Abstract
Rhodoliths are free-living coralline algae (Rhodophyta, Corallinales) that are ecologically important for the functioning of marine environments. They form extensive beds distributed worldwide, providing a habitat and nursery for benthic organisms and space for fisheries, and are an important source of calcium carbonate. The Abrolhos Bank, off eastern Brazil, harbors the world's largest continuous rhodolith bed (of ∼21 000 km2) and has one of the largest marine CaCO3 deposits (producing 25 megatons of CaCO3 per year). Nevertheless, there is a lack of information about the microbial diversity, photosynthetic potential and ecological interactions within the rhodolith holobiont. Herein, we performed an ecophysiologic and metagenomic analysis of the Abrolhos rhodoliths to understand their microbial composition and functional components. Rhodoliths contained a specific microbiome that displayed a significant enrichment in aerobic ammonia-oxidizing betaproteobacteria and dissimilative sulfate-reducing deltaproteobacteria. We also observed a significant contribution of bacterial guilds (that is, photolithoautotrophs, anaerobic heterotrophs, sulfide oxidizers, anoxygenic phototrophs and methanogens) in the rhodolith metagenome, suggested to have important roles in biomineralization. The increased hits in aromatic compounds, fatty acid and secondary metabolism subsystems hint at an important chemically mediated interaction in which a functional job partition among eukaryal, archaeal and bacterial groups allows the rhodolith holobiont to thrive in the global ocean. High rates of photosynthesis were measured for Abrolhos rhodoliths (52.16 μmol carbon m−2 s−1), allowing the entire Abrolhos rhodolith bed to produce 5.65 × 105 tons C per day. This estimate illustrates the great importance of the Abrolhos rhodolith beds for dissolved carbon production in the South Atlantic Ocean.
Keywords: rhodoliths, holobionts, carbon cycle, biomineralization, Abrolhos Bank
Introduction
Eukaryote–microbe associations are increasingly recognized as being essential for eukaryotic host health and metabolism, enabling the expansion of their physiologic capacities (Rosenberg et al., 2007). The term holobiont refers to any organism and all of its associated symbiotic microbes (parasites, mutualists, synergists and amensals) (Rosenberg and Zilber-Rosenberg, 2011), including endobionts and epibionts that perform diverse ecological roles (Wahl et al., 2012). A holobiont occupies and adapts to an ecological niche, and is able to employ strategies unavailable in any one species alone when challenged by environmental perturbations. The holobiont concept, first applied to corals after they were found to host abundant and species-specific microbial communities (Rohwer et al., 2002; Reshef et al., 2006; Rosenberg et al., 2007), has since been extended to the benthic alga (Barott et al., 2011). These holobionts are diverse and distinct from the microbiota in the surrounding seawater and biofilms on abiotic surfaces (Barott et al., 2011; Burke et al., 2011; Barott and Rohwer, 2012). A wide range of beneficial and detrimental interactions have been described between macroalgae and their microbiomes based on the exchange of nutrients, minerals and secondary metabolites (Hollants et al., 2012).
Rhodoliths are biogenic calcareous structures primarily formed by encrusting coralline algae (CCA; Corallinales, Rhodophyta). They provide highly biodiverse and heterogeneous habitats and are distributed worldwide (Foster, 2001; Steller et al., 2003; Nelson, 2009). Despite our vast knowledge concerning the ecophysiology and biogeography of rhodoliths, some aspects of the biology of rhodoliths remain completely unknown. For instance, information on the microbial communities, the microbial metabolic strategies associated with biogeochemical cycles and the biomineralization of CaCO3, and the photosynthetic productivity potential of rhodoliths is absent.
Rhodolith beds constitute one of the Earth's four major macrophyte-dominated benthic communities, along with kelp beds, seagrass meadows and coralline algal reefs. Our group has mapped the largest rhodolith bed in the world, which covers ∼20 900 km2 of the Abrolhos Shelf (16°50′–19°45′S) (Amado-Filho et al., 2012). The Abrolhos Bank is one of the largest marine CaCO3 deposits in the world, with a production of 25 megatons of CaCO3 per year (Amado-Filho et al., 2012). Nevertheless, our limited knowledge of rhodolith biology hinders our ability to develop a broader systemic understanding of their ecological role in the Abrolhos Bank. There are pressing concerns relating to the future of rhodolith beds as global carbon budgets, and ocean acidification may interfere with rhodolith functioning and biogeochemical stability (Webster et al., 2011; Whalan et al., 2012). In addition, because rhodoliths can be applied agriculturally to improve soil pH, rhodolith environments have been suffering extensive commercial pressure. In addition, rhodoliths are a non-renewable resource because of their extremely slow growth rates (Dias, 2001; Barbera et al., 2003; Wilson et al., 2004).
Rhodoliths form biogenic matrices with complex structures in which the interlocking branched thalli can create microhabitats for diverse eukaryotic assemblages, including epiphyte algae, microalgae and different types of invertebrates from both hard and soft benthos (Steller et al., 2003; Kamenos et al., 2004; Figueiredo et al., 2007; Riul et al., 2009; Bahia et al., 2010). Owing to the rich three-dimensional architecture, each rhodolith holobiont may be considered a small individual reef, providing a habitat for the young of various types of marine life (for example, Arthropoda, Nematoda and Cnidaria). Habitat and nursery functions are the most obvious ecological roles that rhodoliths have in the marine realm. Rhodoliths may be key factors in a range of invertebrate-recruitment processes, functioning as autogenic ecosystem engineers by providing three-dimensional habitat structures. A better understanding of the composition of and ecological interactions within the rhodolith holobiont would be a powerful tool for the conservation and sustainable use of these biological resources. This understanding could also provide insights into how cooperation and job partitioning between constituents contribute to holobiont fitness, influence holobiont viability, and consequently affect associated ecosystems and the ubiquitous rhodolith worldwide distribution. We aimed to perform a metagenomic characterization of the Abrolhos rhodoliths to determine the major taxonomic and functional components of these organisms. We also examined the associated fauna diversity and physiologic aspects (photosynthetic capacity and dissolved organic carbon (DOC) productivity) of the rhodoliths.
Materials and methods
Study site and sample collection
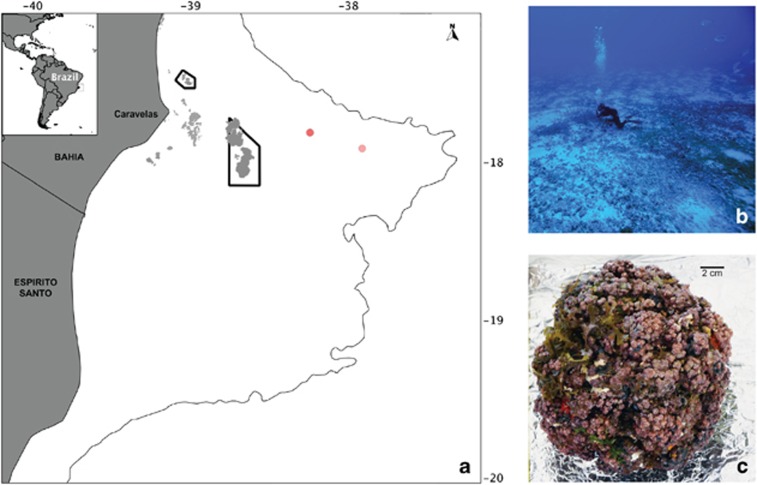
This study was carried out in the Abrolhos Shelf off eastern Brazil. Rhodoliths were collected by scuba diving in December 2010 from three different sites near two recently described sinkhole-like structures called Buracas (Bastos et al., 2013). Buracas are cup-shaped depressions on the seafloor and their suggested function is to trap and accumulate organic matter, thus functioning as productivity hotspots in the mid- and outer shelf of the central portion of the Abrolhos Bank (Cavalcanti et al., 2013). Seven rhodoliths were sampled as follows: two individual rhodoliths from the shallower portion (27 m) (17.81330° S/38.23744° W) outside the first Buraca; three from the inner region of the same Buraca (43 m deep) (17.81399° S/38.24306° W) and two from a deeper point (51 m) (17.91361° S/37.90936° W) near another sinkhole-like structure (Supplementary Table S1) away from the first (Figure 1). Monospecific rhodolith-forming CCA were selected by visual inspection. Immediately after collection, the specimens were frozen in liquid nitrogen in the field. For comparison purposes, surrounding water samples (8 l) collected using sterivex 0.2-μm filters at exactly the same points over the rhodolith beds at the first Buraca (inside—43 m; outside—27 m) and outside the deeper Buraca (51 m) were used. A detailed description of the region of the Buracas, water sampling and metagenomic characterization of the planktonic microbial community is available in the work by Cavalcanti et al. (2013).
Figure 1.
Study site. The world's largest rhodolith bed. (a) The Abrolhos bank, an expanse covering ∼46 000 km2 of the eastern Brazilian continental shelf where the rhodolith bed covers an area of ∼21 000 km2. Rhodoliths were sampled from two different sites surrounding the recently described sinkhole-like structures (red dots). (b) Physiognomy of Abrolhos rhodolith beds. (c) Individual monospecific rhodoliths.
DNA extraction, pyrosequencing and sequence analysis
In the laboratory, a small fragment of each rhodolith sample (∼1 cm2) was sterilely macerated with liquid nitrogen without the separation of epibionts to provide a representation of the entire rhodolith holobiont as previously defined (calcifying algae plus associated microbes, fauna and flora). A subsequent step using CTAB buffer with 100 mM of EDTA and a PowerSoil purification column was used to gather the DNA of high-molecular-weight rhodolith holobionts as described by Garcia et al. (2013). High-quality total DNA extracted from rhodoliths was then sequenced using a 454 GS Jr machine (454 Life Sciences, Branford, CT, USA) and the GS Jr Titanium-sequencing process. Sequences were submitted to the MG-RAST 3.1 server (Metagenomics-Rapid Annotation Using Subsystems Technology) (Meyer et al., 2008) and quality filtered. Post-quality-control (QC) sequences were annotated using the (SEED) Subsystems Technology for functional classification (Overbeek et al., 2005) and the GenBank database for phylogenetic analyses. All BLAST queries were performed with a maximum expected cutoff value of 10–5.
Statistical analyses
The Statistical Analysis of Metagenomic Profiles (STAMP v.2.0.0) software was used for statistical analysis (Parks and Beiko, 2010). For comparison purposes, rhodolith metagenomes were compared with water metagenomes from the same site (Cavalcanti et al., 2013). The water and rhodolith samples were compared by using a two-sided Welch's t-test with 95% confidence intervals calculated by inverting the Welch's test and by using the Benjamin–Hochberg FDR multiple test correction. A principal component analysis was conducted using the STAMP software package to compare the taxonomic grouping based on the taxonomic class contributions of each metagenome from both the rhodolith and the surrounding seawater. To isolate the relative contributions of taxonomic groups within the rhodolith metagenomes, the most abundant groups (Alphaproteobacteria, Gammaproteobacteria, Eukarya, Betaproteobacteria, Deltaproteobacteria, Actinobacteria, Firmicutes, Bacteroidetes, Cyanobacteria, Planctomycetes, Archaea and Others) were functionally re-annotated using the Workbench tool, and their functions were compared with an analysis of variance using a Tukey–Kramer post-hoc test, an eta-squared effect size and a Benjamin–Hochberg multiple test correction. In all these cases, P-values of <5% were considered statistically significant.
Associated fauna analysis
For each sampling point, the fauna associated with the rhodolith was analyzed. These rhodoliths were preserved in 10% formalin, and the faunal organisms were analyzed with regard to the high-level taxonomic groups, such as phylum, class, order and family, when possible using stereomicroscopy.
Primary productivity of rhodoliths
To determine the photosynthetic activity of rhodoliths in the Abrolhos Bank, we applied the pulse-amplitude-modulated method (PAM) that uses variations in the effective quantum yield of photosystem II as a method to assess the photosynthetic performance of photosystem II (Schereiber et al., 1966). Briefly, the change in fluorescence emitted by chlorophyll before and after the stimulation with a saturating light pulse is used to calculate the effective quantum yield (ΔF/Fm′) (Maxwell and Johnson, 2000). The underwater DIVING PAM (Walz, Germany) was used directly in the field with a red light under ambient light. DIVING PAM measurements took place 33 m deep in rhodolith beds at 1300 hours on 17 August 2011. A total of 58 measurements were taken in 12 different rhodolith specimens—five measurements per specimen. A consistent distance of 1 cm between the fiberoptic sensor and the rhodolith surface was maintained using an adaptation of the DIVING PAM sample holder. The yield (ΔF/Fm′) was measured using a 0.8 period of saturating light (ca. 8000 μmol m−2 per s), and an absorption factor of 0.56 was applied for electron transport rate (ETR) calculations, as previously determined for Rhodophyta (Beer and Axelsson, 2004) (ETR=ΔF/Fm′ × PAR × 0.5 × 0.56, where PAR is the photosynthetic active radiation at the sampling site and 0.5 is the factor that accounts for the distribution of electrons between photosystem I and photosystem II). Similarly, the theoretical model of 0.25 mol of O2 for each mol of electrons was also used in the ETR calculations (Beer and Axelsson, 2004).
Rhodolith dissolved organic carbon release
Incubation experiments were performed to determine the amount of fixed carbon released by the rhodoliths in the form of exudates. Single rhodoliths, ∼10 cm in diameter, were randomly collected and placed inside translucent Niskin bottles, which allow photosynthetically active radiation to pass through for light treatment, or inside covered Niskin Bottles (dark) as a control. The treatments and controls were performed in three replicates, and the experiment was repeated twice. Incubation was carried out in situ (at a depth of 30 m) for 2 h from 1300 to 1500 hours using water from near the bottom of the seafloor—the same water mass to which rhodoliths are normally exposed. Dissolved organic carbon (DOC) was measured at time 0 and 2 h later, and the variation in DOC concentrations was analyzed. For the DOC measurements, bottled water samples were filtered using pre-combusted (550 °C) Whatman GF/Filters, preserved using 10% H3PO4, and stored and refrigerated in amber glass bottles. Samples were further acidified with 2 N HCl and sparged with ultrapure air, and the DOC was determined by high-temperature catalytic oxidation in a Shimadzu TOC 5000 Analyzer (Ovalle et al., 1999).
Results
A total of 60.85 million non-redundant nucleotide bases (Mb) were generated in this study. Approximately 165 000 high-quality metagenome sequences from seven different rhodoliths from three different sampling sites in the outer region of the Abrolhos Bank were obtained. After quality control, each rhodolith metagenome contained between 17 863 and 32 488 reads, in which an average of 24% was classified by MG-RAST taxonomically (a total of 41 051 sequences) and functionally (a total of 39 691 sequences) (Supplementary Table S1). The major contributing domain was Bacteria (∼83.64%). The contribution of Archaea was ∼3%, whereas that of the Eukarya domain was the most variable, ranging from 6.5 to 27.4% (Supplementary Table S1).
Taxonomic assignment of rhodolith metagenomes
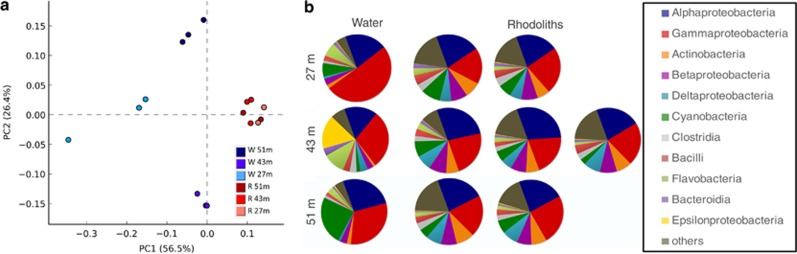
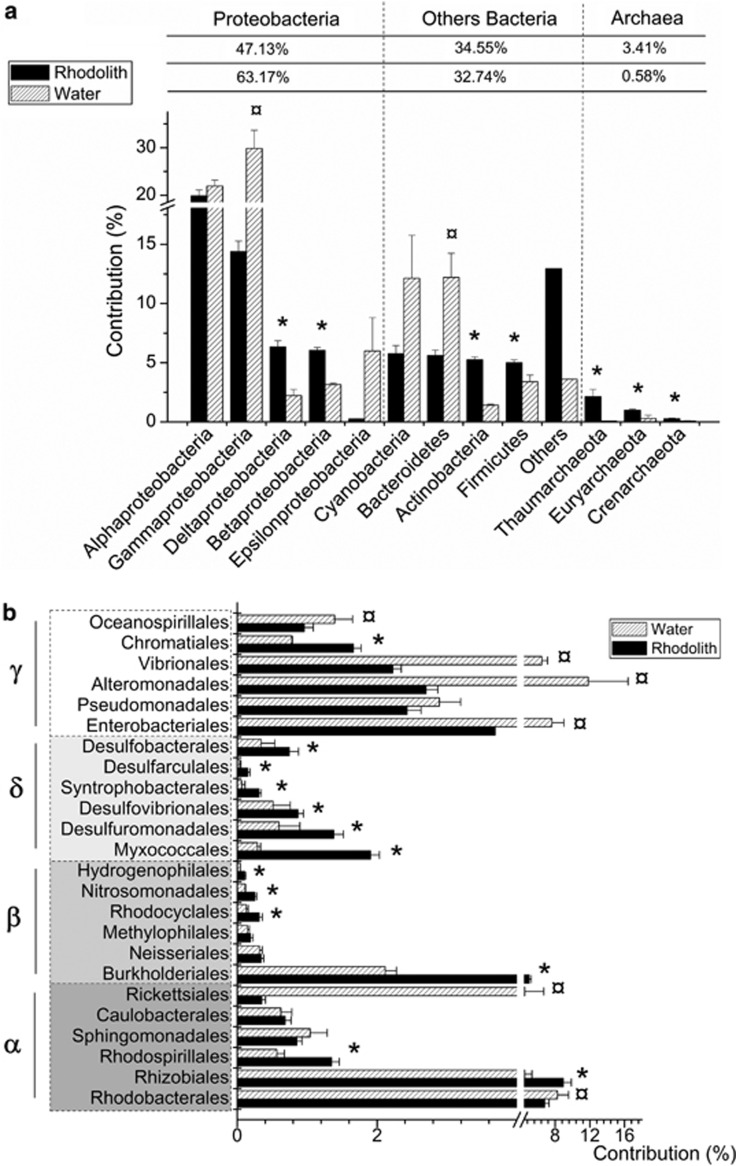
Rhodolith replicates harbor impressive homogenous and characteristic communities of Bacteria (Figure 2). When compared with the surrounding seawater metagenomes, rhodolith metagenomes from different sampling sites clustered closely together based on class-contribution grouping, despite the clustering of surrounding water samples based on site and depth (Figure 2a). The bacterial class composition was extremely consistent among the seven rhodoliths and did not show any site or depth correlations, despite the variability in the surrounding water detailed and discussed by Cavalcanti et al. (2013) (Figure 2b). The majority of bacterial sequences were assigned to the Proteobacteria phylum (an average of 47.13%), followed by Actinobacteria, Firmicutes and Cyanobacteria, contributing ∼5% each (Figure 3a). The Alpha- and Gammaproteobacteria together were responsible for 72.65% of the Proteobacterial sequences; nevertheless, a significantly greater contribution was observed for Beta- and Deltaproteobacterial classes relative to the surrounding water. This increased contribution was observed for the majority of orders of Beta- and Deltaproteobacteria, with a remarkable contribution for Burkolderiales (over 5%) (Figure 3b). The other rhodolith-holobiont components, Archaea and Eukarya, had a more variable constitution between individual rhodoliths. The most abundant archaeal phylum was Thaumarchaeota (ranging from 0.48 to 4.12%), with a greater contribution in the samples obtained from deeper water (51 m). In addition to Thaumarchaeota, both Euryarchaeota and Crenarchaeota phyla (a total of 1.26%) were statistically more abundant in the rhodolith metagenomes compared with the surrounding water (Figure 3a). For the Eukaryota domain—the most variable domain in our study—over 60% of all the sequences were identified as invertebrate marine phyla Chordata, Arthropoda and Cnidaria, and as algal groups Streptophyta and Chlorophyta (Supplementary Table S2).
Figure 2.
Homogeneity of the rhodolith metagenome. Rhodoliths and the surrounding seawater from depths of 27, 43 and 51 m were compared. (a) Principal component analysis (PCA) grouping rhodolith (red) and seawater (blue) metagenomes by class contribution. (b) Taxonomic composition of the rhodolith and surrounding water metagenomes. A taxonomic assignment was performed using MG-Rast based on sequence similarities to the GenBank database.
Figure 3.
Taxonomic composition of rhodolith metagenomes. A taxonomic assignment was performed using MG-Rast based on sequence similarities to the GenBank database. (a) The average percentage of sequences from rhodolith and the surrounding water metagenome with the best BLAST similarity to Archaeal and Bacterial domains (phylum Proteobacteria and other Bacteria phyla). (b) More detailed breakdown of the Proteobacteria group. Relative contribution of the main proteobacterial orders in the rhodolith and the surrounding seawater metagenomes. Comparison of taxonomic contributions between rhodoliths and the surrounding water: (*) represents groups that are significantly over-represented in the rhodolith metagenome (P<0.5); (¤) represents groups that are significantly over-represented in the seawater metagenome (P<0.5).
Metabolic diversity of rhodolith metagenomes
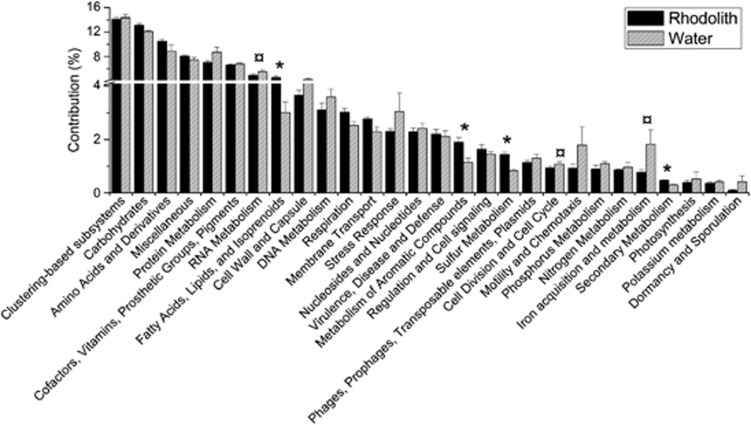
The overall functional annotation (subsystems level 1) of rhodoliths was compared with the surrounding water metagenomes (Figure 4). Rhodoliths presented more sequences related to fatty acids, lipids and isoprenoids (P=4.38 × 10–2), metabolism of aromatic compounds (P=7.9 × 10–3), secondary metabolism (P=4.88 × 10–4) and sulfur metabolism (P=6.97 × 10–3). The water samples had more sequences related to cell division and cell cycle (P=3.76 × 10–2), iron acquisition and metabolism (P=3.31 × 10–2), and RNA metabolism (P=3.47 × 10–2). At the second and third levels, other observed noteworthy differences are listed below, and the rest are summarized in Supplementary Table 3. Briefly, fermentation (P=1.92 × 10–2)—particularly acetone butanol ethanol (ABE) synthesis (P=2.21 × 10–2) and butanol biosynthesis (P=2.09 × 10–2), sulfatases and modifying factors (P=8.31 × 10–4), fatty acids (P=6.24 × 10–3), ABC transporters (P=3.74 × 10–4), metabolism of central aromatic intermediates (P=3.27 × 10–2), peripheral pathways for the catabolism of aromatic compounds (P=3.37 × 10–2), biologically active compounds in metazoan cell defense (P=2.76 × 10–2), steroid sulfates (P=2.06 × 10–2), galactosylceramide and sulfatide metabolism (P=3.05 × 10–3), and organic sulfur assimilation (P=3.36 × 10–2)—was increased in the rhodoliths. Alternatively, DNA replication (P=2.73 × 10–3), the recBCD DNA repair pathway (P=3.97 × 10–2), bacterial cell division (P=2.84 × 10–2), the organic-acid-generating methylcitrate cycle (P=3.41 × 10–3), and the propionate-CoA to succinate module (P=9.34 × 10–3), were reduced in the rhodoliths compared with the surrounding water. The comparisons of the most abundant groups revealed distinguishing features. Most metabolic pathways were shared between all the groups, with the following noteworthy exceptions. Potassium metabolism was higher in Alphaproteobacteria (P=1.15 × 10–5). Respiration (P=2.77 × 10–3) and photosynthesis (P=8.46 × 10–7) were increased in Eukarya, with reasonably large representation in Archaea for respiration and in Cyanobacteria for photosynthesis. Proteolytic pathways were also more abundant in Eukarya (P=9.64 × 10–4). Iron acquisition and metabolism were more prominent in Bacteroidetes (P=7.53 × 10–3), although Gammaproteobacteria were enriched in siderophores (P=5.57 × 10–3), and Alphaproteobacteria had an over-representation of heme and hemin uptake hits (P=1.6 × 10–2). Archaea showed a predominant representation of several subsystems, including organic acids (P=3.3 × 10–2), adhesion (P=1.86 × 10–5), denitrification (P=9.91 × 10–3) and inorganic sulfur assimilation (P=8.71 × 10–7), although none of these roles were exclusive to this domain. Betaproteobacteria were distinguished by fatty-acid degradation (P=7.42 × 10–3), and Deltaproteobacteria were distinguished by the invasion and intracellular resistance subsystem (P=1.3 × 10–2). Some groups (Actinobacteria, Bacteroidetes, Cyanobacteria and Firmicutes) had higher numbers of DNA repair hits (P=3.01 × 10–4), but this subsystem was well represented in most bacterial groups. The cAMP signaling was highly represented in Cyanobacteria and Betaproteobacteria (P=1.75 × 10–5). Type III protein secretion system orphans (P=4.9 × 10–2), type IV secretion systems (P=9.16 × 10–3) and type VIII secretion systems (P=4.4 × 10–2) were also increased in Gammaproteobacteria, Betaproteobacteria and Bacteroidetes, respectively.
Figure 4.
Functional profile. Relative contribution of subsystems (first-level hierarchy) in the rhodolith and surrounding seawater metagenomes. Comparison of functional contributions between rhodoliths and the surrounding water: (*) represents subsystems that are significantly over-represented in the rhodolith metagenome (P<0.5); (¤) represents subsystems that are significantly over-represented in the seawater metagenome (P<0.5).
Fauna taxonomic identification
The most frequent taxonomic groups associated with the sampled rhodoliths were the Foraminiferea, Bryozoa, Annelida and Mollusca phyla, which were observed in all the samples, although represented by different morphotypes. Regarding the epifauna, colonial encrusting forms covered large rhodolith areas, where the most abundant group was Bryozoa, followed by Foraminiferea, particularly Homotrema rubrum. The phylum Annelida was largely represented by the encrusting calcareous tubes of Polychaeta. The occurrence of the phylum Mollusca was observed by the residual shells from Bivalvia and Gastropoda, particularly from the Vermetidae family. The infauna was mainly composed of calcareous tubes of Polychaeta, followed by Bryozoa and Foraminiferea. Different morphotypes (approximately five) of free-living Foraminiferea were observed in all the samples, showing the highest species richness compared with the other groups of fauna associated with rhodoliths.
Rhodoliths from the depths of 43 and 51 m showed lower species richness compared with the 27 m sample. Rhodoliths from a depth of 43 m were mainly associated with filamentous green algae, and the sample from this depth was the only containing one specimen and shell marks of Lithophaga (Bivalvia). On the sample from the depth of 51 m, a morphotype of Brachiopoda and residuals of Echinoidea (Echinodermata) calcareous skeletons were found. The sample from a depth of 27 m presented a different fauna composition from the others, with a large rhodolith surface area covered by colonial forms of Ascidiacea (phylum Chordata, subphylum Tunicata) and Porifera, in addition to Bryozoa, Foraminiferea, Polychaeta and Vermetidae, completing the epifauna. The most abundant groups, primarily because of their encrusting colonial habits, were Ascidiacea and Porifera, followed by Bryozoa and Foraminiferea. In this sample, we observed the occurrence of other groups, including the phyla Platyhelminthes, Nematoda and Arthropoda (subphylum Crustacea), and the class Polyplacophora (phylum Mollusca), although with only one specimen registered for each group.
Primary productivity of rhodoliths
Photosynthetically active radiation from Lithothamnion crispatum at a depth of 33 m, the site of measurements, was 1436±47.84 μmol photons m−2 s−1 (average±s.e.). The effective quantum yield of photosystem II determined for the rhodoliths ranged from 0.206 to 0.78, with a mean value of 0.511, which allowed linear correlations between ETR and CO2 fixation. These values were considerably above the 0.1 critical limit value for these correlations. The ETR was 208.67±10.19 μmol electrons m−2 s−1 (average±s.e.). Therefore, the calculated CO2 fixation rate was 52.16±2.57 μmol carbon m−2 s−1 or 27.03 g carbon m−2 per day (Table 1).
Table 1. Calcifying algae productivity. Photosynthetic capacity of free-living rhodolith holobionts and crustose coralline algae.
| Organism | Depth (m) | Temperature (°C) | Irradiance (μMol photons m−2 s−1) | Fixed carbon (μMol C m−2 s−1) | Reference |
|---|---|---|---|---|---|
| Rhodoliths | 33 | 26 | 1436±47 | 52.1±2.5 | This study |
| CCA | 6–7 | 26 | 9207±907 | 274.5±36.5 | This study |
| P. foecundum (CCA) | 15–18 | 0–1 | <5 | 45.2–66.9 | Roberts et al., 2002 |
| P. tenue (CCA) | 15–18 | 0–1 | <5 | 42.5–47.2 | Roberts et al., 2002 |
| M. Engerlhartii (CCA) | 16–20 | 0–1 | <5 | 9.4–17.9 | Schwarts et al., 2005 |
Rhodolith organic carbon release
The concentration of DOC in the water just above the rhodolith beds was 1.85±0.32 mg l−1 (average±s.d.). The DOC concentration increased after the 2 h of incubation, with ΔDOC of 0.13 and 0.04 mg l−1 for light and dark bottles, respectively. The control bottles had DOC consumptions of 0.26 and 0.28 mg l−1 for light and dark bottles, respectively, likely because of the planktonic activity in the Niskin bottles. Discounting the amount of organic carbon consumed by the plankton, we observed that rhodoliths released 0.39±12 and 0.32±19 mg l−1 of DOC in light and dark incubations, respectively, showing no significant difference between these two treatments (P=0.8). The release rate of organic carbon was 0.04 mg ± 0.01 DOC h−1dm−2 of rhodolith surface area, or 3.58 ± 1.46 μmol h−1 dm−2.
Discussion
Our rhodolith metagenomic analysis demonstrated the wealth of microorganisms that inhabit this holobiont. We verified an extremely similar composition among the microbiomes of the rhodoliths from the three different sampling sites in the Abrolhos Bank—a possible reflection of a long-term host–microbe association. This association may enable the holobiont to expand its physiological capacity and geographic distribution (Hollants et al., 2012). Polychaetes, crustaceans (amphipods), ophiuroids and mollusks, commonly found in the rhodolith infauna of the Abrolhos Bank, were also the main invertebrate taxa inhabiting the rhodoliths analyzed in this study. Our metagenomic analysis extended these previous observations (Figueiredo et al., 2007), detecting other types of flora and fauna (for example, foraminifers).
It is plausible that the genesis and growth of rhodoliths are intimately related to their microbial constituents. The remarkable homogeneity revealed in the rhodolith microbiomes hints at the existence of a close relationship between the microbes and their rhodolith host. Indeed, despite a lack of knowledge of the principles underlying the assembly and the structure of these complex microbial communities, other examples of host specificity have also been illustrated for macroalgal–bacterial interactions (Hollants et al., 2012). Most of the rhodolith metagenomic sequences (over 70%) were identified as bacterial protein-coding sequences. Some bacterial taxa, such as Alphaproteobacteria, Firmicutes and Actinobacteria, are as abundant in the rhodolith holobiont (19.7, 5.8 and 5.8%, respectively) as in other red seaweed–microbe holobionts (13, 10 and 9%, respectively). However, some taxa were particularly enriched in rhodoliths (for example, Betaproteobacteria with 6.5% and Deltaproteobacteria with 5.7%) compared with other red seaweeds (Hollants et al., 2012). The metabolically diverse groups of the aerobic ammonia-oxidizing betaproteobacteria Nitrosomonadales, Rodhocyclales and Hydrogenophylales, and the dissimilative sulfate-reducing deltaproteobacteria Desulfovibrionales, Desulfobacterales and Desulforomonadales were significantly increased in the rhodolith microbiome compared with the surrounding water.
Job partitioning in the rhodolith holobiont
We observed significant contributions of photolithoautotrophs (that is, cyanobacteria (5.09%)), anaerobic heterotrophs (predominantly sulfate-reducing proteobacteria (1.52%)), sulfide oxidizers (0.37%), anoxygenic phototrophs (that is, purple (2.33%) and green sulfur bacteria (1.33%)) and methanogens (0.46%) in the rhodolith metagenome. These guilds with similar metabolic roles operate in collaboration to accomplish the cycling of key elements (O, N, S and C), which may allow them to carry out important tasks, such as biomineralization (Dupraz et al., 2009). Microbially induced carbonate precipitation, which occurs in microbialites, is carried out by the interaction of microbial consortia, their growth and metabolism, cell surface properties, and extracellular polymeric substances (Dupraz et al., 2009). Calcium carbonate formation and precipitation are central processes in rhodolith construction. The corallinaceae algae are considered to be major factors in mineralization in rhodoliths. Comparatively little attention has been paid to the role of rhodolith microbes in calcium carbonate formation and precipitation. Although the diversity and abundance of microbes provide only a cursory indication of what is metabolically feasible, it is noteworthy that in the rhodolith microbiome, we found major functional groups related to microbial-induced organomineralization, which may suggest an important but still unappreciated role of microbes in carbonate precipitation in rhodoliths.
This functional metagenomic profile offers insights into the metabolism of the rhodolith holobiont. Whereas water-column microbes seem more prone to cell division, DNA repair and iron acquisition, rhodolith-associated microbes are more specialized in chemical communication. This interplay is suggested by the increased representation of secondary metabolites, metazoan-active cell compounds and specialized bacterial secretion systems in the rhodolith metagenome. Fatty-acid metabolism and degradation are also important, hinting that an interaction likely occurs in the algal cell membrane. A notable amount of capsular and extracellular polysaccharide hits indicate a structured biofilm in association with this surface. Although the rhodolith biofilm is clearly less impressive than coral mucus or microbial mats, the presence of certain taxonomic groups suggests that a considerable degree of spatial structuring occurs in this biofilm, providing microenvironments that support different metabolic guilds, akin to corals (Garcia et al., 2013), and microbial mats (Dupraz et al., 2009). Interestingly, it has long been known that the cell wall or mucilage is the calcification site in coralline red algae, such as rhodoliths (Borowitzka, 1987), perhaps with an intravesicular stage that could involve the Golgi apparatus (Krumbein and Larkum, 1987). Mineralization in these algae is strictly controlled, as every aspect of the process is regulated by the eukaryotic cell (Borowitzka, 1987; Dupraz et al., 2009). Given this close affinity, it is likely that the microbial composition and metabolic rates associated with this structure will influence its physicochemical characteristics (Dupraz and Visscher, 2005; Dupraz et al., 2009). The promotion (+) or hindrance (−) of calcium carbonate precipitation is determined by the net effect of competing and antagonistic microbial metabolic processes, which cannot be predicted based only on the functions revealed by metagenomics (Khodadad and Foster, 2012). However, our study revealed interesting features of the rhodolith metagenome. For instance, the main energy production and carbon fixation pathways appear to be via algal photosynthesis (+). Cyanobacteria appear to have a secondary role in this system. The main carbon-cycling balance (photosynthesis (+)/respiration(−)) is performed by Eukarya, unlike in stromatolites (Dupraz et al., 2009). Microbial metabolism may still have a role in terms of anoxygenic photosynthesis (+), fermentation (−), and aerobic (−) and anaerobic respirations (+) (sulfate reduction, denitrification) (Krumbein and Larkum, 1987; Visscher et al., 1998; Dupraz and Visscher, 2005; Dupraz et al., 2009). Although some organic-acid-generating metabolic pathways (−) are reduced in rhodoliths, the higher representation of fermentation (−), butanol biosynthesis (−) and ABE synthesis (−) suggest a negative role for microbes in rhodolith calcification. However, sulfate oxidizers (+) and anoxygenic photosynthesis (+) guilds were detected in this holobiont and could significantly promote the precipitation of calcium carbonate in the rhodoliths.
Another potential influence of the microbes in rhodoliths is in sulfur cycling, similar to modern marine stromatolites in the Bahamas (Visscher et al., 1998) and Mexico (Breitbart et al., 2009). The negatively charged sulfated polysaccharides of the algal cell wall are likely the ligands for Ca2+ ions (Borowitzka, 1987). Theoretically, these polysaccharides can also limit carbonate precipitation by sequestering these ions in the organic matrix (Dupraz et al., 2009). We also observed steroid sulfates. The presence of sulfatases, sulfatide metabolism and organic sulfur assimilation pathways indicates a microbial role in the cleavage and cycling of these sulfur radicals, similar to the model proposed for microbialites (Breitbart et al., 2009). The importance of degradation of fatty acids and several aromatic compounds through central and peripheral pathways also suggests a degradation of the mucilage/biofilm interface. The release of the bound Ca2+ ions and carboxyl groups through degradation by aerobic respiration (Breitbart et al., 2009) or consortia between sulfate reducers and fermenters (Dupraz et al., 2009) can also promote mineralization independently of the light/oxygen conditions, which could explain the low rates of dark calcification sometimes observed (Borowitzka, 1987; Krumbein and Larkum, 1987). This accessory role of precipitation should be most prevalent in the space between algal cells and between these cells and the substratum, in which the influence of the coralline algae would be lessened. This collective role would explain the selective nature of some of the microbes associated with this holobiont. Alphaproteobacteria could also be responsible for potassium cycling and iron inputs together with Bacteroidetes and Gammaproteobacteria. Archaea input inorganic sulphur into the system but secrete organic acids, possibly hindering carbonate deposition.
Rhodoliths are the major dissolved carbon biofactories
Notably, the rhodolith holobiont photosynthetic capacity measured in this study via a PAM-based analysis showed high photosynthesis rates for Abrolhos rhodoliths (52.16 μmol carbon m−2 s−1) when compared with other crustose coralline algae, exemplified by Phymatolithon foecundum (45.2–66.9), Phymatolithon tenue (42.5–47.2) and Mesophyllum engelhartii (9.4–17.9) (Table 1) (Roberts et al., 2002; Schwarz et al., 2005). Nevertheless, the gross photosynthetic rate of 27.03 g C m−2 per day calculated for the rhodolith beds was far below the previous values of 462 and 2062 mg C m−2 per day for coastal and open ocean mesotrophic phytoplankton, respectively (Liu et al., 1997; Morán et al., 2002). These data may suggest that the rhodoliths also function as a heterotrophic system because of their large associated fauna, but that part of the fixed carbon is released as DOC. We calculated a rate of 3.58 μmol C h dm−2 organic carbon released in the same range found for crustose coralline algae in Moorea, French Polynesia. On the basis of the 20 902 km2 of bank extension determined previously (Amado-Filho et al., 2012) and the photosynthetic rates calculated here, the estimated total photosynthetic activity for the Abrolhos Bank is calculated to be 56.5 × 104 tons C per day. This estimate may illustrate the great importance of the Abrolhos rhodolith beds for DOC production in the South Atlantic Ocean.
Conclusion
The present study extends the previous studies on rhodoliths (Amado-Filho et al., 2012), exploring the possible roles of microbes in the structure and function of rhodoliths, as well as the systemic roles of rhodoliths in the production of DOC. Calcifying algae have a significant role in the ocean carbon cycle, not only in the deposition of calcium carbonate but also in their photosynthetic capacity. Future studies utilizing metatranscriptomics (Khodadad and Foster, 2012), stable isotopes (Breitbart et al., 2009) or microelectrodes (Visscher et al., 1998) could extend these observations and shed new light on the biology of rhodoliths. Our first attempt to characterize the microbiome of the rhodoliths allowed us to determine the major microbial components of the rhodolith holobiont. The impressively homogeneous bacterial contribution among rhodolith replicates, coupled with the increased representation of pathways associated with aromatic compounds, fatty acids and secondary metabolism, suggests an important chemically mediated interaction within the rhodolith holobiont. We propose that the rhodolith microbial guilds, based on their extended metabolic capacity, have important roles in the rhodolith holobiont functioning—for example, in biomineralization.
Acknowledgments
We acknowledge SISBIOTA CNPq/FAPES, FAPERJ, CNPq and CAPES for funding. We also acknowledge the Project for the International Research Center for Infectious Diseases, Research Institute for Microbial Diseases, Osaka University, from the Ministry of Education, Science, Sports, Culture, and Technology, Japan. FT. Giselle Cavalcanti would like to thank the CNPq for research and PhD fellowships. This paper is part of the DSc requirements for Giselle da Silva Cavalcanti in the Biodiversity and Evolutionary Biology Graduate Program of the Federal University of Rio de Janeiro.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Amado-Filho GM, Moura RL, Bastos AC, Salgado LT, Sumida PY, Guth AZ, et al. Rhodolith beds are major CaCO3 bio-factories in the tropical South West Atlantic. PLoS One. 2012;7:e35171. doi: 10.1371/journal.pone.0035171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia RG, Abrantes DP, Brasileiro PS, Pereira GH, Amado GM. Rhodolith bed structure along a depth gradient on the northern coast of Bahia state, Brazil. Braz J Oceanogr. 2010;58:323–337. [Google Scholar]
- Barbera C, Bordehore C, Borg JA, Glemarec M, Grall J, Hall-Spencer JM, et al. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquat Conserv. 2003;13:S65–S76. [Google Scholar]
- Barott KL, Rodriguez-Brito B, Janouškovec J, Marhaver KL, Smith JE, Keeling P, et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol. 2011;13:1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- Barott KL, Rohwer FL. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 2012;20:621–628. doi: 10.1016/j.tim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Bastos AC, Moura RL, Amado-Filho GM, D'Agostini DP, Secchin NA, Francini-Filho RB, et al. 2013Buracas: novel and unusual sinkhole-like features in the Abrolhos Bank Cont Shelf Res(in press).
- Beer S, Axelsson L. Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances. Eur J Phycol. 2004;39:1–7. [Google Scholar]
- Borowitzka MA. Calcification in Algae—mechanisms and the role of metabolism. Crit Rev Plant Sci. 1987;6:1–45. [Google Scholar]
- Breitbart M, Hoare A, Nitti A, Siefert J, Haynes M, Dinsdale E, et al. Metagenomic and stable isotopic analyses of modern freshwater microbialites in Cuatro Cienegas, Mexico. Environ Microbiol. 2009;11:16–34. doi: 10.1111/j.1462-2920.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011;5:590–600. doi: 10.1038/ismej.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti GS, Gregoracci GB, Longo LL, Bastos AC, Ferreira CM, Francini-Filho RB, et al. 2013Sinkhole-like structures as bioproductivity hotspots in the Abrolhos Bank Continental Shelf Research(in press).
- Dias GTM. Granulados bioclásticos—algas calcárias. BrazJ Geophys. 2001;18:307–308. [Google Scholar]
- Dupraz C, Visscher PT. Microbial lithification in marine stromatolites and hypersaline mats. Trens Microbiol. 2005;13:429–438. doi: 10.1016/j.tim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Dupraz C, Reid RP, Braissant O, Decho AW, Norman RS, Visscher PT. Processes of carbonate precipitation in modern microbial mats. Earth-Sci Rev. 2009;96:141–162. [Google Scholar]
- Figueiredo MADO, Menezes KSD, Costa-paiva EM, Paiva PC, Ventura CRR. Experimental evaluation of rhodoliths as living substrata for infauna at the Abrolhos Bank, Brazil. Cienc Mar. 2007;33:427–440. [Google Scholar]
- Foster MS. Rodotlihs: between rocks and soft places. J Phycol. 2001;37:659–667. [Google Scholar]
- Garcia GD, Gregoracci GB, de OSE, Meirelles PM, Silva GG, Edwards R, et al. Metagenomic analysis of healthy and white plague-affected Mussismilia braziliensis Corals. Microb Ecol. 2013;65:1076–1086. doi: 10.1007/s00248-012-0161-4. [DOI] [PubMed] [Google Scholar]
- Hollants J, Leliaert F, De Clerck O, Willems A. What we can learn from sushi: a review on seaweed-bacterial associations. Fems Microbiol Ecol. 2012;83:1–16. doi: 10.1111/j.1574-6941.2012.01446.x. [DOI] [PubMed] [Google Scholar]
- Kamenos NA, Moore PG, Hall-Spencer JM. Maerl grounds provide both refuge and high growth potential for juvenile queen scallops (Aequipecten opercularis L.) Mar Ecol. 2004;313:241–254. [Google Scholar]
- Khodadad CLM, Foster JS. Metagenomic and metabolic profiling of nonlithifying and lithifying stromatolitic mats of Highborne Cay, The Bahamas. PLoS One. 2012. p. 7. [DOI] [PMC free article] [PubMed]
- Krumbein MA, Larkum AWD. Calcification in algae: mechanisms and the role of metabolism. Crit Rev Plant Sci. 1987;6:1–45. [Google Scholar]
- Liu H, Nolla HA, Campbell L. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Microb Ecol. 1997;12:39–49. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, et al. The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán XAG, Estrada M, Gasol JM, Pedrós-Alió C. Dissolved primary production and the strength of phytoplankton- bacterioplankton coupling in contrasting marine regions. Microbial Ecol. 2002;44:217–223. doi: 10.1007/s00248-002-1026-z. [DOI] [PubMed] [Google Scholar]
- Nelson WA. Calcified macroalgae—critical to coastal ecosystems and vulnerable to change: a review. Mar Freshwater Res. 2009;60:787–801. [Google Scholar]
- Ovalle ARC, Rezende CE, Carvalho CEV, Jennerjahn TC, Ittekkot V. Biogeochemical characteristics of coastal waters adjacent to small river-mangrove systems, East Brazil. Geo-Mar Lett. 1999;19:179–185. [Google Scholar]
- Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Beiko RG. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26:715–721. doi: 10.1093/bioinformatics/btq041. [DOI] [PubMed] [Google Scholar]
- Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. The coral probiotic hypothesis. Environ Microbiol. 2006;8:2068–2073. doi: 10.1111/j.1462-2920.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- Riul P, Lacouth P, Pagliosa PR, Christoffersen ML, Horta PA. Rhodolith beds at the easternmost extreme of South America: community structure of an endangered environment. Aquat Bot. 2009;90:315–320. [Google Scholar]
- Roberts RD, Kuhl M, Glud RN, Rysgaard S. Primary production of crustose coralline red algae in a high Arctic fjord. J Phycol. 2002;38:273–283. [Google Scholar]
- Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser. 2002;243:1–10. [Google Scholar]
- Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Zilber-Rosenberg I. Symbiosis and development: the hologenome concept. Birth Defects Res C Embryo Today. 2011;93:56–66. doi: 10.1002/bdrc.20196. [DOI] [PubMed] [Google Scholar]
- Schereiber U, Schiliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1966;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Schwarz AM, Hawes I, Andrew N, Mercer S, Cummings V, Thrush S. Primary production potential of non-geniculate coralline algae at Cape Evans, Ross Sea, Antarctica. Mar Ecol Prog Ser. 2005;294:131–140. [Google Scholar]
- Steller DL, Riosmena-Rodriiguez R, Foster MS, Roberts CA. Rhodolith bed diversity in the Gulf of California: the importance of rhodolith structure and consequences of disturbance. Aquat Conserv. 2003;13:S5–S20. [Google Scholar]
- Visscher PT, Reid RP, Bebout BM, Hoeft SE, Macintyre IG, Thompson JA. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): The role of sulfur cycling. Am Mineral. 1998;83:1482–1493. [Google Scholar]
- Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F. The second skin: ecological role of epibiotic biofilms on marine organisms. Front Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NS, Cobb RE, Soo R, Anthony SL, Battershill CN, Whalan S, et al. Bacterial community dynamics in the marine sponge rhopaloeides odorabile under in situ and ex situ cultivation. Mar Biotechnol. 2011;13:296–304. doi: 10.1007/s10126-010-9300-4. [DOI] [PubMed] [Google Scholar]
- Whalan S, Webster NS, Negri AP. Crustose coralline algae and a cnidarian neuropeptide trigger larval settlement in two coral reef sponges. PLoS One. 2012;7:e30386. doi: 10.1371/journal.pone.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Blake C, Berges JA, Maggs CA. Environmental tolerances of free-living coralline algae (maerl): implications for European marine conservation. Biol Conserv. 2004;120:279–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.