Summary
Background
Psoriasis is an inflammatory skin disease that may be associated with an adverse cardiometabolic profile including modulated plasma adiponectin and leptin levels. Whether these levels are independent of cardiometabolic risk factors, which are also prevalent in psoriasis, is not known.
Methods
A consecutive sample of 122 participants with varying degrees of psoriasis severity, and a random sample of 134 participants without psoriasis were recruited for this case–control study. Cardiometabolic risk factors including traditional cardiovascular risk factors, waist circumference, insulin resistance, and total plasma adiponectin and leptin were measured. Total plasma adiponectin and leptin levels were compared in unadjusted and adjusted analyses by psoriasis status.
Results
Participants with psoriasis had mostly mild disease and were mainly on topical therapies, but still had a more adverse cardiometabolic profile compared with those without psoriasis. Furthermore, plasma adiponectin levels were significantly lower in participants with psoriasis than those without {7.13 µg/mL [interquartile range (IQR) 4.9–11.3) vs. 14.5 µg/mL (IQR 8.4–24.1); P < 0.001]}. Plasma leptin (ng/mL) levels were higher in the psoriasis group but this did not reach statistical significance [11.3 (IQR 6.4–21.8) vs. 9.8 (IQR 4.9–20.5); P = 0.07]. In multivariable modelling, plasma adiponectin levels were still negatively associated with psoriasis status after adjusting for waist size (% difference = −41.2%, P < 0.001), insulin resistance (% difference = −39.5%, P < 0.001) and both waist size and insulin resistance (% difference = −38.5%, P < 0.001)
Conclusion
Plasma levels of adiponectin were lower in psoriasis, and this relationship persisted after adjusting for cardiometabolic risk factors known to decrease adiponectin levels. These findings suggest that inflammation present in psoriasis may be associated with adipose tissue dysfunction; however, direct studies of adipose tissue are needed to confirm this.
Introduction
Psoriasis is a chronic inflammatory skin disease characterized by T helper (Th) cell dysfunction and overexpression of pro-inflammatory cytokines. The disease is associated with cardiometabolic risk factors, such as dyslipidaemia, central obesity and insulin resistance,1 and psoriasis also increases the risk of cardiovascular disease (CVD) 2. Pro-inflammatory cytokines found to be increased in the blood and skin of patients with psoriasis including tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-173 have been implicated in adipose tissue dysfunction and insulin resistance in central obesity.4 Recent small pilot studies have shown that levels of the adipokines leptin and adiponectin are abnormal in psoriasis,5,6 suggesting the presence of adipose tissue dysfunction. However, whether these perturbations are consequences of psoriatic inflammation or prevalent cardiometabolic risk factors in psoriasis is not known.
The adipokines leptin and adiponectin are key inflammatory mediators secreted by adipose tissue, which have multiple downstream effects, including regulation of insulin sensitivity, inflammation and immunity. Adiponectin has been shown to exert anti-inflammatory, insulin-sensitizing and atheroprotective effects, while leptin has been shown to induce smooth muscle and macrophage proliferation, and to upregulate inflammatory cytokines such as TNF-α.7 Decreased adiponectin and increased leptin have also been shown to be associated with an adverse cardiometabolic profile, including central obesity, insulin resistance, dyslipidaemia and coronary artery disease.8,9 The systemic inflammation, increased adipose mass and changes in adipose cell biology seen in central obesity are thought to result in secretion of abnormally high levels of leptin and abnormally low levels of adiponectin by adipocytes.7 Central obesity burdens the adipose tissue with inflammatory infiltration, hypoxia and oxidative stress, which are thought to result in endoplasmic reticulum stress, insulin resistance and abnormal cytokine secretion by the adipocyte.10 Indeed, pro-inflammatory cytokines suppress adiponectin synthesis,11 and systemic inflammation disturbs leptin and adiponectin signalling.12 The abnormal adipokine levels reported in psoriasis suggest that the systemic inflammation associated with the disease may be associated with adipose tissue inflammation similar to that seen in obesity. Some small studies have shown lower levels of adiponectin5 and higher levels of leptin6 in psoriasis, and a functional polymorphism of the leptin gene resulting in increased leptin has been associated with psoriasis.8 However, whether these differences in adipokine levels are confounded by the high prevalence in psoriasis of central obesity, insulin resistance and dyslipidaemia was not assessed in these previous studies.
We hypothesized that psoriasis may predispose to a more adverse adipokine profile independently of cardiometabolic risk factors, and assessed this in a well-phenotyped, consecutive sample of subjects with and without psoriasis.
Methods
Ethics approval
The Institutional Review Board of the Hospital of the University of Pennsylvania. All participants gave informed consent to use their information and blood.
Participants
We consecutively enrolled participants with psoriasis (n = 122; Ps group) over a 6-month period from the outpatient dermatology clinic at the Hospital of the University of Pennsylvania. The healthy control (HC) group comprised individuals without psoriasis (n = 134) were derived from a random sample of non-diseased subjects recruited as previously described.13 For the Ps group, body surface area (BSA), a measure of psoriasis severity, was ascertained at the time of enrolment by the attending dermatologist, and information on medication was extracted from the electronic medical record.
Procedures
Blood was obtained from participants at the time of enrolment after a 12-h overnight fast, and plasma levels of lipids and glucose were measured enzymatically (Cobas Fara II; Roche Diagnostic Systems, Basel, Switzerland) in lipoprotein fractions after ultracentrifugation (β-quantification technique) for the Ps groups and in whole plasma for the HC group. Plasma insulin levels were measured by a commercial radioimmunoassay (Linco Research, St. Charles, MO, USA) and plasma C-reactive protein (CRP) levels were assayed by high-sensitivity latex turbidimetric immunoassay (Wako Ltd, Richmond, VA, USA). The intra-assay coefficient of variation (CV) was 2.95 ± 2.52% for the insulin standard and the inter-assay coefficient of variation was 4.1% for the insulin standard and 11.6% for pooled human plasma.
The homeostasis model assessment of insulin resistance (HOMA-IR) index [fasting glucose (mmol/l) × fasting insulin (µU/ml)/22.5] was used to estimate the degree of insulin resistance, and waist size was used to approximate the extent of central obesity. Plasma total adiponectin and leptin were measured using ELISA (Linco Research, St. Charles, MO, USA), and the intra-assay CV for adiponectin (6.2 ± 1.5%) and leptin (5.7 ± 1.8%) was within the acceptable range.
Statistical analysis
Unadjusted characteristics for participants with and without psoriasis were assessed by calculating the median and interquartile range (IQR). Rank sum and χ2 tests were used for continuous and categorical variables, respectively. Multivariable linear regression. controlling for age, gender, hypertension, smoking status, low-density and high-density lipoprotein (LDL and HDL), triglycerides, waist size and HOMA-IR was performed using log-transformed adiponectin and leptin. Estimates are reported as % difference in mean adipokine levels between the Ps and HC groups calculated using the formula % difference = ecoefficient − 1.
Analyses were performed using STATA software (version 12; STATA Inc., College Station, TX, USA) and P < 0.05 was considered significant.
Results
Characteristics of the Ps (n = 122) and HC (n = 134) groups are summarized in Table 1. Because all Ps group were all white, we selected only white HCs for comparison. The Ps group had mild to moderate disease (median BSA 2.9%, IQR 1–8.5%) and were mostly on topical therapies. CRP, a crude indicator of systemic inflammation, was higher in the Ps group than in the HC group (P = 0.018). Despite a lower median age in the Ps group compared with the HC group [44.5 years (IQR 34–57) vs. 49 years (IQR 44–54); P = 0.02], the Ps group had greater weight, higher blood pressure, lower median HDL [43 mg/dL (36–58) vs. 49 (42–62); P = 0.008] and higher median HOMA-IR [3.47 (2.33–6.61) vs. 1.425 (0.940–2.131); P = 0.02] reflecting a more adverse cardiometabolic profile. Total cholesterol and LDL were lower in the Ps group compared with the HC group (P < 0.001) and no significant difference in triglycerides was found. There were no differences in the percentage of participants on lipid-lowering therapies between the two groups.
Table 1.
Demographics and clinical characteristics of the patients stratified by psoriasis presence.
| Median (IQR) | |||
|---|---|---|---|
| Psoriasis (n = 122) | No psoriasis (n = 134) | P | |
| Age, years | 44.5 (34–57) | 49 (44–54) | 0.01 |
| Male, n (%) | 68 (60) | 71 (53) | 0.29 |
| Hypertension, n (%) | 39 (35) | 27 (20) | 0.01 |
| Current smoker, n (%) | 8 (7) | 0 (0) | 0.001 |
| Waist size, inches | 40 (35–44) | 35.5 (33–39.5) | < 0.001 |
| BMI, kg/m2 | 29.5 (25.9–33.4) | 26.9 (24.9–30.1) | 0.001 |
| Diabetes mellitus, n (%) | 10 (8.8) | 5 (3.7) | 0.1 |
| CRP, mg/L | 3.3 (.835–9.85) | 1.3 (0.6–2.4) | 0.02 |
| Fasting glucose, mg/dL, n | 88 | 90 | 0.19 |
| Insulin, µU/mL, n (%) | 17.7 | 6.3 | < 0.001 |
| HOMA-IR | 3.5 (2.3–6.6) | 1.4 (.9–2.1) | < 0.001 |
| Total cholesterol, mg/dL | 186 (164–218) | 208.5 (189–231) | < 0.001 |
| LDL (mg/dL) | 106.9 (90–132.5) | 130 (106.4–147.4) | < 0.001 |
| HDL (mg/dL) | 43 (36–58) | 49 (42–62) | < 0.01 |
| Triglycerides (mg/dL) | 127 (78–190) | 130 (91–159) | 0.95 |
| Currently on LLT | 19 (16%) | 22 (16%) | 0.31 |
| Psoriasis severity (% BSA) | 2.9 (1–8.5) | NA | |
| Psoriasis treatment, n (%) | |||
| Topical only | 71 (58) | NA | NA |
| Systemic | 12 (9) | NA | NA |
| Phototherapy | 11 (9) | NA | NA |
BMI, body mass index; BSA, body surface area; CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; LLT, lipid-lowering therapy; NA, not applicable. All data are mean (range) unless otherwise stated.
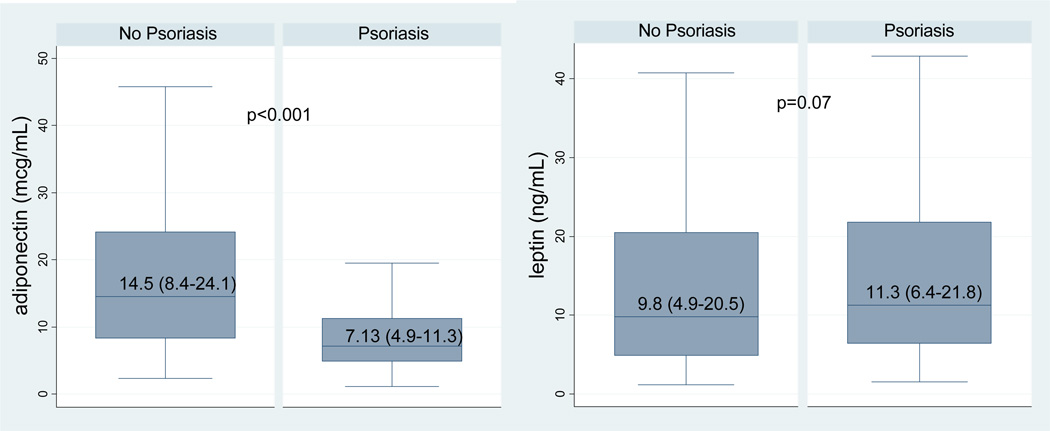
Similar to reports from previous studies, adiponectin was significantly lower in the Ps group than in the HC group [7.13 µg/mL (IQR 4.9–11.3) vs. 14.5 µg/mL (IQR 8.4–24.1); P<0.001] (Figure 1). Leptin was higher in the Ps group than in the HC group, but this did not reach statistical significance [11.3 ng/mL (IQR 6.4–21.8) vs. 9.8 ng/mL (IQR 4.9–20.5); P = 0.07]. After controlling for traditional CVD risk factors (age, gender, smoking status, hypertension and lipids), adiponectin remained significantly lower (% difference = −44.3%; P<0.001), and leptin remained significantly higher (% difference = +40.5%; P = 0.007) in the Ps group. Adiponectin was still negatively associated with Ps status after further adjusting for waist size (% difference = −41.2%, P < 0.001), insulin resistance (% difference = −39.5%; P < 0.001), and both waist size and insulin resistance (% difference = −38.5%; P < 0.001). However, leptin was no longer significantly associated with psoriasis after adjusting for waist size (P = 0.233) and insulin resistance (P = 0.36). Estimates were similar when BMI was substituted for waist size in our multivariable models (data not shown). Differences in adiponectin were not significant between the patients in the Ps group who were receiving or not receiving systemic treatment [4.59 (IQR 3.62–10.47) vs. 7.49 (IQR 5.06–11.43); P = 0.14] or phototherapy [6.44 (IQR 4.9–8.74) vs. 7.13 (IQR 3.78–8.65); P = 0.97].
Figure 1.
Unadjusted Total Plasma Adiponectin and Leptin Levels by Psoriasis Status
Discussion
We found in this consecutive sample of patients with psoriasis and healthy controls that psoriasis is associated with lower levels of plasma adiponectin independently of cardiometabolic risk factors. Our findings suggest that the lower adiponectin levels seen in the Ps group are not completely due to the high comorbidity with cardiometabolic risk factors in psoriasis, but rather may also be a result of a unique effect of psoriasis on adiponectin signalling. We did not find an association between psoriasis and leptin after controlling for central obesity and insulin resistance, so previously reported differences6 in leptin levels between individuals with and without psoriasis may have been a result of the high prevalence of central obesity and insulin resistance in the psoriasis population.
Our findings suggest that the decreased levels of adiponectin seen in the Ps regardless of psoriasis severity may reflect underlying adipose tissue inflammation, possibly driven by psoriatic inflammation. Central obesity is associated with greater amounts of inflammatory visceral fat, which is more hypertrophied, contains more macrophage infiltration, has an increased presence of activated Th cell populations and expresses a more pro-inflammatory cytokine profile, marked by increased TNF-α, IL-6, IL-17, and decreased adiponectin.4,14,15 This pro-inflammatory phenotype induces additional inflammatory stress on adipose tissue, which contributes to a vicious cycle that leads to pathological states such as insulin resistance and CVD, which are strongly associated with central obesity.7 Interestingly, a similar inflammatory state of activated Th cells and increased pro-inflammatory cytokines have been described in psoriasis, and are thought to be responsible for the induction of psoriatic plaque formation.3
Our study indicates that decreased adiponectin is an additional pro-inflammatory feature of psoriasis independent of obesity. Adiponectin has previously been linked to inflammatory activity in psoriasis, as it has been shown in that in patients with psoriasis, successful anti-inflammatory treatments may elevate adiponectin levels.16 Collectively, these studies suggest that Th cell-driven inflammation in psoriasis may be a pathway towards adipose tissue inflammation, independent of obesity.
Our study adds to a growing body of literature exploring the role of inflammation in the pathophysiology of CVD in patients with psoriasis. Psoriasis may be a risk factor for CVD independently of traditional CVD risk factors,17,18 and in fact may be associated with subclinical vascular inflammation in young subjects with severe disease.19. These associations may in part be due to a more atherogenic lipoprotein composition and function.20 Furthermore, this risk of CVD in psoriasis may also be due in part to underlying adipose tissue inflammation and dysfunction, as evidenced by lower plasma adiponectin levels.7 Decreased adiponectin may also lead directly to a more pro-atherogenic phenotype, as adiponectin has been shown to inhibit differentiation of monocyte precursors, synthesis of endothelial adhesion molecules, and formation of foam cells, which are key steps of atherosclerotic plaque formation.7,21 Our study indicates that psoriatic inflammation may predispose this atherogenic phenotype partially through perturbations in adiponectin signalling.
Our study is limited by its correlative nature, which does not permit causal inference, and we did not account for psoriasis severity in our models. Further, 18% of our Ps group were on systemic therapies and phototherapy, which may have had effects on circulating adipokine levels. However, the differences in adiponectin levelsbetween those patients in the Ps group receiving or not receiving systemic therapy were not significant, and our models remained significant after excluding participants on systemic therapy or phototherapy. Our psoriasis participants were derived from a young, real-world consecutive sample that was highly motivated toward lifestyle management, which may explain the lower total cholesterol and LDL levels than in the HC group. Interestingly, the Ps group still displayed a higher metabolic risk profile than the HC group, as evidenced by their lower HDL levels and increased obesity and insulin resistance. Cigarette smoking, which has been shown to be an adiponectin-lowering agent,22 was present in only in our psoriasis group, but was controlled for as a confounder in our adjusted models. Our study is also limited by the use of plasma adipokine levels as a surrogate for adipose tissue characterization and HOMA-IR as a crude estimate of insulin resistance.
Conclusion
Plasma levels of adiponectin were lower in psoriasis, and this relationship persisted after adjusting for cardiometabolic risk factors known to decrease adiponectin levels. These findings suggest that inflammation present in psoriasis may be associated with adipose tissue dysfunction. Based on our findings, larger prospective studies accounting for psoriasis treatment effects and severity that include biopsy studies of adipose tissue are warranted.
What is known about this topic?
Adiponectin has been reported to be decreased in patients with psoriasis.
Decreased adiponectin reflects underlying adipose tissue dysfunction and is associated with adverse cardiometabolic risk factors.
Whether decreased adiponectin in psoriasis is independent of the prevalent cardiometabolic risk factors in psoriasis is unknown.
What does this study add?
Psoriasis is associated with decreased adiponectin independently of cardiometabolic risk factors.
The association between psoriasis and decreased adiponectin suggests an independent link between psoriasis and adipose dysfunction.
Acknowledgement
This work was supported in part by R01 grant HL111293 (to JMG) and by an intramural grant from the National Heart, Lung, Blood Institute of the National Institutes of Health, grant number NHLBI Z99 HL999999 (NNM). NNM was a recipient of a National Psoriasis Foundation Discovery Grant. This work was also supported by a grant from the Doris Duke Charitable Foundation (to RCL). The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
CPD questions
Learning objective
To understand the association between psoriasis and potential adipose tissue dysfunction as estimated by serum adipokines.
Question 1
Which cell type produces adiponectin and leptin?
Myocytes.
Adipocytes.
Neurons.
Endothelial cells.
Osteoblasts.
Question 2
Which of the following has been shown to be associated with decreased adiponectin?
Increased insulin resistance.
Increased mortality.
Lower cholesterol.
Lower body mass index.
Lower blood pressure.
Question 3
Which of the following has been shown to be a function of leptin?
Dissolution of atherosclerotic plaque.
Upregulation of TNF-α.
Vasoconstriction.
Increased heart rate.
Pupillary dilation.
Question 4
Which of the following is considered a cardiometabolic risk factor?
Elevated C-reactive protein.
Elevated erythrocyte sedimentation rate.
Central obesity.
Persistent hypotension.
Persistent facial erythema.
Question 5
Which of the following is associated with an inflammatory phenotype of visceral fat?
Increased synthesis of adiponectin.
Adipocyte atrophy.
Increased IL-4 production.
Adipocyte hypertrophy.
Decreased synthesis of leptin.
Answer 1
Which cell type produces adiponectin and leptin?
Incorrect.
Correct. Adiponectin and leptin are adipokines, which are synthesized by adipocytes.
Incorrect.
Incorrect.
Incorrect.
Answer 2
Which of the following has been shown to be associated with decreased adiponectin?
Correct. Decreased adiponectin has been shown to associate with an adverse cardiometabolic profile, such as insulin resistance, central obesity and dyslipidaemia.
Incorrect.
Incorrect.
Incorrect.
Incorrect.
Answer 3
Which of the following has been shown to be a function of leptin?
Incorrect.
Correct. Leptin has been shown to be associated with an increased inflammatory state, as reflected by elevated TNF-α.
Incorrect.
Incorrect.
Incorrect.
Answer 4
Which of the following is considered a cardiometabolic risk factor?
Incorrect.
Incorrect.
Correct. Cardiometabolic risk factors include age, smoking status, hypertension, dyslipidaemia, central obesity, and insulin resistance.
Incorrect.
Incorrect.
Answer 5
Which of the following is associated with an inflammatory phenotype of visceral fat?
Incorrect.
Incorrect.
Incorrect.
Correct. Inflammatory visceral fat has been shown to exhibit several histological features, including adipocyte hypertrophy and increased macrophage infiltration.
Incorrect.
Footnotes
Conflict of interest: NNM is an employee of the National Institutes of Health. The other authors have no conflicts of interest to declare.
A portion of the data was presented at the 15th International Congress of Endocrinology 2012, Florence, Italy Accepted for publication 24 August 2013
References
- 1.Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol. 2011;147:419–424. doi: 10.1001/archdermatol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 3.Krueger JG. Hiding under the skin: A welcome surprise in psoriasis. Nat Med. 2012;18:1750–1751. doi: 10.1038/nm.3025. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2012;21:449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata S, Saeki H, Tada Y, et al. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci. 2009;55:62–63. doi: 10.1016/j.jdermsci.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Cerman aa, Bozkurt S, Sav A, et al. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br J Dermatol. 2008;159:820–826. doi: 10.1111/j.1365-2133.2008.08742.x. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Hay RM, Rashed L, et al. Association between the leptin gene 2548G/A polymorphism, the plasma leptin and the metabolic syndrome with psoriasis. Exp Dermatol. 2011;20:715–719. doi: 10.1111/j.1600-0625.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 9.Qasim A, Mehta NN, Tadesse MG, et al. Adipokines, insulin resistance, and coronary artery calcification. JACC. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Fasshauer M, Kralisch S, Klier M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 13.Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803–809. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg SP, Mccann D, Desai M, et al. Obesity is associated with macrophage accumulation. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata S, Tada Y, Hau C, et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: induction of elevated serum adiponectin levels following therapy. Br J Dermatol. 2011;164:667–670. doi: 10.1111/j.1365-2133.2010.10123.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(775):e1–e6. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–1039. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–221. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 22.Al-Attas OS, Hussain T, Al-Daghri NM, et al. The relationship between a Mediterranean diet and circulating adiponectin levels is influenced by cigarette smoking. J Atheroscler Thromb. 2013;20:313–320. doi: 10.5551/jat.14837. [DOI] [PubMed] [Google Scholar]