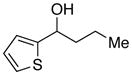
|
83 | 54 | |
| 28 |
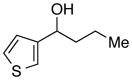
|
94 | 93 | |
All data are the average of two experiments.
Yield of purified product.
Reaction temperature: −35 °C.
Nucleophile: (p-tolyl)ZnI.
Nucleophile: PhZnI.
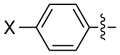
The scope is also fairly broad with respect to the nucleophile (Table 3). Thus, an array of para- and meta-functionalized arylzinc reagents are suitable cross-coupling partners (entries 1–12), including one that bears an aryl iodide (entry 6).16 Furthermore, an indolylzinc reagent can be employed as the nucleophile (entry 15).
Table 3.
Catalytic Asymmetric Synthesis of 1,1-Diarylalkanes from Racemic Benzylic Alcohols: Scope with Respect to the Nucleophilea
| ||||
|---|---|---|---|---|
| entry | Ar1 | ee (%) | yield (%)b | |
| 1 |
|
X = H | 94 | 96 |
| 2c | Me | 93 | 91 | |
| 3 | F | 94 | 95 | |
| 4 | Cl | 94 | 95 | |
| 5 | Br | 94 | 87 | |
| 6c | I | 94 | 66 | |
| 7 | CF3 | 94 | 98 | |
| 8d | CO2Et | 93 | 91 | |
| 9d | CN | 92 | 83 | |
|
|
||||
| 10 |
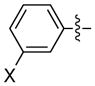
|
X = CF3 | 94 | 96 |
| 11d | CO2Et | 93 | 92 | |
|
|
||||
| 12 |
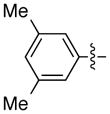
|
93 | 87 | |
| 13e |
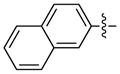
|
92 | 93 | |
| 14d |
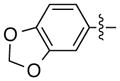
|
94 | 87 | |
| 15d |
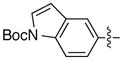
|
93 | 77 | |
All data are the average of two experiments.
Yield of purified product.
Equivalents of arylzinc reagent: 1.5.
Benzylic alcohol coupling partner: 1-phenylpropanol.
Reaction temperature: −30 °C.
As indicated in entry 7 of Table 1, under our optimized asymmetric arylation conditions, the presence of LiI is important for the formation of the desired 1,1-diarylalkane in high ee and good yield. We hypothesize that a nucleophilic iodide reacts with the benzylic mesylate to generate a benzylic iodide in situ, and this is the electrophile that engages with the nickel catalyst in the cross-coupling process.17 Indeed, we have determined that a benzylic mesylate does react with LiI in CH2Cl2/THF at −45 °C to generate a benzylic iodide. Furthermore, cross-couplings of a benzylic mesylate and the corresponding benzylic iodide proceed with similar ee and yield (eq 2).18
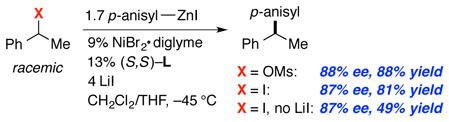 |
(2) |
To illustrate the utility of this catalytic asymmetric method for the formation of 1,1-diarylalkanes, we applied it to a gram-scale synthesis of (S)-sertraline tetralone (B; eq 3), a precursor of sertraline hydrochloride (Zoloft™, a leading antidepressant drug).1a,19 Thus, enantioconvergent Negishi arylation of racemic benzylic alcohol A in the presence of 4.5% of the chiral nickel catalyst, followed by an intramolecular Friedel-Crafts reaction, furnished tetralone B in good ee (92%) and 75% overall yield from the alcohol.
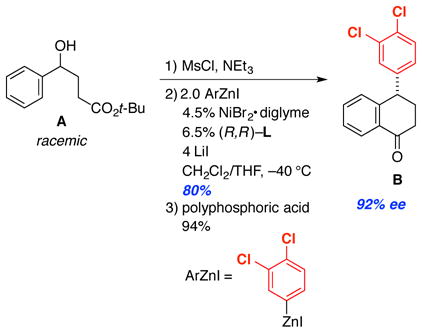 |
(3) |
In conclusion, we have developed a nickel-catalyzed stereoconvergent Negishi cross-coupling of racemic benzylic electrophiles with arylzinc reagents that affords valuable enantioenriched 1,1-diarylalkanes from readily available alcohols in high ee and good yield. Carbon–carbon bond formation occurs under mild conditions that are compatible with a range of functional groups. This method represents an unusual example of the use of an oxygen-based electrophile in an enantioconvergent, nickel-catalyzed cross-coupling, although mechanistic studies are consistent with the possibility that the species that undergoes oxidative addition may be a benzylic iodide that is generated in situ under the reaction conditions. Regardless, this process provides a straightforward approach to enantioenriched 1,1-diarylalkanes from benzylic alcohols, as exemplified by its application to an asymmetric synthesis of (S)-sertraline tetralone, a precursor of Zoloft™. Ongoing studies are focused on the development of additional nickel-catalyzed methods for cross-coupling alkyl electrophiles, including enantioselective reactions and mechanistic investigations.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM62871) and Dr. Reddy’s Laboratories (E.R.R.C.). We thank Dr. Scott C. Virgil (Cal-tech Center for Catalysis and Chemical Synthesis, supported by the Gordon and Betty Moore Foundation) and Dr. Nathan D. Schley for assistance.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews and leading references, see: Khouzam HR, Ernes R, Gill T, Roy R. Comprehensive Therapy. 2003;29:47–53. doi: 10.1007/s12019-003-0007-6.Salvatore S, Serati M, Bolis P. Expert Opinion on Pharmacotherapy. 2008;9:1249–1255. doi: 10.1517/14656566.9.7.1249.Stayrook KR, Carson MW, Ma YL, Dodge JA. In: Vitamin D. Feldman D, Pike JW, Adams JS, editors. Elsevier; London: 2011. pp. 1497–1508.
- 2.For examples and leading references, see: Woodmansee DH, Pfaltz A. Chem Commun. 2011;47:7912–7916. doi: 10.1039/c1cc11430a.Tolstoy P, Engman M, Paptchikhine A, Bergquist J, Church TL, Leung AWM, Andersson PG. J Am Chem Soc. 2009;131:8855–8860. doi: 10.1021/ja9013375. This reference includes a discussion of other approaches to the synthesis of 1,1-diarylalkanes.
- 3.For example, see: Paquin J-F, Defieber C, Stephenson CRJ, Carreira EM. J Am Chem Soc. 2005;127:10850–10851. doi: 10.1021/ja053270w.
- 4.For recent reports and leading references, see: Maity P, Shacklady-McAtee DM, Yap GPA, Sirianni ER, Watson MP. J Am Chem Soc. 2013;135:280–285. doi: 10.1021/ja3089422.Zhou Q, Srinivas HD, Dasgupta S, Watson MP. J Am Chem Soc. 2013;135:3307–3310. doi: 10.1021/ja312087x.Harris MR, Hanna LE, Greene MA, Moore CE, Jarvo ER. J Am Chem Soc. 2013;135:3303–3306. doi: 10.1021/ja311783k. (doubly benzylic electrophiles)
- 5.For an example of an umpolung process that employs an enantioenriched benzylic nucleophile, see: Imao D, Glasspoole BW, Laberge VS, Crudden CM. J Am Chem Soc. 2009;131:5024–5025. doi: 10.1021/ja8094075.
- 6.For recent examples and leading references, see: Binder JT, Cordier CJ, Fu GC. J Am Chem Soc. 2012;134:17003–17006. doi: 10.1021/ja308460z.Choi J, Fu GC. J Am Chem Soc. 2012;134:9102–9105. doi: 10.1021/ja303442q.Wilsily A, Tramutola F, Owston NA, Fu GC. J Am Chem Soc. 2012;134:5794–5797. doi: 10.1021/ja301612y.Oelke AJ, Sun J, Fu GC. J Am Chem Soc. 2012;134:2966–2969. doi: 10.1021/ja300031w.
- 7.For a recent application in a total synthesis of a natural product (carolacton), see: Schmidt T, Kirschning A. Angew Chem Int Ed. 2012;51:1063–1066. doi: 10.1002/anie.201106762.
- 8.Arp FO, Fu GC. J Am Chem Soc. 2005;127:10482–10483. doi: 10.1021/ja053751f. [DOI] [PubMed] [Google Scholar]
- 9.Under the conditions described in Ref. 6a, the cross-coupling of a benzylic halide with an arylzinc reagent proceeded in poor yield and poor ee. See also footnote 6c in Ref. 8.
- 10.For a previous example of a nickel-catalyzed cross-coupling of an alkyl sulfonate, see: Terao J, Watanabe H, Ikumi A, Kuniyasu H, Kambe N. J Am Chem Soc. 2002;124:4222–4223. doi: 10.1021/ja025828v.
- 11.For leading references, see: Kochi JK, Hammond GS. J Am Chem Soc. 1953;75:3443–3444.Hammond GS, Peloquin J, Fang FT, Kochi JK. J Am Chem Soc. 1960;82:443–445.Coates RM, Chen JP. Tetrahedron Lett. 1969;10:2705–2708.
- 12.For example, see: Smith SW, Fu GC. J Am Chem Soc. 2008;130:12645–12647. doi: 10.1021/ja805165y.Lundin PM, Esquivias J, Fu GC. Angew Chem Int Ed. 2009;48:154–156. doi: 10.1002/anie.200804888.(c) Ref. 6b and 6d.
- 13.In a preliminary experiment under our standard conditions, a highly hindered electrophile (R = isopropyl) was not a suitable cross-coupling partner.
- 14.Benzylic bromides and iodides wherein the aromatic group is electron-rich can be highly reactive and therefore difficult to isolate.
- 15.In preliminary experiments under our standard conditions, an electrophile in which the aromatic group was hindered (o-tolyl) or very electron-poor (cyano- or ester-substituted) was not a suitable cross-coupling partner.
- 16.For leading references to nickel-catalyzed cross-coupling reactions of aryl electrophiles, including processes that involve C– O bond cleavage, see: Yamaguchi J, Muto K, Itami K. Eur J Org Chem. 2013:19–30.
- 17.For a suggestion of a tosylate-to-iodide substitution in an iron-catalyzed cross-coupling reaction, see: Ito S, Fujiwara Y-i, Nakamura E, Nakamura M. Org Lett. 2009;11:4306–4309. doi: 10.1021/ol901610a.
- 18.(a) The improved yield for the cross-coupling of the benzylic iodide in the presence of LiI (eq 2) may be due, for example, to an increase in the dielectric constant of the reaction medium or to nucleophilic activation of the organozinc reagent. (b) Under otherwise identical conditions, a benzylic bromide cross-coupled in comparable yield but slightly lower ee, whereas a benzylic chloride was not a suitable electrophile. (c) In preliminary studies, ZnPh2 was not an effective cross-coupling partner.
- 19.Quallich GJ. Chirality. 2005;17:S120–S126. doi: 10.1002/chir.20113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


