Abstract
Despite often showing behaviorally typical levels of social cognitive ability, unaffected siblings of children with autism spectrum disorder have been found to show similar functional and morphological deficits within brain regions associated with social processing. They have also been reported to show increased activation to biological motion in these same regions, such as the posterior superior temporal sulcus (pSTS), relative to both children with autism and control children. It has been suggested that this increased activation may represent a compensatory reorganization of these regions as a result of the highly heritable genetic influence of autism. However, the response patterns of unaffected siblings in the domain of action perception are unstudied, and the phenomenon of compensatory activation has not yet been replicated. The present study used functional magnetic resonance imaging to determine the neural responses to intentional biological actions in 22 siblings of children with autism and 22 matched controls. The presented actions were either congruent or incongruent with the actor’s emotional cue. Prior studies reported that typically developing children and adults, but not children with autism, show increased activation to incongruent actions (relative to congruent), within the pSTS and dorsolateral prefrontal cortex. We report that unaffected siblings did not show a compensatory response, or a preference for incongruent over congruent trials, in any brain region. Moreover, interaction analyses revealed a sub-region of the pSTS in which control children showed an incongruency preference to a significantly greater degree than siblings, which suggests a localized deficit in siblings. A sample of children with autism also did not show differential activation in the pSTS, providing further evidence that it is an area of selective disruption in children with autism and siblings. While reduced activation to both conditions was unique to the autism sample, lack of differentiation to incongruent and congruent intentional actions was common to both children with ASD and unaffected siblings.
Keywords: action perception, autism, endophenotype, superior temporal sulcus, fMRI
1. Introduction
Research focusing on unaffected siblings of individuals with autism spectrum disorder (ASD) is critical to understanding how the disorder impacts both individuals and families. Recently published studies have examined not only quantitative measures of mental health in siblings, but also their subjective experiences and perceptions (Petalas et al. 2012; Shivers et al. 2012; Angell et al. 2012). Furthermore, study of the “broader phenotype” of autism traits has consistently shown that siblings of children with autism exhibit behavioral deficits in social, communication, and learning domains (see Dawson et al., 2002, for a review). Moreover, Kates et al. (2004) reported shared structural deficits in frontal, temporal, and occipital lobes between discordant twin pairs (one child diagnosed with autism and one unaffected). In this study, all but one of the nine twin pairs contained a unaffected sibling who exhibited the broad autism phenotype, which was defined as showing a language or social delay that was either subclinical (undiagnosed but indicating mild impairment), or clinical (diagnosed as developmental delay or pervasive developmental disorder, but not as autism).
Neuroimaging data from unaffected siblings have also been presented in terms of their similarities to and differences from control children, as well as children with ASD. Dalton et al. (2007) found that unaffected siblings showed decreased fixations onto faces, decreased fusiform gyrus activation, and decreased amygdala volume compared with controls; all of these deficits were also present in the autism group. Belmonte et al. (2010) showed that both unaffected siblings and children with ASD were behaviorally impaired in a non-social visual attention task and also showed atypical brain activations in frontal and cerebellar regions. However, they also noted that a measure of overall functional correlation was decreased in autism but not in siblings. Barnea-Goraly et al. (2010) reported that deficits shared between children with ASD and siblings extend to the structural modality (measured by significantly reduced white matter fractional-anisotropy values compared to controls) in regions of the brain associated with social cognition. Baron-Cohen’s group reported that neural activations in unaffected siblings are similar to individuals with autism in a face processing task (Spencer et al., 2011). In a visual search task, Spencer et al. (2012) showed not only that unaffected siblings and children with autism show similar reductions in activation, but also that these reductions were correlated with behavioral measures of social interaction. These studies demonstrate that unaffected siblings expressing the broader autism phenotype also have structural and functional neurological deficits, some of which they share with children with ASD.
Taking a different approach, Kaiser et al. (2010) used a social task (point-light displays of biological motion). Crucially, the unaffected siblings were matched with controls on measures of social responsiveness and lacked characteristics of the broader autism phenotype. Thus, any shared neural response patterns could be classified as an endophenotype reflecting ASD vulnerability rather than an epiphenomenon resulting from subclinical behavioral features of the disorder. In that study, children with ASD and unaffected siblings both showed hypoactivations in cortical regions, consistent with prior work. However, they also reported atypical hyperactivations, relative to controls, that were unique to the unaffected siblings. Kaiser et al. hypothesized that these hyperactivations may be neural “compensatory” mechanisms.
Further exploration of similarities and differences in neuroimaging measures between individuals with ASD and their siblings seems pertinent. Given the trajectory of research in ASD, it is natural that many of the previous functional neuroimaging experiments have focused on face processing in unaffected siblings. However, no published studies have extended these findings into the domain of intentional action perception. This extension is required if we are to translate current findings from the low-level social cognition to the experience of actively participating in a social world (a much more complex and higher-level activity).
Prior studies of both children and adults show that brain activations in individuals with ASD differ from controls when viewing actions that are congruent with a prior displayed emotion versus those that are incongruent (Pelphrey et al., 2011; Vander Wyk et al., 2012). The pSTS showed differential activations depending on the congruency of the action, suggested that the region is involved in extracting social meaning (intention) from bodily action. We used functional magnetic resonance imaging (fMRI) to investigate brain activations, as measured by the blood oxygen level-dependent (BOLD) response, to congruent and incongruent intentional actions in unaffected siblings of children with ASD, a matched group of control children, and an unmatched, smaller sample of children with ASD.
The primary hypothesis concerns the comparison of differential activation to incongruent actions in unaffected siblings relative to controls. If the hypothesis concerning compensatory activation is correct, unaffected siblings may show overall greater activation to observed actions, regardless of congruency. However, they may also show increased differential activation to incongruent actions relative to congruent actions. The secondary hypothesis concerns the comparison between the control and ASD groups. Based on prior literature, we expect that they ASD sample will show decreased or no differential activation, and lower activation overall, as a function of congruency.
2. Methods
2.1. Subjects
Three groups of participants were recruited. The first group consisted of 22 unaffected siblings (US) of children with ASD with no history of psychiatric disorders (mean age = 12.58, SD = 2.43, range = 9.08-17.25). The second was an age-, gender-, and sample size-matched group of control children (CC) with no presence of ASD in the family and no psychiatric history (mean age = 11.63, SD = 1.85, range = 7.67-15.17). The third group consisted of 14 children diagnosed with ASD (mean age = 10.92, SD = 3.94, range = 5.67-18). Complete sample statistics are presented in Table 1. Between US and CC groups, there were no significant differences in age (t(39.205) = −1.4703, p = 0.1495)) or IQ as measured by the DAS-II’s General Conceptual Ability standard score (t(41.596) = −0.461, p = 0.6472)). Moreover, they did not differ in gender-normed T-scores on the Social Responsiveness Scale (SRS; t(36.779) = 1.0717, p = 0.2909). The SRS is a 65-item parent-report questionnaire that indexes atypical social behavior and responsiveness (Constantino & Todd, 2003). While it is not intended to be diagnostic of ASD, a score of 60 (one standard deviation above the mean) or higher implies risk for the disorder. Because the US and CC groups tested in the typically developing range for social responsiveness, we account for the possibility that neural differences within US are epiphenomenal to subclinical social impairments.
Table 1.
Sample statistics for the control, unaffected sibling, and ASD groups. Average values are given, with standard deviations in parentheses, for age in years, DAS-II Early Years General Conceptual Ability (GCA) standard scores, and SRS T-scores. A GCA score was not available for one participant in the ASD group. Race information was not reported for two participants in the CC group.
| Group | n | Gender distribution |
Racial distribution |
Average Age |
Age Range |
Average GCA Score |
Average SRS T- score |
|---|---|---|---|---|---|---|---|
| Control children (CC) |
22 | 14 males, 8 females |
16 White, 4 African American, 2 Not reported |
11.63 (1.85) |
7.67 - 15.17 |
105.95 (16.11) |
45.55 (9.33) |
| Unaffected Siblings (US) |
22 | 14 males, 8 females |
19 White, 2 More than one race, 1 Asian |
12.58 (2.43) |
9.08 – 17.25 |
108.09 (14.59) |
49.36 (13.87) |
| Children with autism spectrum disorder (ASD) |
14 | 12 males, 2 females |
12 White, 1 African American, 1 More than one race |
10.92 (3.94) |
5.67 – 18 |
89.62 (22.85) |
73.21 (11.0) |
| Control children comparison sub-group |
14 | 12 males, 2 females |
- | 11.61 (1.80) |
9.08 – 15.17 |
101.71 (14.88) |
46.29 (10.57) |
Diagnosis of individuals in the ASD group was determined using the Autism Diagnostic Observation Schedule (Lord et al., 2000) and/or the Autism Diagnostic Interview-Revised (Lord et al., 1994) along with the judgment of expert clinicians. A complete list of scores on diagnostic measures for the ASD group is shown in Table 2. The ASD group showed elevated SRS scores, as expected, with an average of 73.21 (SD = 11.0). Analysis of the ASD sample was primarily intended to provide a confirmation of disorder relevance via a qualitative comparison of the brain response of individuals with ASD, within regions identified in direct comparisons between the other two groups. The ASD group was not matched to the other two groups in gender distribution, IQ, or sample size; however, there was no significant difference in age when comparing the ASD group with the CC group (t(16.698) = −0.6249, p = 0.5405) and with the US group (t(19.361) = −1.4146, p = 0.1731). In one analysis, a smaller sub-group of control subjects was created for direct comparison with the ASD group (see section 2.8).
Table 2.
Average scores across three characterization measures administered to participants in the ASD group, with standard deviations in parentheses, rounded to two decimal places. All subjects received the SRS (parent report). Ten participants received scores for both ADOS 3 and ADI-R, two received only ADOS 3, one received only ADOS 4, and one received ADOS 4 and ADI-R. All measures resulted in a classification of autism or autism spectrum, with the exception of one participant (who was diagnosed with PDD-NOS).
| ADOS 3 (n = 12) | |
| Social Affect | 7.58 (1.73) |
| Restricted and Repetitive Behaviors |
2.42 (1.44) |
| Communication and Social Interaction |
9.75 (2.38) |
| ADOS 4 (n = 2) | |
| Communication | 1.5 (2.12) |
| Social Interaction | 8 (1.41) |
| Imagination and Creativity | 1.5 (0.71) |
| Stereotyped Behaviors and Restricted Interests |
2 (0) |
| ADI-R (n = 11) | |
| Social Interaction | 20.64 (5.10) |
| Communication and Language (verbal) |
16.73 (3.93) |
| Communication and Language (non-verbal) |
9.82 (3.49) |
| Restricted and Repetitive Behaviors |
5.64 (3.20) |
The sample sizes and age distributions reported are the final groups after motion correction of the fMRI data was performed. Motion correction led to the removal of some subjects from the analyses (for more details, see section 2.4). Written informed consent was required from the parents or legal guardians of all child participants, age-appropriate explanations were given to children and their written assent was obtained, and research protocols were approved by the Yale Human Investigation Committee.
2.2. Paradigm
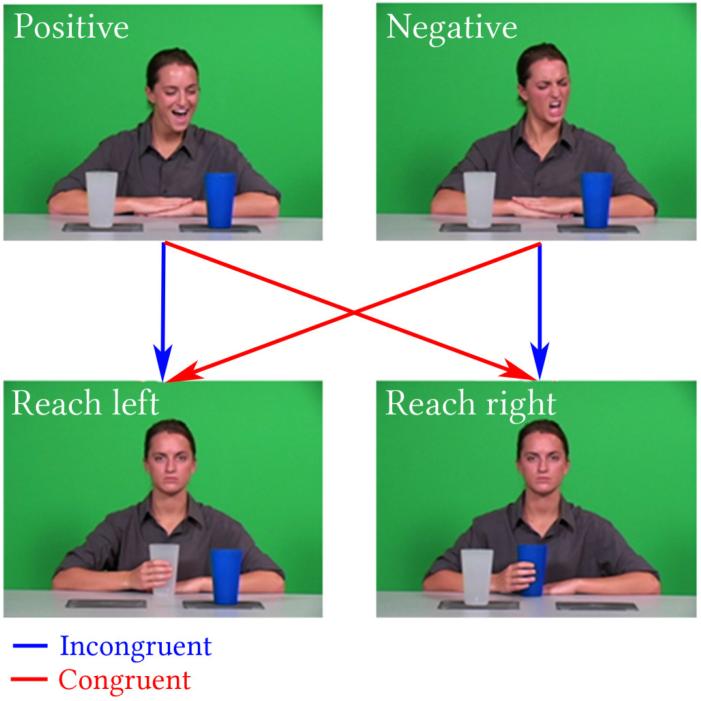
Subjects were first instructed to watch a DVD movie of their choice while anatomical MRI images were acquired (see below for acquisition details). Afterward, they were instructed to passively view a 6 minute, 16 second paradigm, during which functional images were acquired. A visual depiction of the paradigm is displayed in Figure 1. A human actor displayed either positive or negative affect towards a cup, and disregards the other. The actor then reaches for the same cup she regarded or the other. The resulting two-by-two design (positive-same, positive-other, negative-same, negative-other) allowed for the comparison of goal-driven actions that were incongruent to intention (positive-other, negative-same) versus those that were congruent (positive-same, negative-other).
Figure 1.
Diagram depicting the paradigm used in the present study. The actor orients towards a cup and displays a positive or negative emotion, then a reach in either direction while maintaining a neutral expression. Four outcomes are possible, two of which are congruent with intention and two are not.
One full run consisted of 32 trials of 6 seconds each. The actor begins in a neutral expression looking straight ahead, turns and emotes (two seconds), returns to neutral, and then reaches for a cup and places it in front of her, all while retaining a neutral expression (four seconds). Jittered fixation periods were present between each trial, and were 2 to 8 seconds long.
2.3. fMRI data acquisition
Imaging data were collected on a Siemens 3T Tim Trio scanner. T1-weighted anatomical images were acquired using an MP-RAGE sequence (TR = 1900 ms; TE = 2.96 ms; FOV = 256 mm; image matrix 256 × 256; 1 mm3). Whole-brain functional images were acquired with a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 × 64; voxel size = 3.4 mm × 3.4 mm × 3.4 mm; 34 slices) sensitive to the blood oxygenation level-dependent (BOLD) contrast.
2.4. fMRI data preprocessing
Data were processed and analyzed using the BrainVoyagerQX software package, version 2.4 (Brain Innovation, Maastricht, The Netherlands). The first five functional volumes were discarded. The data were preprocessed with the following steps: slice scan timing correction using sinc interpolation, 3D motion correction using trilinear and sinc interpolation with 3 translations and 3 rotations, spatial smoothing using a Gaussian filter (FWHM = 7 mm) and high-pass filtering using a GLM-Fourier basis set with 2 cycles per time course (0.00546 Hz). Each subject’s data were coregistered to their own anatomical data and converted to Talairach standard space (Talairach and Tournoux 1988). The processed data were passed through an in-house motion correction algorithm that detected motion exceeding 3 millimeters of translational motion (or 3 degrees of rotational motion) in any direction relative to the starting position. These “bad” volumes were excised from the data. Only subjects who had 90% or more of their data pass this threshold were allowed into the analysis; this forced the exclusion of 1 control child, 8 children with ASD, and 6 unaffected siblings.
2.5. fMRI data analysis
2.5.1. Waveform definition
A random-effects general linear model was computed using the motion-corrected functional time courses, deconvolved (single-volume) binary predictors at each timepoint, and motion regressors computed from BrainVoyager’s 3D motion correction algorithm (Formisano et al. 2006). A deconvolution design was created to model all runs, with a binary predictor defined at each timepoint from the initial onset of the stimulus for a total of ten timepoints. Each timepoint corresponded to an acquired volume, which were taken two seconds apart. Extracting the data from each of these predictors in a region-of-interest analysis yielded event-related waveforms that represent each subject’s BOLD response to the stimulus. This was done for both the incongruent and congruent conditions. The first signal value was then subtracted from the subsequent signal values to normalize them to begin at zero, resulting in waveforms that show relative changes from onset. For statistical and quantitative comparisons, we focus on timepoint 5, which represents the relative change in BOLD signal 10 seconds after stimulus onset. The segment of the trial during which the intentional action is presented – that is, when the actor reaches towards a cup after displaying her emotional cue – occurs 2 seconds after the initial onset. The BOLD response has been shown to exhibit an initial 2-3 second delay and peaks over the course of 6-12 seconds (Logothetis et al., 2001). It follows that the relevant component of the waveform should be about 6-12 seconds after the critical component of our stimulus (the intentional reach). Our assumed peak of 10 seconds after onset therefore falls within a theoretically plausible range. Moreover, in prior studies, it was observed that the pSTS and dlPFC maximally and significantly differentiated incongruent and congruent conditions at timepoint 5, suggesting that this chronological window best represents the aspect of the BOLD response relevant to the processing of biological motion and intentionality for these stimuli (Vander Wyk et al., 2012).
2.5.2. Condition and timepoint contrasts
Having computed the condition level event waveforms, data were analyzed in two ways. First, congruent actions were contrasted against incongruent actions to test whether longitudinal responses to biological actions varied based on a matching or non-matching intentional context. Second, we defined an aggregate response to intentional action as the average of incongruent and congruent conditions. This was done in order to quantify the BOLD response to these observed reaches, without regard to congruency.
2.6. Region-of-Interest (ROI) definition
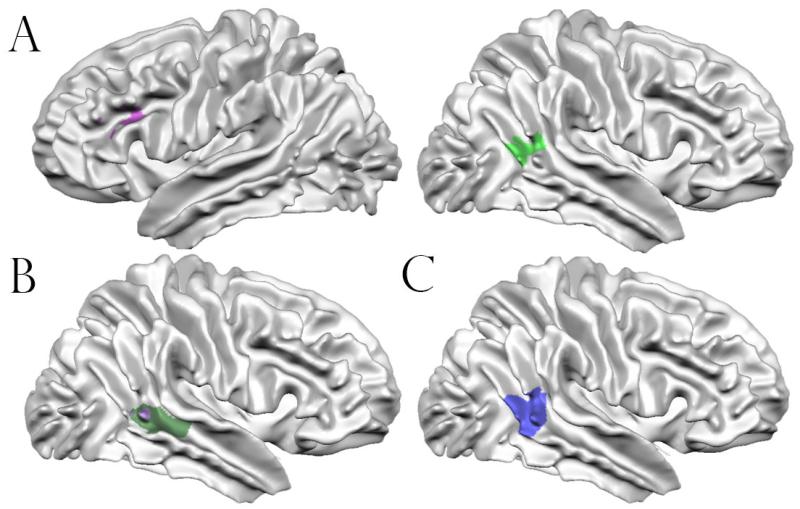
A priori defined ROIs for the posterior superior temporal sulcus (pSTS) and dorsolateral prefrontal cortex (dlPFC) were taken from Kaiser et al. (2010), in which a point-light display of human biological motion was contrasted with one of scrambled motion. Specifically, we focused on a region of the pSTS in which unaffected siblings responded more strongly to biological motions than children with ASD and typically developing individuals. Because unaffected siblings of children with autism may have been engaging the region to compensate for the genetic influence of autism, Kaiser et al. labeled it a “compensatory region.” We also chose a region in the left dorsolateral prefrontal cortex in which both children with ASD and unaffected siblings showed atypical patterns of brain activation (no preferential response to biological motion) relative to typical individuals. Deficits in this region seemed to be associated with the genetic presence of autism because they were present in children with the disorder and their unaffected siblings, and it was thus labeled a “trait region.” The location and extent of the trait-defined dlPFC and compensatory-defined pSTS are visually depicted in Figure 4A. In addition, quantitative details for these and all other ROIs used or created in this study are presented in Table 3.
Figure 4.
Surface representations for ROIs used or created in the current study. (A): Representation of the “compensatory” right hemisphere pSTS ROI (right) and “trait” left hemisphere dlPFC (left). (B): Regions defined by a whole-brain analysis. The purple region represents the area where control children showed an incongruent > congruent effect most strongly. The green region represents the area where control children showed an incongruency preference to a significantly greater degree than unaffected siblings did. No brain regions existed where unaffected siblings responded significantly more strongly to incongruent relative to congruent trials. The green region includes areas that are occluded by the purple region. (C): Visual depiction of the pSTS region defined using activation-likelihood estimation meta-analysis.
Table 3.
Center of gravity table for all regions of interest. Anatomical labels were found by inputting mean Talairach co-ordinates of each region (with dimensions rounded to nearest whole number) into the Talairach Client, version 2.4.2.
| Region Name | Source | Mean x (SD) |
Mean y (SD) |
Mean z (SD) |
Anatomical Label |
Extent (1mm cubic voxels) |
|---|---|---|---|---|---|---|
| “Compensatory” Right pSTS |
Kaiser et al., 2010 | 47.37 (4.41) |
−51.87 (4.35) |
10.67 (2.66) |
Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus, BA39 |
703 |
| “Trait” Left dlPFC |
Kaiser et al., 2010 | −42.92 (2.71) |
23.97 (5.48) |
24.62 (2.30) |
Left Cerebrum, Frontal Lobe, Middle Frontal Gyrus, BA46 |
860 |
| Right pSTS | Whole-brain ANCOVA, Incongruent > Congruent, CC subjects only |
59.74 (3.85) |
−43.02 (2.67) |
10.43 (2.80) |
Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus, BA22 |
955 |
| Right pSTS | Whole-brain ANCOVA, Incongruent > Congruent, CC > US |
50.81 (6.39) |
−37.85 (4.74) |
2.88 (3.74) |
Right Cerebrum, Temporal Lobe, Middle Temporal Gyrus, BA22 |
2432 |
| Right pSTS | Activation likelihood estimation meta- analysis |
52.20 (4.33) |
−47.74 (4.34) |
9.74 (4.13) |
Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus, BA22 |
3229 |
The motivation for using these regions is two-fold. First, we wanted to assess whether unaffected siblings respond in a complex manner (decreased activation in some regions and increased activation in others) to intentional biological motion. Secondly, we intended to investigate the extensibility of those findings, that is, whether or not the compensatory/trait patterns discussed above persist in the context of our current paradigm, which presents human biological motion with a greater amount of social complexity and real-world similarity. Although multiple regions have been implicated, we focus on the pSTS and dlPFC because these two areas of the brain were responsive to the current paradigm in prior studies focusing on typically developing adults and children (Vander Wyk et al., 2012).
In order to maintain statistical independence for some analyses, an empirical ROI was created using an activation-likelihood estimation meta-analysis implemented in GingerALE, version 2.1 (Eickhoff et al. 2009; Turkeltaub et al. 2012; Eickhoff et al. 2012). Voxels of peak activation were taken from the literature and fed into an algorithm that creates a probabilistic map of regions most likely to be active. Studies were retrieved using a PubMed literature search with the keywords “superior temporal sulcus,” “social cognition,” and “fMRI.” In sum, 12 studies with 140 subjects contributed to the meta-analysis. The resulting whole-brain map was thresholded with a false-discovery rate of q = 0.01, with 11 of the 12 studies providing foci (peak co-ordinates) towards the definition of the region; these studies are listed in Table 4. The region had a volume of 3600 cubic millimeters, and was centered at (51.19, −47.71, 9.85) in Talairach standard space. It is visually depicted on a display brain in Figure 4C.
Table 4.
List of published studies examining perception of faces whose co-ordinates contributed to the meta-analytically defined right pSTS region-of-interest. Each study contributed one peak co-ordinate to the analysis.
| Author | Year published |
Contrast or stimulus of interest |
|---|---|---|
| Saxe | 2004 | Long occlusion vs. short occlusion |
| Pelphrey | 2004 | Goal direction vs. non-goal directed motion |
| Grossman | 2004 | Biological vs. scrambled motion |
| Thompson | 2005 | Intact mannequins vs. pulled apart mannequins |
| Carter | 2006 | Biological vs. non-biological motion |
| Vander Wyk | 2009 | Congruent vs. incongruent actions |
| Jastorff | 2009 | Full vs. scrambled motion |
| Shultz | 2010 | Failed vs. successful goal directed actions |
| Safford | 2010 | Biological motion vs. tool motion |
| Zucker | 2011 | Incongruent vs. congruent motion |
| Kim | 2011 | Biological vs. scrambled motion |
2.7. Whole-brain analysis
Prior studies suggested that the violation of social cues evoked through intentional biological action engages similar brain regions as the low level perception of a point-light display of biological motion (pSTS, dlPFC). However, a whole-brain ANCOVA analysis was also carried out in which group (US or CC) was defined as a between-subjects factor, and relative activation at timepoint 5 during each condition (incongruent or congruent intentional motion) was defined as a within-subjects factor. Three contrasts of interest were performed on the resulting data: regions in which CC subjects showed greater activation to incongruent trials relative to congruent trials, the same contrast for the US group, and the difference between the two (regions where CC subjects differentiated the two conditions to a greater degree than US). The resulting maps of activation were then thresholded to an uncorrected p-value of 0.001. A cluster threshold estimation algorithm was then performed on each map separately; 10,000 iterations of a Monte-Carlo simulation were performed to determine the threshold value that corresponds to a cluster-level multiple comparisons correction of p < 0.05. Applying these thresholds to the corresponding maps resulted in the final regions reported for each contrast.
2.8. ASD group analyses
We examined whether children with ASD differentiate congruent and incongruent actions the pSTS. However, the intentional information in our paradigm was conveyed only through facial expression, and prior research has established a deficit in face processing in children with ASD (Klin & Jones, 2006; New et al., 2010). Therefore, in addition to defining intentional congruency by the emotional content in the face (as done throughout the study), we also defined it by the actor’s head turn. Trials where the actor made a head turn to the right, and subsequent reach in the same direction, were labeled “congruent” regardless of whether the actor’s facial affect suggested a different intention. Normalized waveforms were extracted using the procedures outlined in section 2.5 for both definitions of congruency and plotted for comparison. To investigate whether the ASD group showed preferential brain responses to incongruent actions in any region, two analyses were performed using a random-effects general linear model, one for the emotion-based definition of congruency and one for the head turn-based definition, using the methods described in section 2.5. Analyses were computed at the whole-brain level and within ROIs derived from comparisons between controls and siblings.
An analysis directly comparing children with ASD and control children was performed using a sub-group of the CC group that matched, as closely as possible, the gender distribution, IQ scores, and age of the ASD subjects. Details on the sample statistics of the control children sub-group can be found in Table 1. Exact matches between the groups were obtained for sample size (14 subjects) and gender distribution (12 males, 2 females). No statistically significant difference between the ASD group and the CC sub-group was detected in mean age (t(18.18) = 0.5963, p = 0.5583), or in mean IQ (t(20.39) = −1.6169, p = 0.1213). For the direct comparison of control and ASD groups, the peak BOLD responses to incongruent and congruent trials were extracted from the empirically-defined pSTS ROI, which was created using the procedure described in section 2.6. This was done because we wished to avoid making interferences based on data from the pSTS region defined using the same US and CC subjects (see section 2.7), which would have violated statistical independence. The relative BOLD signal change values at peak timepoint 5 were extracted from the empirical ROI and used as the response variable in an ANOVA design with one between-subjects variable (group) and one within-subjects variable (condition).
3. Results
3.1. Region-of-Interest analysis
3.1.1. pSTS “compensatory region”
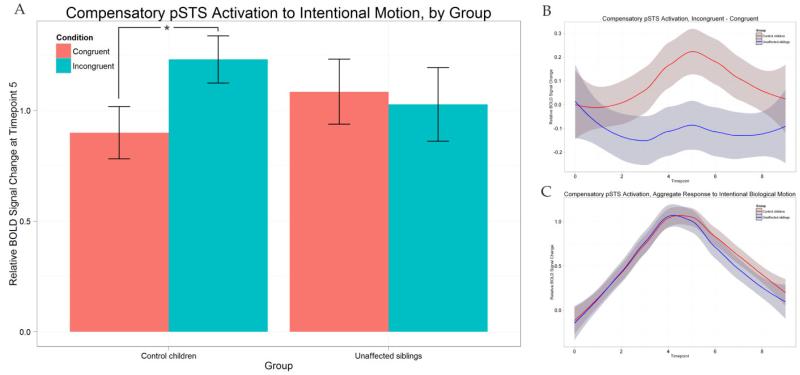
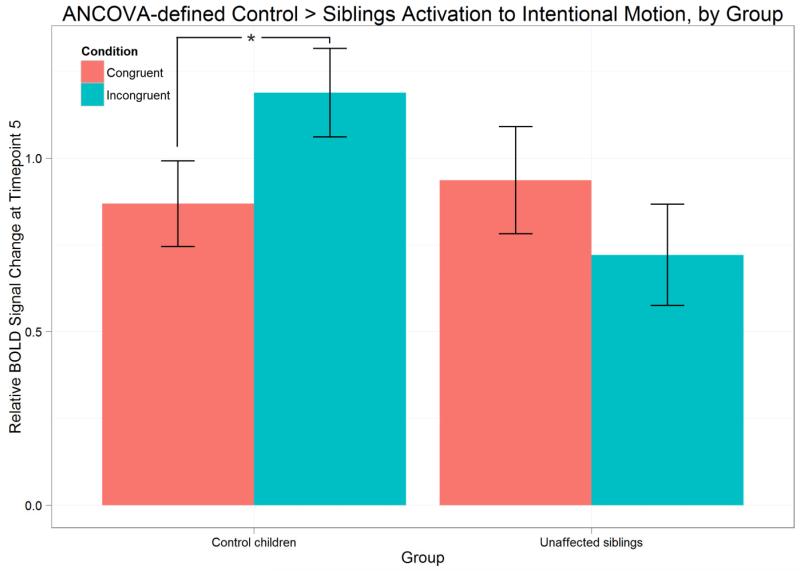
The US and CC group-averaged differential (incongruent – congruent) and aggregate (average of both conditions) waveforms for the compensatory-defined pSTS are displayed in Figure 2 (B and C). We also show a bar graph representing the peak BOLD response at timepoint 5 relative to onset for the conditions, separated by group (Figure 2A). We observe control participants showing a high degree of preference to incongruent trials in this region, while unaffected siblings do not; the lack of differentiation in the sibling group persists throughout the waveform (Figure 2B). To test this relationship for significance, an ANOVA was performed with relative BOLD activation at timepoint 5 in this region as the response variable, condition as a within-subjects factor, and group as a between-subjects factor. The main effect of condition on the BOLD response was significant (F(1,42) = 4.201, p < 0.05). Between the groups, there were no significant differences in this region’s activation to congruent trials (t(40.188) = −0.981, p = 0.3324) and incongruent trials (t(35.835) = 1.0279, p = 0.3109). However, there was a significant interaction between group and condition (F(1,42) = 8.429, p < 0.05). Post-hoc tests were performed using a Bonferroni correction. As shown in Figure 2A, control children showed significantly greater BOLD activation to incongruent trials than to congruent trials (paired t(21) = 3.6388, p < 0.05). Unaffected siblings, however, responded equivalently to the two conditions (paired t(21) = −0.5826, p = 0.5664). The groups did not differ in their overall activation to intentional biological motion (the average of both conditions) in this region (Figure 2C).
Figure 2.
(A): Peak activations (relative rise from onset to timepoint 5) in compensatory pSTS for both groups and separated by group. Also shown are the differential (B) and aggregate (C) group-averaged waveforms for control children (red line) and unaffected siblings (blue line).
3.1.2. dlPFC “trait region”
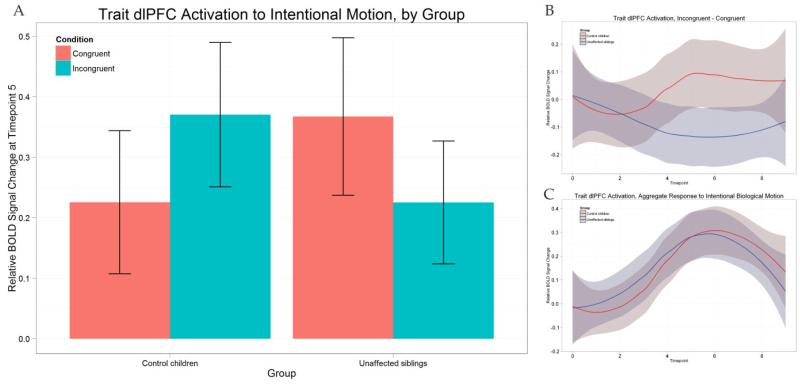
The same plots as for the compensatory pSTS above are displayed for the trait-defined dlPFC ROI in Figure 3. The region shows a similar response pattern as the pSTS, although greatly reduced in both groups; CC subjects show maximum group-averaged aggregate responses of 0.32 and 1.13 in the dlPFC and pSTS, respectively (0.30 and 1.14 for the US group). Despite this reduction, a similar pattern of differentiation is present, with CC subjects preferring incongruent stimuli at peak and US deflecting in the opposite direction. An ANOVA with equivalent design to the one described above for the compensatory region was performed. The main effect of condition on the dlPFC response was not significant (F(1,42) = 0.000, p = 0.9864). The interaction between group and condition was marginal but did not reach significance (F(1,42) = 3.967, p = 0.0529). If group is ignored, the opposite responses of the groups to the individual conditions cancel, leading to an elimination of the main effect. There were no significant differences in peak BOLD activation in dlPFC to the two conditions, in either CC subjects (paired t(21) = 1.3102, p = 0.2043) or CU subjects (paired t(21) = −1.5374, p = 0.1391).
Figure 3.
(A): Peak activations (relative rise from onset to timepoint 5) in the trait-defined dlPFC for both groups and separated by group. Also shown are the differential (B) and aggregate (C) group-averaged waveforms for control children (red line) and unaffected siblings (blue line).
3.2. Whole-brain analysis
In the CC group, activation to incongruent trials was significantly greater than congruent trials in the purple pSTS region depicted in Figure 4B (uncorrected p < 0.001, cluster-level multiple-comparisons corrected at p < 0.05, cluster threshold k = 15 functional voxels). In US, the same contrast did not yield any statistically significant regions. Lowering the threshold to an uncorrected p < 0.05 did not result in any region in which activation to incongruent trials was greater at peak than congruent trials, suggesting that the lack of expected activation in unaffected siblings is not due to our choice of threshold.
A direct comparison of the magnitude of the condition differentiation (incongruent - congruent) revealed a distinct sub-region of the pSTS in which the CC group showed significantly greater differentiation than US (uncorrected p < 0.001, cluster-level multiple-comparisons corrected at p < 0.05, cluster threshold k = 16 functional voxels). This region, shown in green in Figure 4B, was located anterior and ventral to the differentiation region for CC subjects alone. The response variable (mean BOLD activation at timepoint 5) for this region is plotted for both groups and separated by group in Figure 5. Post-hoc t-tests were performed using a Bonferroni correction. In this region, CC subjects showed significantly greater BOLD activation to incongruent trials than to congruent trials (paired t(21) = −3.9229, p < 0.05). After correction for multiple comparisons, CU subjects showed no significant difference in BOLD activation to incongruent trials relative to congruent (paired t(21) = 2.4531, p(uncorrected) = 0.023). Finally, the difference in activation to the two conditions (incongruent - congruent) was significantly lower in CU subjects relative to CC subjects (t(41.39) = −3.5312, p < 0.05).
Figure 5.
Peak activations (relative rise from onset to timepoint 5) in the pSTS region in which control children showed a preference in activation to incongruent over congruent trials to a significantly greater degree than unaffected siblings.
3.3. Incongruent action processing in participants with ASD
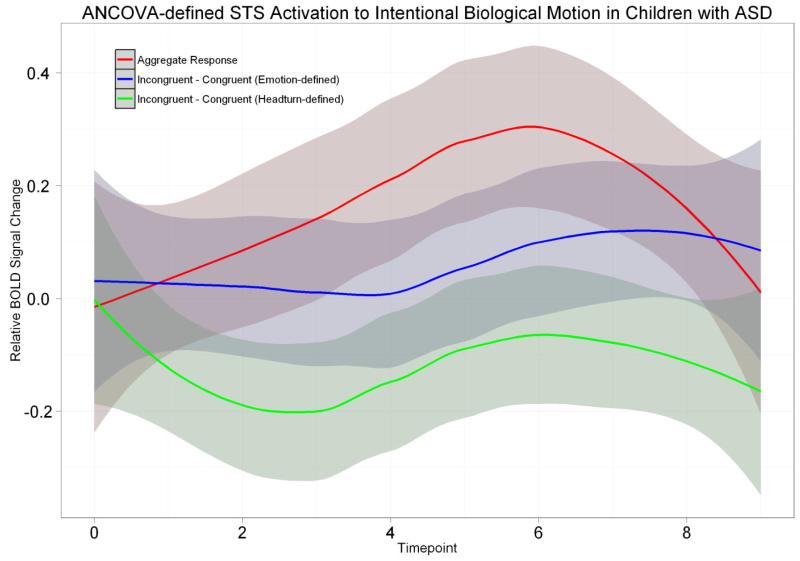
Brain responses to intentional biological action in children with ASD were extracted from the region where the CC group showed a preference to incongruent trials over congruent trials to a significantly greater degree than the US group. Since the ASD group is unmatched to the CC and US groups, we restricted ourselves to only within-group observations. Collapsing across all trials, overall activation to intentional biological actions peaks at timepoint 6 (Figure 6, red line), and is significantly greater than zero in a one sample t-test (t(13) = 2.1778, p < 0.05). Differentiation between incongruent and congruent is plotted as the blue line in Figure 6, and is not significantly greater than zero at peak (t(13) = 1.0778, p = 0.3007).
Figure 6.
Aggregate and differential waveforms for the ASD group in the pSTS region in which control children showed a preference in activation to incongruent over congruent trials to a significantly greater degree than unaffected siblings. The two differential waveforms correspond to two distinct definitions of congruency. The blue line is the waveform for the same definition used throughout the study (congruency is based on the intention derived from the emotional expression of the actor). The green line disregards emotion and defines intention by head turn, such that looking at a cup and reaching for it is labeled a congruent action, regardless of the preceding emotion.
We then tested the hypothesis that children with ASD are not attending to the emotional properties of the face but rather to the spatial properties of the head, thus leading to a lack of expected differentiation in the pSTS. Trials where the actor made a head turn to the right, and subsequent reach in the same direction, were labeled “congruent” regardless of whether the actor’s facial affect suggested a different intention. A differential waveform was plotted using this other definition of congruency is plotted as the green line in Figure 6. No pattern of differentiation inactivation to incongruent and congruent trials is evident in either definition of congruency. Participants with ASD therefore showed a lack of differentiation in activation to the two conditions in the same region of the pSTS as unaffected siblings did.
At a whole-brain level, no regions showed increased activation to incongruent trials relative to congruent trials at peak, even at an uncorrected threshold of p < 0.05, regardless of which definition of congruency was used (emotion-based or head turn-based). However, this finding should be considered alongside the fact that the sample size of the ASD group was small relative to the other groups.
3.4. Comparisons of the CC sub-group and ASD group
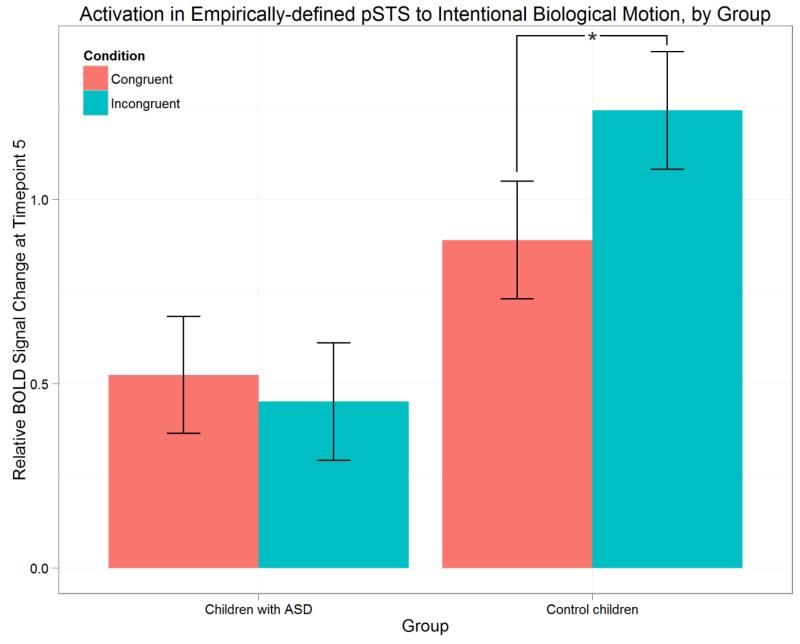
A sub-group of control children was compared with the ASD group; control children in the sub-group were matched with the ASD participants on age, gender, sample size, and IQ. An ANOVA revealed a significant main effect of group on the peak BOLD response to intentional motion in the pSTS (F(1,26) = 8.137, p < 0.01) and a significant group by condition interaction (F(1,26) = 2.002, p < 0.05). In order to further explore these effects, post-hoc t-tests were performed using a Bonferroni correction for multiple comparisons. Activation in the pSTS to all trials was significantly lower in the ASD group relative to control children (p < 0.05). Moreover, the difference in pSTS activation between incongruent and congruent trials was significant in control children (paired t(13) = 2.8242, p < 0.05), but not in the ASD group (paired t(13) = 0.472, p = 0.645). Peak BOLD responses of the groups are plotted by group in Figure 7.
Figure 7.
Peak BOLD response values in the pSTS (defined empirically via activation likelihood-estimation meta-analysis) to intentional biological motion in the ASD group and the sub-group of control children, matched on age, gender, sample size, and IQ.
4. Discussion
Siblings of individuals with an ASD presumably share some genetic risk for ASD. However, few studies have targeted this group, although there appears to be not only a complex pattern of common deficits, but also preserved and perhaps even heightened brain function. The current study directly compared unaffected siblings and control children during action perception, in a paradigm that includes stimuli previously used to examine action understanding and intentional processing in both neurotypical children and children with ASD. The differentiations between incongruent and congruent conditions shown within the pSTS may underlie social cognitive deficits in the disorder, namely, using visual information to understand the intentions of others (Pelphrey et al., 2011).
We targeted two regions, right pSTS and left dlPFC, which are key nodes in the action perception network. In the left dlPFC, we observed deficits in differentiation of incongruent and congruent trials in unaffected siblings but not in typically developing subjects, consistent with its label as a “trait region” in Kaiser et al. (2010). Such trait regions were areas in which ASD and US groups shared atypical patterns of activation to biological motion. However, we cannot confirm that these findings were replicated in our sample because the analysis did not reveal a significant group by condition interaction. It was, however, strongly trending. These results, along with those from the “compensatory” pSTS, indicate a discrepancy between neural processing based on type of biological motion stimuli presented. The compensatory pSTS ROI analysis, in which we would expect siblings to respond to the contrast of interest to a greater degree than control children, shows a preference in activation to incongruent motion in CC but not US participants. Moreover, we predicted that US would be impaired in the trait-defined dlPFC, but no significant effects were found. These data suggest that the “compensatory” and “trait” distinctions defined using a point-light human motion paradigm may not extend to our paradigm, which targets perception of intentional human motion. A whole-brain analysis was then carried out to explore group differences that the ROI analyses may have missed.
The whole-brain analysis revealed a distinct sub-region of the pSTS in which unaffected siblings showed a lack of differentiation of incongruent and congruent intentional actions with respect to a matched group of typical children. To confirm the region’s relevance to ASD, we tested its response properties in a group of children with ASD. Within this sample, the region did not differentiate congruent from incongruent trials regardless of whether congruency was defined by emotion (as it was in the other groups), or by head-turn (which would not require attention to facial and emotional features but rather to the movement of the head). This region, in which both unaffected siblings of children with ASD and children with the disorder show a lack of differentiation, is likely a location of selective disruption resulting from hereditary risk for the disorder (a neuroendophenotype).
Based on previous studies, we predicted that there would be some regions in which US would respond more strongly – in a possibly compensatory fashion – to intentional biological motion than CC subjects. However, US subjects did not respond preferentially to the action stimuli overall, nor did they respond differentially to incongruent intentional action to a higher degree than CC subjects did, in either a whole brain analysis or within specific regions-of-interest. In fact, the US group showed significantly less differentiation of incongruent and congruent actions within the pSTS. We propose two possible explanations for this. Unaffected siblings may exhibit compensatory activity in lower-level social perception tasks, but increased neurotypical activation in the pSTS of siblings is nonexistent (or too subtle to be detected) when perceptual demand is increased. Alternatively, repeated exposure to incongruent actions may attenuate and eventually eliminate the differential activation to incongruent actions. It is possible that the nature of compensatory processing in the pSTS in siblings enables rapid habituation during the experiment. We could not examine within-run habituation within the design of the current experiment, but future studies could examine this hypothesis.
Our results reinforce the notion that the pSTS is a region of selective disruption in individuals with autism and unaffected siblings. Different regions of the pSTS show atypical activations based on whether low-level biological motion perception (point-light walkers) or high-level intentional biological action perception (human actors) are presented. Although unaffected siblings responded to intentional biological motion stimuli to a comparable magnitude as controls, the siblings did not differentiate congruent and incongruent actions. Moreover, not only did children with ASD respond equivalently to the two conditions, but they also responded significantly less strongly to all trial types compared to controls. Performing this analysis required a reduced sample of14 control children (with 12 males and 2 females to match the gender distribution of the ASD group). While these results should be interpreted with caution, they support prior findings indicating reduced overall pSTS activation (and no differentiation) to incongruent and congruent intentional actions in adults with autism (Pelphrey et al., 2011). Because the lack of differentiation of incongruent and congruent actions seems to be a shared deficit of the US and ASD groups, it may be endophenotypic in nature. The accompanying reduced activation, furthermore, may be idiosyncratic of individuals with clinical levels of autistic traits. This is consistent with multiple prior studies reporting lower activation in children diagnosed with ASD, relative to controls, in a variety of brain regions across multiple cognitive modalities, including attention, social information integration, emotion attribution, and social processing (Fan et al., 2012; Groen et al., 2010; Greimel et al., 2012; Piggot et al., 2004).
There has been a move to use neuroimaging data to characterize complex disorders. Because traits are continuously distributed throughout the general population, this “dimensional view of psychopathology” places all individuals on the same quantitative spectrum, and those with clinical disorders lie at the extremes (Hudziak et al., 2007). Samples of typically developing individuals, therefore, can be useful in identifying and investigating possible “neural signatures” of clinical disorders (Wallace et al., 2012). These ideas have been successfully implemented in other neurological disorders, such as Alzheimer’s disease. Kohannim et al. (2012) reported a substantial increase in the number of genes identified in connection to Alzheimer’s disease in a group of patients with the disorder, relative to a standard genome-wide association study, when a measure of temporal lobe volume was used as a quantitative endophenotype. Furthermore, the most significant gene’s influence on temporal lobe morphology was replicated in an independent sample of young adults. These methods, which have produced demonstrable increases in our ability to detect tractable brain changes resulting from genetic influences, are starting to be applied to autism (see Ameis & Szatmari, 2012, for a review). One of the most striking lines of inquiry centers on the infant siblings of children with autism, who are at a high risk to develop the disorder. Wolff et al. (2012) showed that siblings who were eventually diagnosed with autism at two years of age had altered white matter development at six months, relative to those who were not. Combining these results with imaging-genetics methods could allow for the introduction of targeted therapies and early interventions.
Functional and structural imaging studies focusing on children with autism and unaffected siblings, particularly in exploring their unique and shared deficits across childhood, could guide and form the theoretical bases for these investigations. More work is needed to characterize the developmental trajectories of these deficits, and under what circumstances unaffected siblings compensate for, or otherwise avoid, expressing the social and behavioral symptoms associated with autism. Such findings could lead to a reliable set of neuroendophenotypes that could be used to longitudinally track genetic influences upon the brain from infancy to adulthood, thus making progress toward disentangling an extremely heterogeneous and phenotypically complex disorder.
5. Conclusion
Intentional action perception is critical for making sense of social situations, and these cognitive processes, along with the neural bases underlying them, are deeply relevant to our understanding of autism spectrum disorders. Furthermore, exploring the response patterns of unaffected siblings in action perception would be informative from the perspective of discovering neuroendophenotypes, but they are not yet well understood. We report that unaffected siblings of children with autism did not show any compensatory patterns of activation, which we defined as showing a preference for incongruent intentional actions to a significantly greater degree than controls. We also found that a region within the right pSTS differentiated unaffected siblings and control children in its preferential activation to incongruent intentional actions relative to congruent actions; control children showed the effect to a significantly greater degree than siblings. The region also showed no differentiation to the two conditions in a smaller, unmatched sample of children with autism. When an empirical region-of-interest in the pSTS was used to compare ASD subjects with a smaller, matched sub-group of controls, we found significant differentiation in control children only, along with significantly reduced activation to all conditions in children with ASD. Our results suggest that irregular pSTS differentiation in activation to congruent and incongruent intentional actions may be a locus of disruption in both children with autism and siblings.
Intentional action perception is critical for understanding social situations.
Unaffected siblings of children with autism showed deficits in activation.
Deficits in pSTS activation were also present in children with autism.
Acknowledgments
The authors would like to sincerely thank the children and families who contributed to this research for their time, patience, and cooperation.
Funding
This research was funded by NIHM grant R01MH084080 and the Simons Foundation.
Abbreviations
- (ASD)
autism spectrum disorder
- (US)
unaffected siblings
- (CC)
control children
- (pSTS)
posterior superior temporal sulcus
- (dlPFC)
dorsolateral prefrontal cortex
- (BOLD)
blood oxygen level-dependent
- (ROI)
region-of-interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameis SH, Szatmari P. Imaging-genetics in autism spectrum disorder: advances, translational impact, and future directions. Frontiers in Psychiatry. 2012;3:46. doi: 10.3389/fpsyt.2012.00046. doi:10.3389/fpsyt.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell ME, Meadan H, Stoner JB. Experiences of siblings of individuals with autism spectrum disorders. Autism Research and Treatment. 2012;2012(1):1–11. doi: 10.1155/2012/949586. doi:10.1155/2012/949586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar White Matter Aberrations in Children With Autism and Their Unaffected Siblings. Archives of General Psychiatry. 2010;67(10):1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. doi:10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Gomot M, Baron-Cohen S. Visual attention in autism families: “unaffected” sibs share atypical frontal activation. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(3):259–76. doi: 10.1111/j.1469-7610.2009.02153.x. doi:10.1111/j.1469-7610.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524–30. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61(4):512–20. doi: 10.1016/j.biopsych.2006.05.019. doi:10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14:581–611. doi: 10.1017/s0954579402003103. doi:10.1017.S0954579402003103. [DOI] [PubMed] [Google Scholar]
- Fan J, Bernardi S, Dam NT, Anagnostou E, Gu X, Martin L, Park Y, et al. Functional deficits of the attentional networks in autism. Brain and Behavior. 2012;2(5):647–60. doi: 10.1002/brb3.90. doi:10.1002/brb3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. doi:10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. doi:10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Di Salle F, Goebel R. Advanced imaging processing in magnetic resonance imaging. CRC Taylor & Francis; Boca Raton (FL): 2005. Fundamentals of data analysis methods in functional MRI; pp. 481–503. [Google Scholar]
- Greimel E, Nehrkorn B, Fink GR, Kukolja J, Kohls G, Müller K, Piefke M, et al. Neural mechanisms of encoding social and non-social context information in autism spectrum disorder. Neuropsychologia. 2012;50(14):3440–9. doi: 10.1016/j.neuropsychologia.2012.09.029. doi:10.1016/j.neuropsychologia.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Groen WB, Tesink C, Petersson KM, Van Berkum J, Van der Gaag RJ, Hagoort P, Buitelaar JK. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cerebral Cortex. 2010;20(8):1937–45. doi: 10.1093/cercor/bhp264. doi:10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16(S1):S16–23. doi: 10.1002/mpr.217. doi:10.1002/mpr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Deen B, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(49):21223–8. doi: 10.1073/pnas.1010412107. doi:10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, et al. Neuroanatomic Variation in Monozygotic Twin Pairs Discordant for the Narrow Phenotype for Autism. American Journal of Psychiatry. 2004;161(3):539–546. doi: 10.1176/appi.ajp.161.3.539. doi:10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W. Attributing social and physical meaning to ambiguous visual displays in individuals with higher-functioning autism spectrum disorders. Brain and Cognition. 2006;61(1):40–53. doi: 10.1016/j.bandc.2005.12.016. doi:10.1016/j.bandc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, Toga AW, et al. Discovery and Replication of Gene Influences on Brain Structure Using LASSO Regression. Frontiers in Neuroscience. 2012;6:115. doi: 10.3389/fnins.2012.00115. doi:10.3389/fnins.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. [PubMed] [Google Scholar]
- New JJ, Schultz RT, Wolf J, Niehaus JL, Klin A, German TC, Scholl BJ. The scope of social attention deficits in autism: prioritized orienting to people and animals in static natural scenes. Neuropsychologia. 2010;48(1):51–9. doi: 10.1016/j.neuropsychologia.2009.08.008. doi:10.1016/j.neuropsychologia.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(6):631–44. doi: 10.1111/j.1469-7610.2010.02349.x. doi:10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petalas MA, Hastings RP, Nash S, Reilly D, Dowey A. The perceptions and experiences of adolescent siblings who have a brother with autism spectrum disorder. Journal of Intellectual and Developmental Disability. 2012;37(4):303–314. doi: 10.3109/13668250.2012.734603. doi:10.1155/2012/949586. [DOI] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, Bookheimer S, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(4):473–80. doi: 10.1097/00004583-200404000-00014. doi:10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Shivers CM, Deisenroth LK, Taylor JL. Patterns and Predictors of Anxiety Among Siblings of Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1685-7. doi:10.1007/s10803-012-1685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Suckling J, Calder AJ, Bullmore ET, Baron-Cohen S. A novel functional brain imaging endophenotype of autism: the neural response to facial expression of emotion. Translational Psychiatry. 2011;1(7):e19. doi: 10.1038/tp.2011.18. doi:10.1038/tp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Calder AJ, Suckling J, Bullmore ET, Baron-Cohen S. Atypical activation during the Embedded Figures Task as a functional magnetic resonance imaging endophenotype of autism. Brain: A Journal of Neurology. 2012;135:3469–3480. doi: 10.1093/brain/aws229. doi:10.1093/brain/aws229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. Thieme; Stuttgart: 1988. [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2012;33(1):1–13. doi: 10.1002/hbm.21186. doi:10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk BC, Voos A, Pelphrey KA. Action representation in the superior temporal sulcus in children and adults: An fMRI study. Developmental Cognitive Neuroscience. 2012;2(4):409–16. doi: 10.1016/j.dcn.2012.04.004. doi:10.1016/j.dcn.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Lee NR, Clasen LS, Raznahan A, Lenroot RK, Martin A, et al. Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. The Journal of Neuroscience. 2012;32(14):4856–60. doi: 10.1523/JNEUROSCI.6214-11.2012. doi: 10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botterson KN, Dager SR, Dawson G, Estes AM, Evans A, Hazlett HC, Kostopoulos P, McKinstry SJ, Shultz RT, Zwaigenbaum L, Piven J. Differences in White Matter Fiber Tract Development Present from 6 to 24 Months in Infants with Autism. American Journal of Psychiatry. 2012;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. doi:10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]