Abstract
Study Objectives:
The primary objective was to systematically review the literature on how sleep disordered breathing (SDB) affects recurrence and death among stroke or transient ischemic attack (TIA) patients. A secondary objective was to evaluate how treatment of SDB with continuous positive airway pressure (CPAP) affects the risk of recurrence and death in these patients.
Methods:
Adults (18+) with a stroke or TIA diagnosis were eligible for inclusion. Case groups consisted of patients with a sleep disorder. The outcomes of interest were all-cause mortality, recurrent vascular events, and case fatality.
Results:
Ten articles covering 1,203 stroke and TIA patients were included in the review. The results generally support a dose-response relationship between severity of SDB and risk of recurrent events and all-cause mortality in stroke and TIA patients. Three small-scale articles with substantial risk of bias evaluated the effects of CPAP therapy, and the results are inconclusive. Data on case fatality is too sparse to be conclusive.
Conclusions:
Existing studies provide sufficient data to establish obstructive SDB as a negative predictor of all-cause mortality and recurrent vascular events following stroke or TIA. The ability of CPAP treatment to lower the risk of serious adverse outcomes after stroke remains controversial because of substantial risk of bias identified in most of the eligible studies addressing this relation. Additional studies are needed.
Citation:
Birkbak J; Clark AJ; Rod NH. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med 2014;10(1):103-108.
Keywords: Stroke, sleep apnea, transient ischemic attack, outcome, systematic review, continuous positive airway pressure, sleep disorders
Sleep disordered breathing (SDB) is a term used to describe nocturnal breathing and ventilatory problems. SDB includes obstructive sleep apnea, which is defined as partial or complete closure of the upper airways and central sleep apnea, which describes apneic events due to lack of respiratory effort.1 The severity of SDB is measured by the number of apneas and hypopneas per hours of sleep, the apneahypopnea index (AHI).
SDB has been associated with 2-3 times higher risk of incident stroke in several large well-designed prospective studies.2–5 Moreover, a recent meta-analysis concludes that SDB with an AHI > 5 events/hour is seen in 72% and with AHI > 20 events/hour in 38% of all stroke or transient ischemic attack (TIA) patients.6 The highest prevalence of SDB is seen among male patients, patients with recurrent strokes, and those with stroke of unknown etiology. The underlying mechanisms explaining a high prevalence of SDB among stroke and TIA patients remain to be established, but the hypothesized mechanisms behind a deleterious effect of SDB on stroke incidence and prognosis include hypertension, endothelial damage, cardiac arrhythmias, variability in cerebral blood flow, and oxygen desaturation.7–9
Two previous reviews have found that SDB in stroke patients leads to poorer outcomes and increased risk of recurrent strok e.10,11 Another review is inconclusive about the benefits from treatment of SDB after stroke.12 The relationship between stroke and SDB is being increasingly discussed in the scientific literature,12,13 and we aimed to systematically evaluate serious adverse outcomes in stroke and TIA patients related to SDB. This review differs from previous reviews by focusing exclusively on the effects of SDB after stroke, and by reviewing the literature systematically. The systematic design ensures that all relevant studies are included regardless of outcome, which is necessary to draw conclusions about the state of the art of current literature.
Continuous positive airway pressure (CPAP) can be applied to patients with obstructive SDB. The rationale is that the closure of the upper airway can be prevented by the applied airway pressure when given overnight. CPAP treatment has been shown to reduce the severity of SDB in stroke patients from an AHI > 30 events/hour to 10 events/hour or less.14,15 The ability of CPAP to reduce recurrent events and death in stroke patients is still uncertain due to small and, in some cases, methodologically weak intervention studies.16,17
The objective of the present study is to systematically review the literature on how SDB affects the probabilities of recurrence and death among patients diagnosed with stroke or TIA. A secondary objective is to evaluate how treatment of SDB with CPAP affects the risk of recurrence and death in stroke or TIA patients. Our goal is to systematically review the literature and to investigate whether the present data are sufficient to draw conclusions. The methodology of this systematic review follows the PRISMA statement for reporting systematic reviews and meta-analysis.18
METHODS
Initially, a review protocol specifying the criteria of the review was developed. The protocol was changed once when the area of interest was restricted to only include stroke and TIA in order to get a reasonable number of studies for the qualitative analysis. The protocol can be assessed by contacting the corresponding author.
The eligibility criteria for inclusion were: adults (18+) with stroke or TIA diagnosed by qualified personnel (in an emergency department, stroke center, department of neurology, or a similar unit). Case groups had to consist of patients with a sleep disorder (e.g., obstructive or central sleep apnea, Cheyne-Stokes respiration). In order to comprehensively review the literature on sleep and stroke outcomes, our initial protocol included no restrictions on sleep disorder measurements, and patients suffering from sleep impairment (e.g., long/short sleep, snoring, nightmares, poor sleep, and insomnia) were also eligible for inclusion into the review. However, no relevant articles on sleep impairment and adverse outcomes of stroke and TIA were identified, and assessment of these associations was therefore omitted from the review.
The outcomes of interest were serious adverse events, including case fatality (death within 28 days), all-cause mortality, and recurrent vascular events. Experimental studies and observational studies were eligible for inclusion (i.e., cohort design, case-control design, cross-sectional studies, and case-studies with ≥ 10 cases).
A systematic search of articles using MEDLINE, EMBASE, PsycINFO, and The Cochrane Library was conducted using the following search terms: Sleep; sleep apnea; sleep disorders; insomnia; nightmare AND stroke; transient ischemic attack AND prognosis; disease progression; recurrence; case fatality. The searches were conducted in the total time span available in the respective databases with no language restrictions. In order to identify unpublished data, we contacted the corresponding authors of all included studies.
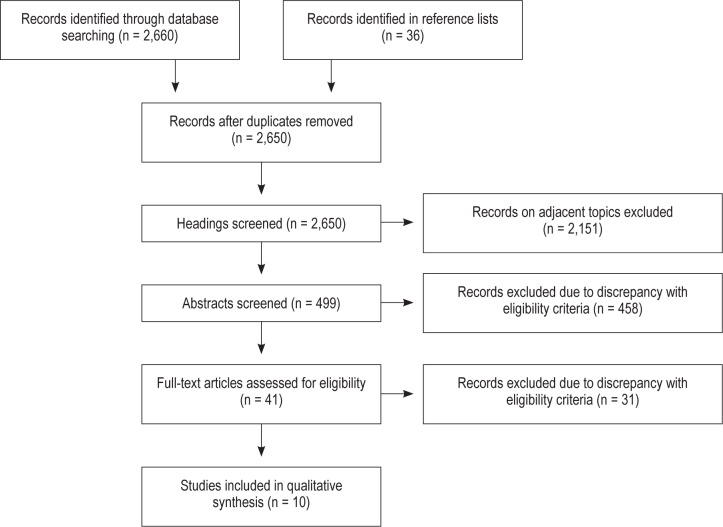
Database searches were conducted on March 5, 2012, and updated on November 28, 2012. The study selection process is illustrated in Figure 1. Eight articles met the eligibility criteria and two additional articles were identified and included by screening reference lists of the full-text articles and reviews on adjacent topics found by a search in The Cochrane Library. Thus, a total of 10 studies were included in the review. During the selection process, studies were excluded on the grounds of: not performing a sleep study in all patients, mixing stroke or TIA patients with other diagnoses without performing separate analyses, and not investigating the outcomes of interest.
Figure 1. Search strategy.
As we wanted to include all types of sleep disorders and sleep impairment, we did not make any restrictions about sleep exposure and sleep exposure measurements. However, all studies that met the eligibility criteria focused on obstructive or central sleep apnea or habitual snoring, which is included in the term SDB. The severity of SDB (the AHI) was measured with sleep study recorders of different brands, henceforth known as partial polysomnography (PSG), in all studies except two: Good et al. used overnight oximetry,19 and Mansukhani et al. used a questionnaire.20
The literature search and the screenings of headings and abstracts were made by JB. Data on methods, participants, exposure, outcome, and results was extracted using a data extraction sheet developed by inspiration of The Cochrane Consumers and Communication Review Group's data extraction template.21 The data extraction sheet template used is available from the corresponding author upon request. The extraction of data and the assessment of bias were done by JB. AC read all 41 full-text articles that were assessed for eligibility and verified all data extraction sheets. If consensus was not achieved, NR was included for a final decision.
The risk of bias was systematically evaluated in each eligible study. This included limitations related to study design and study population, randomization to treatment, proportion lost to follow-up, degree of adequate compliance, adjustment for potential confounding factors, and possible conflicts of interest.
We found great differences in the characteristics of the eligible studies regarding measurements of outcome, assessment of sleep exposure, eligibility criteria for participants, and follow-up time. This lack of comparability between the studies was expected; thus we focused on qualitative analyses rather than conducting a meta-analysis.
RESULTS
SDB and Serious Adverse Events in Stroke and TIA Patients
We identified eight studies on SDB and serious adverse events in stroke and TIA patients. Study characteristics and main results for these are presented in Table 1. Six studies were European22–27 and two were North American.19,20 The sample sizes were small, ranging from 47 to 174 stroke or TIA patients. The study designs included seven prospective cohort studies19,20,23–27 and one cross-sectional study.22 Two studies presented stroke recurrence as a primary outcome,22,25 five studies addressed all-cause mortality as primary outcome,19,20,23,26,27 and one study evaluated both fatal and nonfatal cardiovascular events.24 Two studies had case-fatality as secondary outcomes.20,25 The definition of SDB varied from AHI of 5 events/hour to 20 events/hour. An apnea episode was defined as 10 seconds of cessation of airflow,22,23,26,27 > 75% decrease,24 or > 50% decrease in airflow.25 A hypopnea was defined as a 25% to 50%,24 ≥ 50%,22,26,27 or a discernible23 or clear25 reduction in airflow or thoraco-abdominal amplitude for ≥ 10 seconds. In most cases, the hypopnea had to be associated with a 3% to 4% oxygen desaturation.23–27
Table 1.
Overview of studies addressing the effect of sleep disordered breathing on stroke and TIA outcome
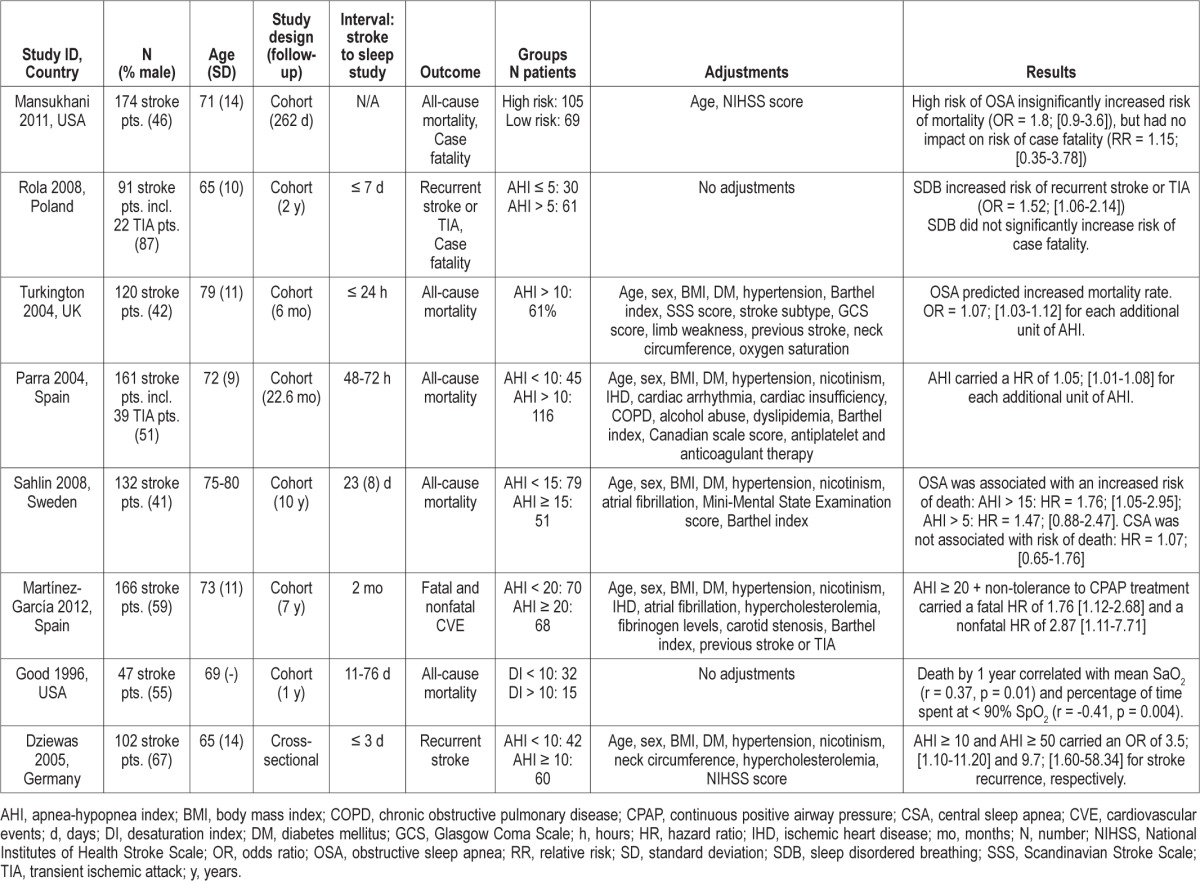
Two cohort studies and one cross-sectional study found SDB to be associated with higher risk of recurrent events.24,25 The study by Rola et al. included 91 stroke patients and found a higher risk of recurrent stroke or TIA after 24 months of follow-up (odds ratio [OR], 1.52; 95% confidence interval [CI], 1.06-2.14).25 They defined SDB as AHI > 5, which is markedly lower than in any of the other included studies. The mean AHI in patients with SDB was 20.8 ± 15.8 compared with 1.75 ± 1.4 in patients without SDB (p < 0.05). The relatively low cut-point for SDB could have overestimated the fraction of patients with SDB and consequently underestimated the effect. The study by Martinez-Garcia et al. included 166 stroke patients; they found that an AHI ≥ 20 and intolerance to CPAP treatment carried a hazard ratio (HR) of 2.87 (95% CI, 1.11-7.71) for a recurrent nonfatal cardiovascular event.24 In a cross-sectional study, Dziewas et al. showed a dose-response relation between the severity of SDB and the risk of stroke recurrence.22
Six cohort studies have addressed the relationship between SDB and all-cause mortality19,20,23,26,27 or fatal cardiovascular events.24 Three of these studies found an exposure-dependent relationship between the severity of SDB and risk of death, within six months,27 two years,23 and 10 years26 follow-up. Martinez-Garcia et al. found an increased risk of premature death among stroke patients with AHI ≥ 20 and intolerance to CPAP treatment (HR, 1.76; 95% CI, 1.12-2.68) after seven years follow-up.24 Good et al. found nocturnal oxygen desaturations to be associated with mortality after a one year follow-up.19 In a similar vein, Mansukhani et al. showed a nonsignificant association between SDB assessed by a questionnaire and mortality (OR, 1.8; 95% CI, 0.9-3.6) after 262 days follow-up.20 In summary, despite differences in study designs, definition of SDB, and length of follow-up, all of the included studies supported a higher risk of mortality among stroke and TIA patients with SDB.
Two studies included analyses on case fatality.20,25 Mansukhani et al. found no clear association between SDB and case fatality based on 11 cases (relative risk [RR], 1.15; 95% CI, 0.35-3.78).20 Rola et al. found that SDB was related to a nonsignificant higher occurrence of case fatality based on six cases.25
The study by Sahlin et al. found no association between central SDB and increased risk of death (HR, 1.07; 95% CI, 0.65-1.76).26 There was, however, limited statistical power to assess this association. In the study by Parra et al., 39% of the participants had central SDB, but they were not analyzed separately in the study.23
How CPAP Affects Stroke and TIA Outcome in Patients with SDB
The three studies, which addressed the effect of CPAP on adverse outcomes in stroke and TIA patients with SDB are summarized in Table 2. All 3 studies were European, involving 376 participants in total.24,28,29 The definition of SDB varied from an AHI of 10 events/hour in combination with excessive daytime sleepiness to 20 events/hour. Compliance rates varied from 11%28 to 72%,29 with a mean of 37%.
Table 2.
Overview of studies addressing how CPAP affects stroke and TIA outcome in patients with sleep disordered breathing
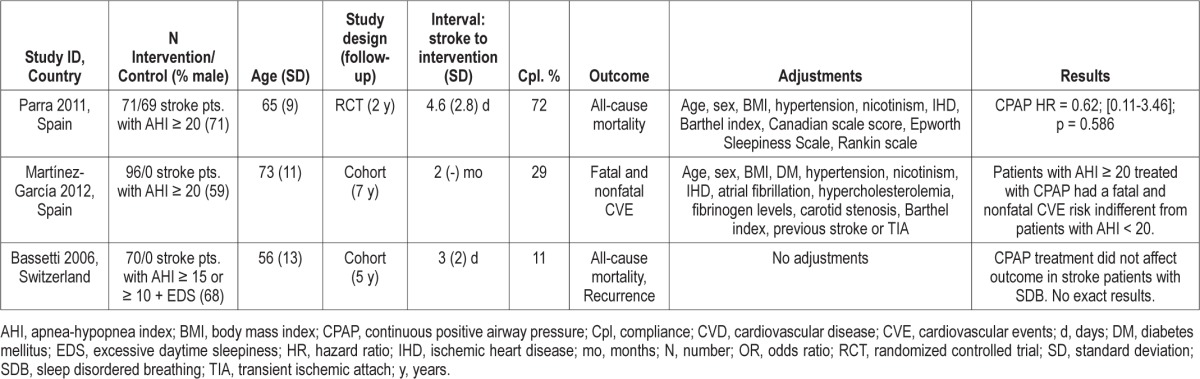
Parra et al. contributed with the only randomized controlled trial identified on this topic. They found no significant impact of CPAP treatment on all-cause mortality after two years of follow-up (HR, 0.62; 95% CI, 0.11-3.46).29
The two other intervention studies are cohort studies with follow-up between five and seven years.28,24 The study by Martínez-García et al. concludes that patients with an AHI ≥ 20 and adequate CPAP compliance had hazard ratios that were not different from patients with an AHI < 20 in terms of both fatal and nonfatal cardiovascular events.24 In the final intervention study by Bassetti et al., only eight of 70 intervention patients had adequate compliance, leaving limited strength to assess the association.28
DISCUSSION
Summary of Evidence
Based on this systematic review of the literature SDB is identified as a risk factor for recurrence and mortality following stroke or TIA. Central SDB is only seen in few cases and its impact on recurrence and mortality following stroke remains controversial. The evidence suggests that there is a dose-response relationship between the severity of obstructive SDB and the risk of serious adverse outcomes in stroke and TIA patients. The presented data are too sparse to draw conclusions about the risk of case fatality.
In terms of CPAP treatment, the only randomized controlled trial showed no protective effect of CPAP usage among stroke patients with SDB.29 Martínez-García et al. found that adequate CPAP treatment neutralized the excessive risk of cardiovascular events associated with SDB.24 The cohort study by Bassetti et al. did not prove any benefit of CPAP treatment in stroke patients with SDB.28 The evidence is too sparse to draw conclusions. Low compliance rates, mainly because of discomfort with the machine, are a major problem even when the patients in the worst mental and physical conditions are left out.24,28 Further studies are needed to clarify the role of CPAP treatment among stroke patients with SDB.
Risk of Bias within Studies
Six of the included studies were well adjusted for baseline characteristics and known cardiovascular and cerebrovascular risk factors; one study adjusted for only age and stroke severity20; while three studies investigated for differences in the distribution of confounding factors among the study groups, but made no adjustments (Tables 1 and 2).19,25,28 In studies with insufficient adjustments cerebrovascular risk factors such as hypertension, smoking, obesity, and arrhythmias could be distributed unevenly among the study groups and potentially introduce confounding.
None of the studies used full PSG to measure SDB in all patients. Instead, different validated partial PSG systems were used. Good et al. used a simple overnight oximetry to calculate the desaturation index (DI) on basis of episodes with ≥ 4% desaturation from baseline and time with SpO2 < 90%.19 The authors recognize that the fraction of patients having SDB could be underestimated. In the Mansukhani study20 SDB was assessed by the Berlin Sleep Questionnaire, which has previously been validated to have a positive predictive value between 89% and 96% in identifying patients with sleep apnea.30,31 While the use of alternatives to full PSG might be an economic necessity when classifying SDB, low diagnostic agreement with full PSG may lead to misclassification of some SDB patients and probably underestimation of the associations.
The time interval from stroke onset until sleep study varied significantly among the studies - from 24 hours to 76 days. The previously mentioned meta-analysis by Johnson et al.6 found no difference in SDB frequency related to the timing of the sleep study in a review of 29 studies. A few studies have found spontaneous remission of SDB, and we cannot exclude that the variations in time interval could possibly influence the findings.
Two studies included both stroke and TIA patients, but the results were not subdivided by these diagnoses.23,25 There is no indication that stroke and TIA differ in risk of serious adverse outcomes in relation to SDB, but a separation of patient types would have been preferable. Few studies included both central and obstructive SDB, with up to 39% of the patients having central SDB,23 without conducting separate analyses. If central SDB is not a predictor of serious adverse outcomes, as the Sahlin study26 suggests, these studies potentially underestimate the deleterious effects of obstructive SDB.
Risk of Bias Related to CPAP Studies
In terms of bias associated with study design, the two non-randomized intervention studies had control groups consisting of patients who did not tolerate CPAP treatment. Even though the groups were adjusted for potential cerebrovascular confounders, it is likely that patients who tolerated CPAP had better mental, cognitive, or social status, and potentially a better prognosis before treatment, thus introducing selection bias. This could have led to an overestimation of the protective effect of CPAP treatment.
Low compliance rates caused substantial risk of bias in most of the intervention studies. When a great deal of the intervention group, and most likely those in the worst condition, is left out of the analyses, the beneficial effect of treatment is likely to be overestimated.
The only potential conflicts of interest declared in the included studies were related to Bassetti, who is member of the medical advisory board of ResMed, the company that produced the CPAP device used in the study.28 The role of this relationship is unknown, however, the results of the study did not show any beneficial effects of CPAP treatment in stroke patients with SDB.28
Risk of Bias across Studies
The AHI score used to classify SDB was very variable, and while some studies found a higher risk of adverse outcomes even at low cut points for SDB,22,23,25,27 other studies only found an effect in the more severe cases of SDB.24,26 The definitions used to classify apneas and hypopneas and the partial PSG recorders differed slightly across the studies. Such variations and their possible influence on the results are inevitable. No studies used placebo CPAP, also known as sham CPAP, though this is available.32 Given the major impact on daily living associated with CPAP usage, long-term trials with placebo CPAP could very well be considered unethical, though a demand for these trials is proposed by many authors.
Patients with a diagnosis of previous stroke are expected to have a worse prognosis than first-time stroke victims. However, four studies screened for and included patients with previous stroke at baseline,19,24,26,27 and two studies did not assess the occurrence of previous stroke in their baseline characteristic.20,28
It is well known that positive results are more likely to be published than negative results, especially in terms of small studies as the ones included in this review. By making a broad search in different electronic databases, and by contacting authors of eligible studies, we did our utmost to assess all existing relevant studies, but the existence of publication bias cannot be fully excluded.
In summary, based on this systematic and comprehensive review of the literature, we conclude that existing studies provide sufficient data to establish obstructive SDB as a negative predictor of serious adverse outcomes following stroke or TIA in terms of all-cause mortality and recurrent vascular events. The evidence on central SDB is insufficient, and additional studies are needed to address this relationship. The present data on case fatality are also very sparse and inconclusive. The ability of CPAP treatment to lower the risk of serious adverse outcomes after stroke remains controversial because of substantial risk of bias identified in most of the eligible studies addressing this relation. Intolerance to CPAP treatment among stroke patients is high, and alternative ways of treating SDB should be considered including, for example, positional therapy, which has been shown to reduce SDB by changing sleeping position,33 and usage of therapeutic pillows.34 In relevant cases, weight loss intervention should be considered as a way to reduce SDB severity and cerebrovascular risk. Given the results of this systematic review, we suggest that SDB should be recognized as a predictor of worse outcome among stroke patients, but further studies are necessary in order to recommend an appropriate intervention for these patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- DI
desaturation index
- HR
hazard ratio
- OR
odds ratio
- PSG
polysomnography
- RR
relative risk
- SDB
sleep disordered breathing
- TIA
transient ischemic attack
REFERENCES
- 1.Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Can Respir J. 2006;13:387–92. doi: 10.1155/2006/627096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Marin JM. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Netzer N, Werner P, Jochums I, Lehmann M, Strohl KP. Blood flow of the middle cerebral artery with sleep-disordered breathing: correlation with obstructive hypopneas. Stroke. 1998;29:87–93. doi: 10.1161/01.str.29.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Goyal SK, Sharma A. Atrial fibrillation in obstructive sleep apnea. World J Cardiol. 2013;5:157–63. doi: 10.4330/wjc.v5.i6.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrscher TE, Overland B, Sandvik L, Westheim AS, Akre H. High cardiovascular risk profile in patients with sleep apnea. Laryngoscope. 2013 Jul 12; doi: 10.1002/lary.24304. doi: 10.1002/lary.24304. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Portela PC, Fumado JC, Garcia HQ, Borrego FR. Sleep-disordered breathing and acute stroke. Cerebrovasc Dis. 2009;27(Suppl 1):104–10. doi: 10.1159/000200447. [DOI] [PubMed] [Google Scholar]
- 11.Brown DL. Sleep disorders and stroke. Semin Neurol. 2006;26:117–22. doi: 10.1055/s-2006-933315. [DOI] [PubMed] [Google Scholar]
- 12.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–77. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke. 2012;7:231–42. doi: 10.1111/j.1747-4949.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnerup J, Ritter MA, Wersching H, et al. Continuous positive airway pressure ventilation for acute ischemic stroke: a randomized feasibility study. Stroke. 2012;43:1137–9. doi: 10.1161/STROKEAHA.111.637611. [DOI] [PubMed] [Google Scholar]
- 15.Wessendorf TE, Wang YM, Thilmann AF, Sorgenfrei U, Konietzko N, Teschler H. Treatment of obstructive sleep apnoea with nasal continuous positive airway pressure in stroke. Eur Respir J. 2001;18:623–9. doi: 10.1183/09031936.01.00057201. [DOI] [PubMed] [Google Scholar]
- 16.Levy P, Pepin JL. CPAP treatment of sleep apnoea in the early phase of stroke: growing evidence of effectiveness. Eur Respir J. 2011;37:997–9. doi: 10.1183/09031936.00182810. [DOI] [PubMed] [Google Scholar]
- 17.Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke. 2012;7:231–42. doi: 10.1111/j.1747-4949.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The PRISMA Statement website. [Accessed February, 2012]. http://www.prisma-statement.org.
- 19.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–9. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 20.Mansukhani MP, Bellolio MF, Kolla BP, Enduri S, Somers VK, Stead LG. Worse outcome after stroke in patients with obstructive sleep apnea: an observational cohort study. J Stroke Cerebrovasc Dis. 2011;20:401–5. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Data Extraction Template for Cochrane Reviews. The Cochrane Consumers & Communication Review Group. Updated May 3, 2011. [Accessed March, 2012]. http://www.latrobe.edu.au/chcp/assets/downloads/DET_2011.doc.
- 22.Dziewas R, Humpert M, Hopmann B, et al. Increased prevalence of sleep apnea in patients with recurring ischemic stroke compared with first stroke victims. J Neurol. 2005;252:1394–8. doi: 10.1007/s00415-005-0888-7. [DOI] [PubMed] [Google Scholar]
- 23.Parra OA. Sleep-related breathing disorders: Impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24:267–72. doi: 10.1183/09031936.04.00061503. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Garcia MA, Campos-Rodriguez F, Soler-Cataluna JJ, Catalan-Serra P, Roman-Sanchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39:906–12. doi: 10.1183/09031936.00011311. [DOI] [PubMed] [Google Scholar]
- 25.Rola R, Jarosz H, Wierzbicka A, et al. Sleep disorderd breathing and recurrence of cerebrovascular events, case-fatality, and functional outcome in patients with ischemic stroke or transient ischemic attack. J Physiol Pharmacol. 2008;59(Suppl 6):615–21. [PubMed] [Google Scholar]
- 26.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 27.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004;59:367–71. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–72. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 29.Parra O, Sanchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128–36. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 30.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124:281–90. [PubMed] [Google Scholar]
- 32.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33:260–6. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dziewas R, Hopmann B, Humpert M, et al. Positional sleep apnea in patients with ischemic stroke. Neurol Res. 2008;30:645–8. doi: 10.1179/174313208X289598. [DOI] [PubMed] [Google Scholar]
- 34.Svatikova A, Chervin RD, Wing JJ, Sanchez BN, Migda EM, Brown DL. Positional therapy in ischemic stroke patients with obstructive sleep apnea. Sleep Med. 2011;12:262–6. doi: 10.1016/j.sleep.2010.12.008. [DOI] [PubMed] [Google Scholar]