Abstract
Phytoplankton produce large amounts of polysaccharide gel material known as transparent exopolymer particles (TEP). We investigated the potential links between phytoplankton-derived TEP and microbial community structure in the sea surface microlayer and underlying water at the English Channel time-series station L4 during a spring diatom bloom, and in two adjacent estuaries. Major changes in bacterioneuston and bacterioplankton community structure occurred after the peak of the spring bloom at L4, and coincided with the significant decline of microlayer and water column TEP. Increased abundance of Flavobacteriales and Rhodobacterales in bacterioneuston and bacterioplankton communities at L4 was significantly related to the TEP decline, indicating that both taxa could be responsible. The results suggest that TEP is an important factor in determining microbial diversity in coastal waters, and that TEP utilisation could be a niche occupied by Flavobacteriales and Rhodobacterales.
Keywords: bacterioplankton, bacterioneuston, transparent exopolymer particles, ion torrent, coastal, estuarine
Bacterioplankton communities at the English Channel L4 time-series station have repeating seasonal-scale diversity patterns (Gilbert et al., 2012). Rickettsiales dominate during the winter when primary production is low, whereas Rhodobacterales dominate during the spring and autumn when primary production is high (Gilbert et al., 2009, 2012). Similar changes in diversity during and following spring diatom blooms in the North Sea indicate that specialised bacterioplankton populations are adapted to utilise phytoplankton-derived organic matter (Teeling et al., 2012). Bacterioplankton carbohydrate-active enzymes (CAZymes) increase with phytoplankton blooms, suggesting that phytoplankton-derived polysaccharides are particularly important in affecting bacterioplankton diversity (Teeling et al., 2012).
Transparent exopolymer particles (TEP) are polysaccharide gels that are formed from phytoplankton-derived precursors, are abundant in seawater and typically peak in concentration towards the end of phytoplankton blooms (Passow, 2002). TEP from the water column can rise up to the air–sea interface (sea surface microlayer; Azetsu-Scott and Passow, 2004), and form directly in the microlayer (Wurl et al., 2011). TEP in the sea surface microlayer are important because they contribute to the physicochemical structure of the microlayer, and could provide habitat and resource for microlayer bacteria (bacterioneuston; Cunliffe et al., 2013).
This study aimed to determine potential links between the bacterioplankton and bacterioneuston communities at L4 station with TEP. We also incorporated samples collected from two estuaries in close proximity to L4 in order to establish a broader geographical perspective (Figure 1a). 16S rRNA genes were amplified from extracted DNA using primers 515F and 806R (Caporaso et al., 2011), sequenced using the Ion Torrent PGM and analysed with the QIIME software package (Caporaso et al., 2010; Supplementary Materials and Methods).
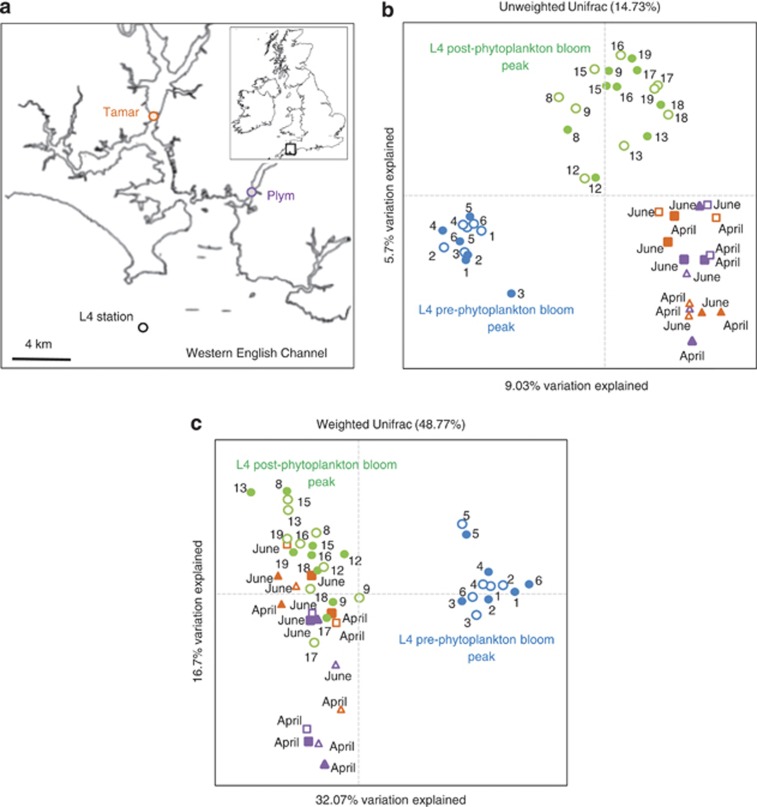
Figure 1.
(a) Location of L4 station in the Western English Channel and estuarine sampling sites on the Tamar and Plym estuaries. (b) Principal coordinates analysis plot describing betadiversity using the unweighted UniFrac distance matrix generated from OTU (97% similarity) presence/absence. Fifteen surveys were conducted over 19 weeks between 9 February and 2 July 2012 (numbers indicate week surveyed). Microlayer-enriched samples were collected using a mesh screen (sampling depth ⩽400 μm; open shapes) and underlying water samples were collected from 2 m (closed shapes). Estuarine microlayers and underlying water were sampled twice during the study period; 25 April and 27 June Tamar high tide (orange squares) and Tamar low tide (orange triangles), and 26 April and 29 June Plym high tide (purple squares) and Plym low tide (purple triangles). (c) The same analysis as b except using a weighted UniFrac distance matrix generated from OTU relative abundance.
Comparison between L4 operational taxonomic unit (OTUs) showed that bacterioplankton and bacterioneuston communities changed considerably following the peak of the spring diatom bloom (Figures 1b and c, Supplementary Figure 1). Based on the presence/absence of OTUs, estuarine bacterioplankton and bacterioneuston communities were distinct from the L4 communities (Figure 1b), however, some estuarine communities became similar to the L4 post-phytoplankton bloom communities when the relative abundance of OTUs was considered (Figure 1c).
Previous studies have raised the issue of the representativeness of L4 station (Caporaso et al., 2012; Gilbert et al., 2012). This study indicates that the L4 station community during periods of high primary productivity is similar to the communities from neighbouring estuarine ecosystems.
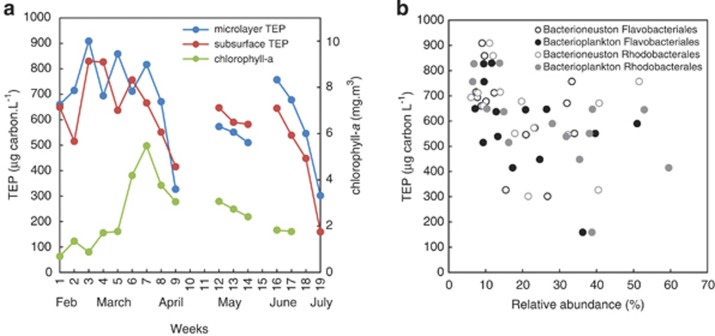
In parallel with microbial community analysis, TEP were quantified using the alcian blue dye-binding assay (Passow and Alldredge, 1995). L4 microlayer and underlying water TEP concentrations were coupled throughout the study (Spearman Correlation r=0.77, P<0.01, n=16), increasing with the spring bloom up to week 3, and rapidly decreasing between weeks 7 and 9 (Figure 2a). The same pattern of increase and rapid decrease in TEP concentration also occurred during the secondary phytoplankton bloom. TEP was significantly lower (t-test; P<0.01) in both the microlayer and underlying water after the spring bloom peak when the microbial communities had changed. The decline in TEP was not related to changes in phytoplankton biomass (chlorophyll-a) at L4. Average estuarine TEP concentrations were 799±210 μg carbon per l and 954±237 μg carbon per l in the subsurface and microlayer, respectively, and were significantly higher than the TEP concentrations at L4 during the same period (t-test; P<0.01).
Figure 2.
(a) Transparent exopolymer particle (TEP) concentration in the microlayer and underlying water at L4 station. Surveys were conducted over 19 weeks between 9 February and 2 July 2012. Phytoplankton biomass at L4 station was determined from chlorophyll-a concentration in underlying water samples. (b) Relationship between the increase in abundance of the orders Flavobacteriales and Rhodobacterales in both bacterioneuston and bacterioplankton communities and the decline of TEP in the microlayer and underlying water at L4 station.
We compared changes in TEP concentration (Figure 2a) with microbial community composition (Supplementary Figure 2) to identify potential groups linked to the rapid decreases in TEP at L4. The orders Flavobacteriales and Rhodobacterales in the subsurface and microlayer communities were negatively correlated with TEP concentration (Flavobacteriales, Spearman Correlation r=−0.50, P<0.01, n=28; Rhodobacterales, Spearman Correlation r=−0.55, P<0.01, n=28), both significantly increasing in abundance when TEP was depleted (Figure 2b). No components of the estuarine microbial communities showed similar links to TEP.
Genome analysis of Flavobacteriales isolates has identified high numbers of genes encoding the CAZymes glycoside hydrolases (Fernández-Gómez et al., 2013), and a mesocosm study has shown that extracellular glycoside hydrolases degrade diatom TEP (Smith et al., 1995). TEP are sulfated polysaccharides that are enriched with fucose (Passow, 2002), and Flavobacteriales sulfatases and fucosidases increase with spring diatom blooms (Teeling et al., 2012). Combined with the evidence from our study, we propose that the Flavobacteriales at L4 are degrading and utilising phytoplankton-derived TEP.
The Rhodobacterales at L4 were primarily members of the Marine Roseobacter Clade (MRC). The MRC are a metabolically diverse and opportunistic bacterioplankton group (Newton et al., 2010), which are known to form close associations with phytoplankton blooms (Geng and Belas, 2010). MRC responding to diatom blooms in the German Bight increase expression of low-molecular-weight substrate transporters (Teeling et al., 2012). It is possible that the Rhodobacterales at L4 could be utilising low-molecular-weight substrates produced from the Flavobacteriales-degraded TEP via cross-feeding. Supporting this idea, Gilbert et al. (2012) showed that bacteria–bacteria interactions are very important at L4 station.
Caporaso et al. (2012) have proposed that L4 bacterioplankton diversity is a result of induced changes in the abundances of a persistent and resident community. Here we show that phytoplankton-derived TEP could contribute to some of the induced community diversity responses at L4, namely, the seasonal increase of the orders Flavobacteriales and Rhodobacterales.
There is a need to connect seasonal changes in microbial community composition with geochemical processes (Giovannoni and Vergin, 2012). Marine gel particles are released from the sea into the atmosphere by fragmentation of the sea surface microlayer during bubble bursting (Russell et al., 2010), and are subsequently a source of aerosols and cloud condensation nuclei (Orellana et al., 2011). The realisation of the importance of marine gels in climate regulation has forced a fundamental paradigm shift away from an exclusively dimethylsulfide-controlled marine boundary layer, to a complex system where marine gels are integral (Quinn and Bates, 2011). This study is the first to link changes in microbial diversity with marine gels, and therefore supports a multidisciplinary understanding of marine gel biogeochemical cycling. The coupling between microlayer and underlying water TEP concentrations and bacteria community dynamics indicate that microbial TEP processing could be similar in both systems.
Acknowledgments
We thank the crew of the RV Plymouth Quest for help with sampling. Supporting data (chlorophyll-a) used in this study were provided by the Western Channel Observatory that is funded as part of the UK Natural Environmental Research Council's National Capability. This research was funded primarily through a MBA Fellowship awarded to MC.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Azetsu-Scott K, Passow U. Ascending marine particles: significance of transparent exopolymer particles (TEP) in the upper ocean. Limnol Oceanogr. 2004;49:741–748. [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;15:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Paszkiewicz K, Field D, Knight R, Gilbert JA. The Western English Channel contains a persistent microbial seed bank. ISME J. 2012;6:1089–1093. doi: 10.1038/ismej.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe M, Engel A, Frka S, Gašparović B, Guitart C, Murrell JC, et al. Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface. Prog Oceanogr. 2013;109:104–116. [Google Scholar]
- Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas SG, González JM, et al. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J. 2013;7:1026–1037. doi: 10.1038/ismej.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Belas R. Molecular mechansims underlying roseobacter-phytoplankton symbioses. Curr Opin Biotechnol. 2010;21:332–338. doi: 10.1016/j.copbio.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Field D, Swift P, Newbold L, Oliver A, Smyth T, et al. The seasonal structure of microbial communities in the Western English Channel. Environ Microbiol. 2009;11:3132–3139. doi: 10.1111/j.1462-2920.2009.02017.x. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Steele JA, Caporaso JG, Steinbrück L, Reeder J, Temperton B, et al. Defining seasonal marine microbial community dynamics. ISME J. 2012;6:298–308. doi: 10.1038/ismej.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni SJ, Vergin KL. Seasonality in ocean microbial communities. Science. 2012;335:671–676. doi: 10.1126/science.1198078. [DOI] [PubMed] [Google Scholar]
- Newton RJ, Griffin LE, Bowles KM, Meile C, Gifford S, Givens CE, et al. Genome characteristics of a generalist marine bacterial lineage. ISME J. 2010;4:784–798. doi: 10.1038/ismej.2009.150. [DOI] [PubMed] [Google Scholar]
- Orellana MV, Matrai PA, Leck C, Rauschenberg CD, Lee AM, Coz E. Marine microgels as a source of cloud condensation nuclei in the high Arctic. Proc Natl Acad Sci USA. 2011;108:13612–13617. doi: 10.1073/pnas.1102457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow U, Alldredge AL. A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP) Limnol Oceanogr. 1995;40:1326–1335. [Google Scholar]
- Passow U. Transparent exopolymer particles (TEP) in aquatic environments. Prog Oceanogr. 2002;55:287–333. [Google Scholar]
- Quinn PK, Bates TS. The case against climate regulation via oceanic phytoplankton sulphur emissions. Nature. 2011;480:51–56. doi: 10.1038/nature10580. [DOI] [PubMed] [Google Scholar]
- Russell LM, Hawkins LN, Frossard AA, Quinn PK, Bates TS. Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting. Proc Natl Acad Sci USA. 2010;107:6652–6657. doi: 10.1073/pnas.0908905107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Steward GF, Long RA, Azam F. Bacterial mediation of carbon fluxes during a diatom bloom in a mesocosm. Deep Sea Res Part 2 Top Stud Oceanogr. 1995;42:75–97. [Google Scholar]
- Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, Bennke CM, et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science. 2012;336:608–611. doi: 10.1126/science.1218344. [DOI] [PubMed] [Google Scholar]
- Wurl O, Miller L, Vagle S. Production and fate of transparent exopolymer particles in the ocean. J Geophys Res. 2011;116:C00H13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.