Abstract
Study Objectives:
To evaluate sleep modifications induced by chronic benzodiazepine (BDZ) abuse.
Methods:
Cohort study, comparison of sleep measures between BDZs abusers and controls. Drug Addiction Unit (Institute of Psychiatry) and Unit of Sleep Disorders (Institute of Neurology) of the Catholic University in Rome. Six outpatients affected by chronic BDZ abuse were enrolled, (4 men, 2 women, mean age 53.3 ± 14.8, range: 34-70 years); 55 healthy controls were also enrolled (23 men, 32 women, mean age 54.2 ± 13.0, range: 27-76 years). All patients underwent clinical evaluation, psychometric measures, ambulatory polysomnography, scoring of sleep macrostructure and microstructure (power spectral fast-frequency EEG arousal, cyclic alternating pattern [CAP]), and heart rate variability.
Results:
BDZ abusers had relevant modification of sleep macrostructure and a marked reduction of fast-frequency EEG arousal in NREM (patients: 6.6 ± 3.7 events/h, controls 13.7 ± 4.9 events/h, U-test: 294, p = 0.002) and REM (patients: 8.4 ± 2.4 events/h, controls 13.3 ± 5.1 events/h, U-test: 264, p = 0.016), and of CAP rate (patients: 15.0 ± 8.6%, controls: 51.2% ± 12.1%, U-test: 325, p < 0.001).
Discussion:
BDZ abusers have reduction of arousals associated with increased number of nocturnal awakenings and severe impairment of sleep architecture. The effect of chronic BDZ abuse on sleep may be described as a severe impairment of arousal dynamics; the result is the inability to modulate levels of vigilance.
Citation:
Mazza M; Losurdo A; Testani E; Marano G; Di Nicola M; Dittoni S; Gnoni V; Di Blasi C; Giannantoni NM; Lapenta L; Brunetti V; Bria P; Janiri L; Mazza S; Della Marca G. Polysomnographic findings in a cohort of chronic insomnia patients with benzodiazepine abuse. J Clin Sleep Med 2014;10(1):35-42.
Keywords: Abuse, arousal, benzodiazepines, cyclic alternating pattern, polysomnography, sleep
Benzodiazepines (BDZs) are used as anxiolytics, hypnotics, anticonvulsants, muscle relaxants, and to induce anesthesia. Although guidelines emphasize that BDZs are not drugs of first choice and should only be used short term (recommended use should not exceed 4-6 weeks' duration), their use beyond the licensed duration is common.1 Within weeks of chronic use, tolerance may occur, leading to unwanted dose increases, and withdrawal becomes apparent once the drug is no longer available: these conditions are both indicative of BDZ dependence.2 BDZs are also drugs of abuse, either on their own or in conjunction with opioids and stimulants. The diagnosis of addiction is made in presence of compulsive use of the drug despite negative consequences.3 Recently it has been suggested that specific psychological and situational factors differentiate benzodiazepine addicts from non-addicted benzodiazepine users; in particular, benzodiazepine addiction might be associated with higher neuroticism, introversion, less effective coping mechanisms, previous accumulation of adverse life events, and/or inadequate BDZ treatment.4 These drugs may initially lead to prolonged total sleep time as a desired effect. Acute and short-term usage of BDZs is usually associated with a reduction of nocturnal wake time, subjective improvements of quality and depth of sleep, as well as improved sleep continuity and total sleep time as reported by polysomnography.5,6
BRIEF SUMMARY
Current Knowledge/Study Rationale: Chronic use and abuse of benzodiazepines is common in chronic insomnia patients, and it can lead to modifications of sleep patterns. The aim of this study was to evaluate the modifications of sleep, at macro- and microstructural level, in patients with abuse of benzodiazepines.
Study Impact: The study demonstrates that abuse of benzodiazepines has a severe impact on sleep: in particular, abusers have a marked reduction of arousals associated with increased number of nocturnal awakenings. These effects of chronic abuse may be related to a modification of the thalamic GABAergic gating of incoming stimuli during sleep.
Regarding the microstructure of sleep, BDZs lead to a reduction of slow frequencies and an increase of fast frequencies in the EEG.7–12 It has been demonstrated that BDZ users have less delta and theta activity than good sleepers.7 When compared to drug-free insomniacs, chronic BDZ users have less delta and theta activity only within the second sleep cycle.7 The effect of BDZs on sleep microstructure have been tested in a model of situational insomnia, i.e., a condition in which sleep is disrupted by exposing normal subjects to variable levels of noise.3 In this model, BDZs (as well as other non-BDZ hypnotics) are able to reduce the instability of NREM sleep (CAP rate) and have a protective action against the perturbation induced by white noise. All hypnotic drugs, including BDZs, may determine a significant decrease in EEG arousals, as measured by CAP parameters.13 The intake of BDZs may have a negative long-term effect on sleep.8
The aim of the present study was to evaluate the sleep structure and the pattern of arousability in a cohort of patients with chronic abuse of BDZs. For this reason, we evaluated the macrostructure of sleep (by means of nocturnal polysomnographic recordings), EEG power spectral analysis, pattern of arousal (by means of the cyclic alternating pattern [CAP] analysis), and autonomic activity (by means of heart rate variability analysis).
MATERIALS AND METHODS
Patients
Six patients affected by chronic BDZ abuse were enrolled: 4 men and 2 women, mean age 53.3 ± 14.8 (range: 34-70 years). Patients were recruited consecutively from the Drug Addiction Unit of the Catholic University in Rome over 12 months (January to December 2012). Inclusion criterion was a diagnosis of by chronic BDZ abuse according to the criteria of the DSM-IVTR,14 not associated with other drug or substance dependence or abuse. Exclusion criteria were: presence of other medical, neurologic, or psychiatric diseases; presence of heart disease, arrhythmias, intake of cardiovascular active drugs; diabetes; uncontrolled hypertension; severe obesity (BMI > 35 kg/m2); chronic respiratory disease; obstructive sleep apnea syndrome; restless legs syndrome; and thyroid diseases. All patients underwent a full psychiatric, medical, and neurological evaluation. The diagnosis of BDZ abuse was assessed on a clinical basis, according to DSM-IV-TR criteria.14 All patients were still taking BDZs at the time of the sleep study.
Control Group
Patients were compared with a control group of 55 healthy subjects, matched for age and sex: 23 men and 32 women, mean age 54.2 ± 13.0 (range: 27-76 years). Control subjects were healthy volunteers. Controls underwent a full medical and neurological evaluation and a hypnological interview to rule out present or previous history of sleep disorders. The same exclusion criteria were applied to patients and controls. To compare patients with controls of same age, we chose from the entire control group 3 subgroups, defined by age ranges: age 30-40 years (n = 12), age 50-60 years (n = 17), and age > 65 (n = 14). The study was approved by the local ethics committee; the study was designed according to the Helsinki Declaration of 1975. All patients and control subjects were fully informed, and all gave a written consent to participate.
Psychological Functioning Measures
All patient underwent a full clinical psychiatric evaluation, followed by a psychometric evaluation which included the following self-administered scales: Self-Administered Anxiety Scale (SAS#54),15 Beck Depression Inventory (BDI),16 Maudsley Obsessive Compulsive Inventory (MOCI),17 and Snaith-Hamilton Pleasure Scale (SHAPS).18
The SAS #54 is used in order to measure anxiety-related symptoms. It consists in a 4-point Likert-type scale, ranging from 1 to 4; higher scores correspond to higher levels of anxiety. The BDI is a 21-item validated instrument which measures characteristic attitudes and symptoms of depression. Scores range from 0 to 36; scores > 9 indicate mild to severe depression. The MOCI is a questionnaire with true-false format developed for evaluating obsessive-compulsive symptoms. The total score ranges between 0 (absence of symptoms) and 34 (maximum presence of symptoms). The SHAPS is a 14-item instrument that is used to measure hedonic capacity. Total scores range from 0 to 14; a higher total SHAPS score indicates higher levels of anhedonia.
Subjective Sleep Evaluation
Subjective evaluation of sleep quality was performed using the validated Italian version of the Pittsburgh Sleep Quality Index (PSQI).19 A global score > 5 was considered an indicator of poor sleep quality.20 The validated Italian version of the Epworth Sleepiness Scale (ESS)21 was used for the evaluation of excessive daytime sleepiness (EDS). A score > 9 was considered indicative of EDS. In all participants, patients and controls, an evaluation of the symptoms and clinical signs predictors of obstructive sleep apnea syndrome (OSAS) was performed by means of the Berlin Questionnaire.22 The clinical evaluation included the measure of neck circumference, body mass index (BMI), presence of habitual snoring, nocturia, morning headache, arterial hypertension, and apneas reported by the bed partner.
Polysomnography
Twenty-four hour ambulatory (home-based) polysomnography was recorded. This recording technique was chosen in order to allow the patients to sleep in their habitual home setting.23 Recording montage included EEG leads filled with electrolyte applied to the following locations: F4, C4, O2 or F3, C3 O1; reference electrodes applied to the contralateral mastoid (M1); 2 EOG electrodes applied to the outer ocular canthus and referred to the contralateral mastoid, surface EMG of submental muscles, and EKG. Patients were asked to indicate in a sleep log the times of lights-off and lights-on. Patients were not asked to keep a defined schedule, but were left free to follow their spontaneous sleep-wake cycle. Sleep recordings were analyzed on computer monitor, and sleep stages were visually classified according to the criteria of the American Academy of Sleep Medicine (AASM).24
In order to compare subjective sleep quality with results of PSG, in the morning after the PSG recording, all subjects were asked to make an estimate of their sleep latency, sleep duration, number of awakenings, and sleep quality (according to a visual analogue scale [VAS] ranging from 0 to 100). In order to quantify the degree of sleep misperception we calculated a misperception index (MI),25 which was computed using the following formula25:
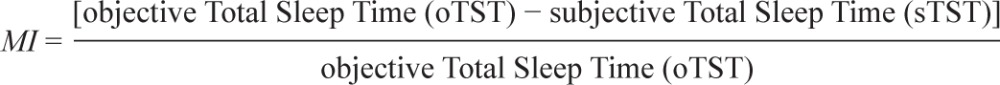 |
Sleep microstructure was evaluated by means of the detection of the fast-frequency EEG arousals and the analysis of CAP. Arousal were visually detected and quantified in accordance to the rules of the ASDA26; separate arousal indexes (number of arousals/time) were calculated for the entire sleep period time, NREM sleep, and REM. To evaluate the dynamics of arousal, we quantified the arousal fluctuations during sleep by means of CAP. CAP scoring was performed visually, according to the criteria established by Terzano et al.27 We quantified, within NREM sleep stages, the percentage of NREM sleep occupied by CAP. This ratio (CAP duration/NREM sleep), referred to as CAP rate, is the expression of the percentage of NREM sleep spent in a state of arousal instability.
Power Spectral Analyses
Sleep EEG power spectral analysis was performed using a central monopolar scalp derivation (C4 or C3 referred to the contralateral mastoid M1 or M2). Recordings from leads placed centrally reflect EEG activity summed from both frontal and parietal regions and are considered the most sensitive for recording sleep related activity.28–31 Sleep power spectral analysis was performed on all NREM and REM stages. Each 30-sec epoch was visually screened for artifacts (EMG, temporary disconnect spikes, sweating, body movements), and epochs with artifacts were removed from further analysis. The remaining data were extracted from the scored sleep data file by a software program (Rembrandt SleepView, Medcare) and stored in separate ASCII file. Spectral analysis was performed on 1-sec windows with a frequency resolution of 0.5 Hz using a discrete Fourier transform algorithm. Four spectral bands (delta: 0.5-4 Hz, theta: 4.5-7.5 Hz, alpha: 8-13 Hz, beta: 13.5-30 Hz) were computed per 30-sec epochs for the entire recording period. These spectra were converted to data file for statistical analysis. To compensate for variability among subjects and across the night in EEG power, the spectra were normalized: values in each frequency band were expressed as percentage of the total power.
Heart Rate Variability
Physiological Correlates of Heart Rate Variability Analysis
Heart rate variability (HRV) analysis is the measure of the variations of the interval between consecutive heart beats. It is widely accepted that HRV represents a quantitative marker of autonomic activity.32,33 The variations in heart rate may be evaluated by time domain methods and frequency domain methods.
The time domain methods are based on the detection of the QRS in a normal EKG and on the determination of normal-to-normal (NN) intervals, which are all the intervals between adjacent QRS sinusal complexes. Time domain variables used in the present study were heart rate and heart rate standard deviation.
The frequency domain methods consist in the calculation of the power spectral density analysis of a plot of consecutive NN intervals, called the tachogram. The auto-regressive method used in this study allowed an accurate estimation of power spectral density even on a small number of samples on which the signal is supposed to maintain stationarity. Two major spectral components were computed: low-frequency (LF) and high-frequency (HF). The HF component of the spectrum is widely recognized as a measure of vagal activity; the significance of LF component is more debated and seems to reflect at the same time both vagal and sympathetic activity. Overall, the LF/HF ratio may provide a quantitative estimation of the balance of the 2 branches of the autonomic nervous system (sympathovagal balance). The power of LF and HF bands was expressed in normalized units (nu), and the LF/HF ratio was calculated. Normalization was performed according to the formula:
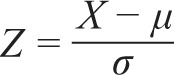 |
where μ = E[X] is the mean and the standard deviation of the probability distribution of X. A detailed description of HRV analysis, standards of measurement, physiological interpretation, and clinical use is available in the report of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.32,33
Statistical Analysis
Data obtained from the patient group were compared to those obtained from controls. The following sleep variables were compared: sleep latency (subjective and objective), total sleep time (subjective and objective), number of awakenings (subjective and objective), sleep efficiency, percentages of each sleep stage (N1, N2, N3, REM), sleep quality (subjective VAS), and CAP parameters. The HRV parameters considered were: HR, HR standard deviation, power of LF and HF bands in normalized units, and the LF/HF ratio. All sleep parameters and HRV measures were compared in these 2 groups by means of a nonparametric test (Mann-Whitney U-test). To avoid type I errors, a formal Bonferroni correction was applied to each family of comparisons. The threshold for significance was p = 0.05.
RESULTS
The mean duration of BDZ abuse was 3.5 years (range 2-6 years); BDZs used were lorazepam in 3 cases (mean daily dose: 7.8 mg), lormetazepam in 1 case (10 mg/day), alprazolam in 1 case (9 mg/day), and bromazepam in 1 case (31 mg/day). In all cases, BDZs were initially prescribed for the treatment of chronic insomnia.
Psychometric and Subjective Sleep Evaluation
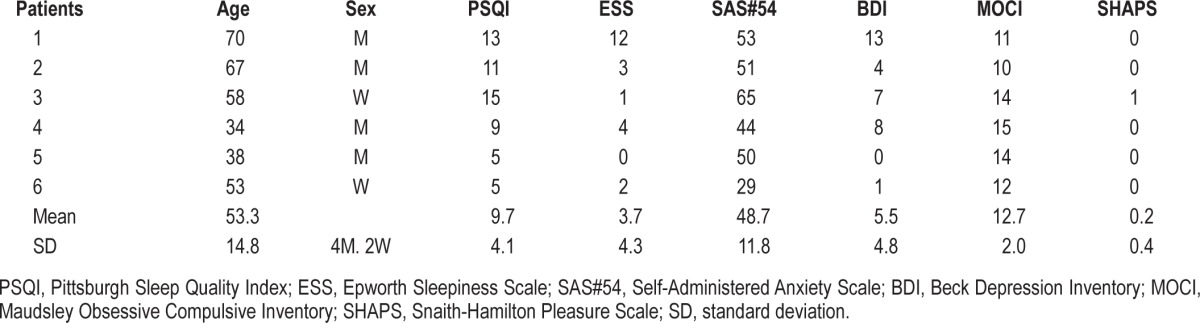
All patients completed the study. In the subjective sleep evaluation, the mean PSQI score was 9.7 ± 4.1; all patients had PSQI ≥ 5, indicating poor subjective sleep quality. The ESS mean score was 3.7 ± 4.3; only one patient had ESS > 9, indicating excessive daytime sleepiness. As concerns the evaluation of anxiety symptoms, the mean SAS score was 48.7 ± 11.8 (2 patients were in the normal range, 3 had mild to moderate anxiety levels, 1 had a score indicating severe anxiety). Mean BDI was 5.5 ± 4.8: all patients but one were below the threshold indicating mild depression symptoms. The mean score of the MOCI was 12.7 ± 2.0; all patients had scores ≥ 10; these scores appear greater that those reported in literature for normal subjects.34 SHAPS scores were normal in all subjects. Results of psychometric and sleep quality tests are in Table 1.
Table 1.
Results of subjective sleep evaluation and psychometric tests in BDZ abusers
Polysomnographic Scores
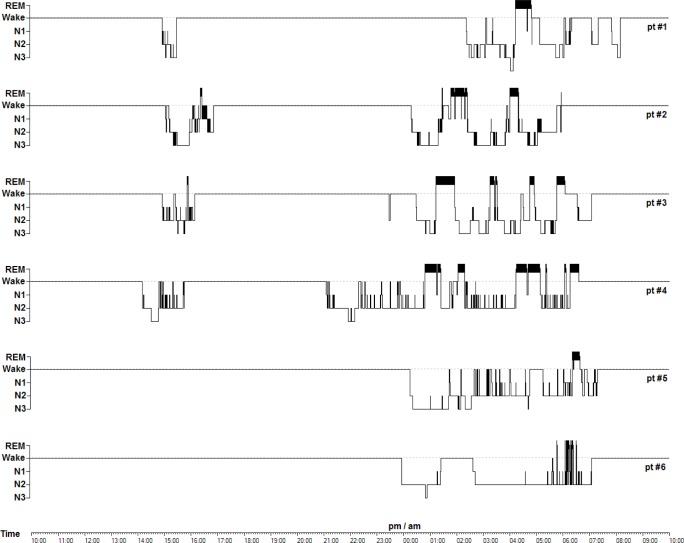
As concerns sleep macrostructure, BDZ abusers, compared to controls, had shorter SOL (patients: 14.8 ± 18.0 min, controls: 31.3 ± 23.7, U-test: 249, p = 0.042) and increased WASO (patients: 133.4 ± 54.9 min, controls: 54.3 ± 40.7 min, U-test: 49, p = 0.005); no differences were observed in sleep stage percentages. Three patients had negative MI (indicating underestimation of sleep duration), and 2 patients had positive MI (indicating overestimation of sleep duration). The most relevant differences between the groups were observed in sleep micro-structure: BDZ abusers had lower indexes of fast-frequency EEG arousal in total sleep (patients: 7.0 ± 3.2 events/h, controls 13.6 ± 4.6 events/h, U-test: 292, p = 0.002), NREM (patients: 6.6 ± 3.7 events/h, controls 13.7 ± 4.9 events/h, U-test: 294, p = 0.002), and REM (patients: 8.4 ± 2.4 events/h, controls 13.3 ± 5.1 events/h, U-test: 264, p = 0.016). Moreover, BDZ abusers showed much lower levels of CAP time (patients: 46.5 ± 26.3 min, controls: 169.9 ± 49.5 min, U-test: 325, p < 0.001) and CAP rate (patients: 15.0% ± 8.6%, controls: 51.2% ± 12.1%, U-test: 325, p < 0.001). Results of polysomnographic and subjective sleep evaluations and the MI are in Table 2. Detailed results of sleep scoring and of CAP analysis are shown in Table 3 and Table 4. Sleep hypnograms of all patients enrolled are shown in Figure 1.
Table 2.
Objective and subjective measures in BDZ abusers
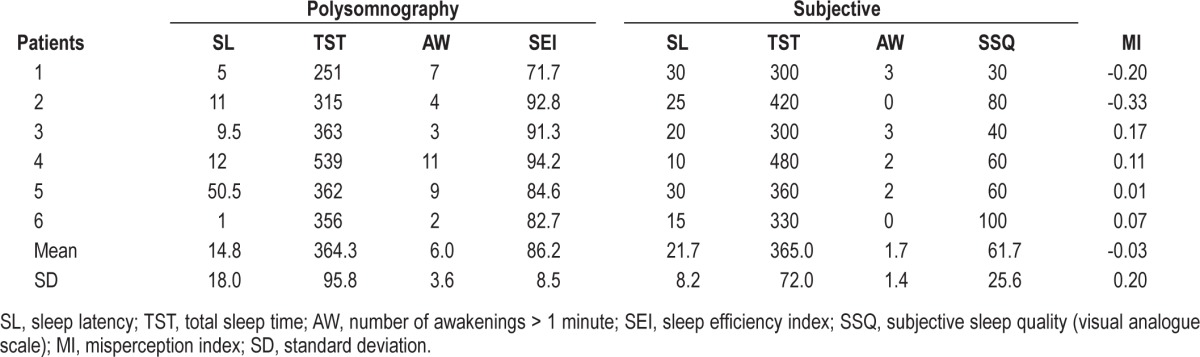
Table 3.
Results of polysomnographic analysis, arousal and CAP scores, and HRV analysis in patients and controls, and statistical comparison
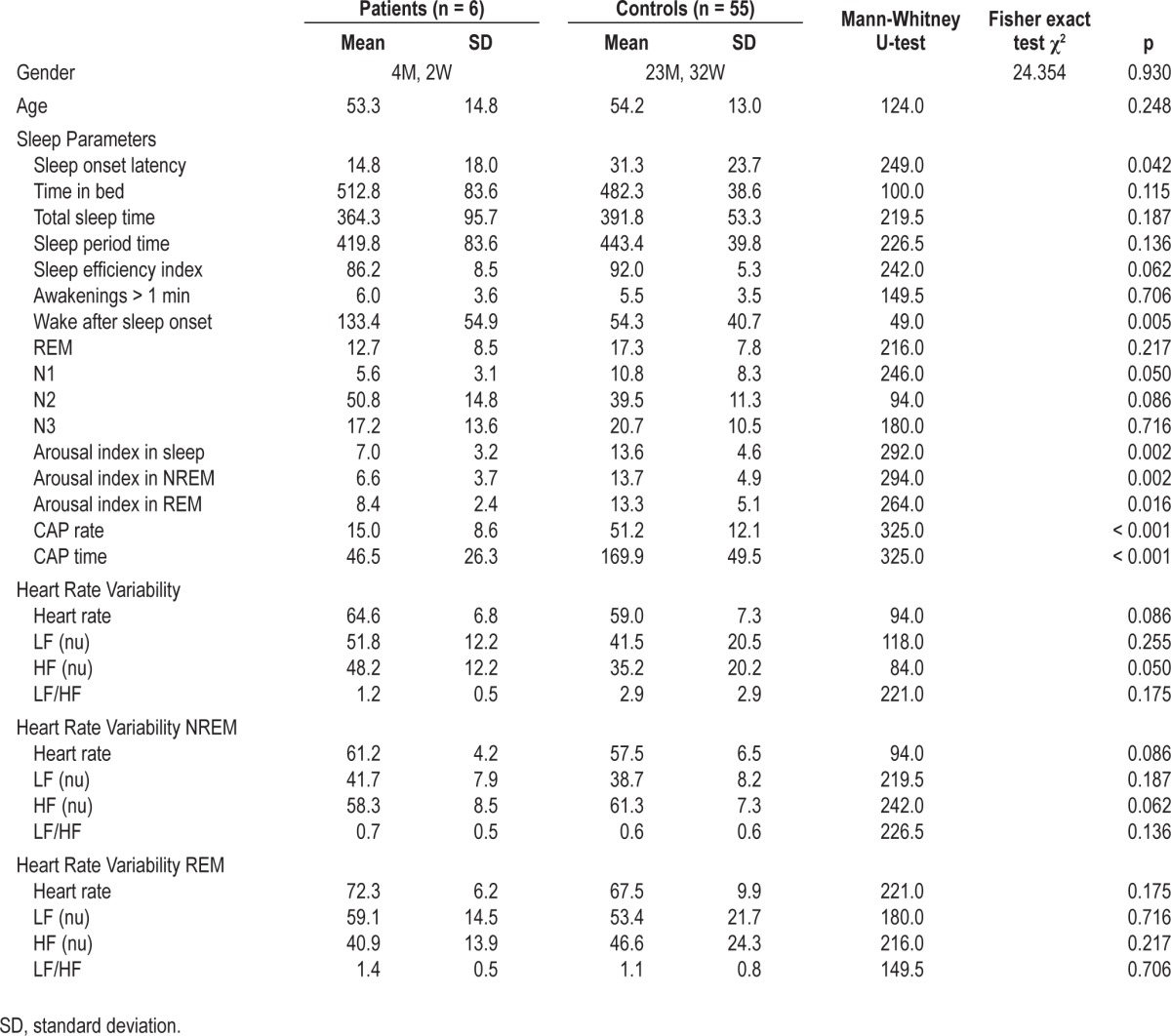
Table 4.
Results of polysomnographic analysis, arousal and CAP scores, and HRV analysis in patients and controls
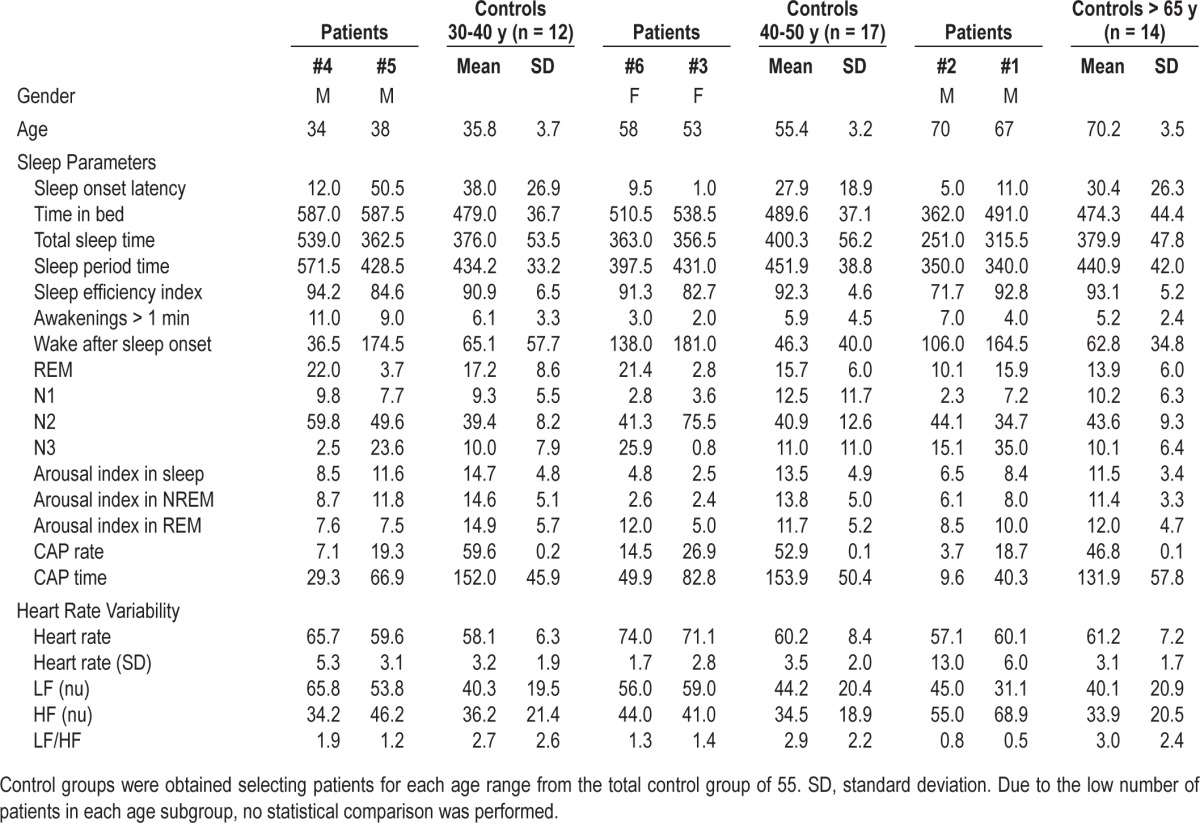
Figure 1. Twenty-four-hour sleep hypnograms in BDZ abusers.
EEG Power Spectral Analysis
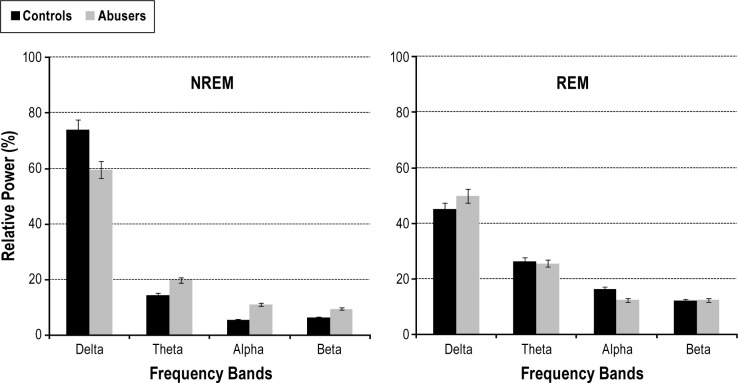
Relative spectral power analysis showed that abusers, as compared to controls, had more beta activity (patients 13.7% ± 11.3%, controls: 6.3% ± 5.7%, U-test: 44, p = 0.028) and less theta activity (patients 7.1% ± 4.4%, controls: 14.1% ± 4.0%, U-test: 179, p = 0.004), whereas no significant differences were measured in the relative amount of delta and alpha frequency bands. Detailed results of EEG power spectral analysis and comparison between patients and controls are shown in Figure 2.
Figure 2. Histograms of relative power spectra in BDZ abusers and controls.
HRV Analysis
No significant differences were measured between BDZ abusers and controls in HRV parameters, with the exception of an increased HF component (patients: 48.2 ± 12.2 nu, controls: 35.2 ± 20.2 nu, U-test: 84, p = 0.050). Results of HRV analysis and comparison are shown in Table 3.
DISCUSSION
The objective of the present study was to investigate the modification of sleep pattern in chronic benzodiazepine abusers. Our results, though obtained from a small cohort of patients, seem to indicate that chronic BDZ abuse is associated with a peculiar pattern of sleep modification involving all levels of sleep organization.
As concerns sleep macrostructure, all patients showed poor subjective sleep quality, and polysomnography showed a marked increase in wake after sleep onset, associated with a gross disruption of the ultradian NREM/REM cycle (Figure 1). Despite these modifications in sleep macrostructure, the most relevant findings observed in the abuse cohort concerned microstructure: in particular, abusers, compared to controls had significantly lower indexes of EEG arousal in all sleep stages and lower indexes of NREM sleep instability, measured by CAP. It seems likely that also HRV modifications, and in particular an increased HF component (a marker of vagal tone) reflect the reduced activity of the autonomic branch of the arousal system.35 It has been reported that physiological fluctuations of the EEG arousal level influence cardiac autonomic activity in normal subjects during sleep.36
Arousal mechanisms comprise ascending networks projecting to the cerebral cortex, which stimulate cortical activation reflected as fast EEG activity, and descending networks project to the spinal cord, stimulating sensory-motor activation.37 Nevertheless, the role of arousal systems is not simply to induce and maintain wake and EEG activation. Arousal modulations act as a filter that gates the flux of information from the peripheral receptors to the cortex; anatomically, this filter is situated in the thalamocortical connections where the incoming signals are blocked or attenuated via synaptic inhibition.35 The major role in this mechanism is played by the thalamic reticular nucleus (TRN), which is a GABAergic nucleus placed between the thalamus and the cortex; it receives excitatory afferents from both cortical and thalamic neurons and sends inhibitory projections to nuclei of the dorsal thalamus. The TRN is involved in the regulation of bottom-up activities, including sensory gating and the transfer to the cortex of sleep spindles.38,39 This mechanism modulates the susceptibility of the cerebral cortex to all the activating stimuli. Seen in this view, the modulation of arousal levels during sleep have a complex role: that is, to allow prompt awakening from sleep, but at the same time, to allow processing of incoming inputs and to define a threshold of intensity or relevance, above which the stimulus may cause awakening and below which the stimulus is dumped and sleep may go on.
Fluctuations of arousal, measured by CAP, constitute therefore an essential mechanism in sleep regulation. CAP acts as a constitutive element of sleep architecture, since arousal fluctuations are essential to allow the physiological balance between slow wave and desynchronized sleep (NREM/REM). Moreover, arousal fluctuations are also essential to dump the effect of incoming disruptive stimuli and to protect sleep from external perturbations.
Our patients, overall, did not show abnormal scores on tests of psychological measures, with the exception of the MOCI. MOCI score is a measure of obsessive-compulsive behaviors. Several data indicate a strong association between obsessive-compulsive disorder (OCD) and drug abuse. Obsessive-compulsive personality disorder (OCPD) has been reported as the most common personality disorder in hypnotic-dependent adults40; the co-occurrence of substance abuse in OCD is higher than in other psychiatric disorders41; and, in a neuroimaging study, orbitofrontal connectivity was reduced in both OCD and drug abusers, suggesting that the two conditions share important cognitive and neurobiological substrates.35,41 Anxious hyper-arousability, a hallmark of Cluster C personality, and in particular of OCPD and avoidant features, is a sensitive risk marker for chronic insomnia, which was the initial reason for BDZ intake in all our patients.
Seen in this view, the effect of chronic BDZ abuse on sleep may be described as a severe modification of the thalamic gating of incoming stimuli and, consequently, of arousal dynamics. As result, abusers seem to have a reduced ability to elaborate afferent stimuli during sleep. Incoming stimuli in normal subjects can induce increased amount of arousal and CAP rate, without causing awakenings (as happens in the experimental model of noise-induced situational insomnia13,42). Conversely, in BDZ abusers, the chronic GABAergic stimulation makes the thalamic filter less adaptive: when exposed to stimuli, abusers either produce no response (and keep sleeping without arousal) or fully awaken. As a consequence, abusers have a marked reduction of arousals associated with increased number of nocturnal awakenings without relevant modifications of sleep macroarchitecture.
Study Limitations
The main limitation of the present study is the small number of patients enrolled. This is a consequence of the strict inclusion criteria: we decided to study sleep in a cohort of patients with pure BDZ abuse not associated with consumption of other drugs or substances. Moreover, the sleep study lasted 24 hours: this could have prevented evaluation of the circadian sleep-wake cycle and its variability.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lader M. Dependence and withdrawal: comparison of the benzodiazepines and selective serotonin re-uptake inhibitors. Addiction. 2012;107:909–10. doi: 10.1111/j.1360-0443.2011.03736.x. [DOI] [PubMed] [Google Scholar]
- 2.Lalive AL, Rudolph U, Luscher C, Tan KR. Is there a way to curb benzodiazepine addiction? Swiss Med Wkly. 2011;141:w13277. doi: 10.4414/smw.2011.13277. [DOI] [PubMed] [Google Scholar]
- 3.Terzano MG, Parrino L, Fioriti G, et al. Variations of cyclic alternating pattern rate and homeostasis of sleep organization: a controlled study on the effects of white noise and zolpidem. Pharmacol Biochem Behav. 1988;29:827–9. doi: 10.1016/0091-3057(88)90218-3. [DOI] [PubMed] [Google Scholar]
- 4.Konopka A, Pelka-Wysiecka J, Grzywacz A, Samochowiec J. Psychosocial characteristics of benzodiazepine addicts compared to not addicted benzodiazepine users. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:229–35. doi: 10.1016/j.pnpbp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Mendelson WB. Effects of flurazepam and zolpidem on the perception of sleep in insomniacs. Sleep. 1995;18:92–6. doi: 10.1093/sleep/18.2.92. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 7.Bastien CH, LeBlanc M, Carrier J, Morin CM. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26:313–7. doi: 10.1093/sleep/26.3.313. [DOI] [PubMed] [Google Scholar]
- 8.Ferrillo F, Balestra V, Carta F, Nuvoli G, Pintus C, Rosadini G. Comparison between the central effects of camazepam and temazepam. Computerized analysis of sleep recordings. Neuropsychobiology. 1984;11:72–6. doi: 10.1159/000118055. [DOI] [PubMed] [Google Scholar]
- 9.Tan KR, Brown M, Labouebe G, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–74. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–97. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida S, Okudaira N, Nishihara K, Iguchi Y, Tan X. Flunitrazepam effects on human sleep EEG spectra. II: Sigma and beta alterations during NREM sleep. Life Sci. 1996;59:PL117–20. doi: 10.1016/0024-3205(96)00367-0. [DOI] [PubMed] [Google Scholar]
- 12.Uchida S, Okudaira N, Nishihara K, Iguchi Y. Flunitrazepam effects on human sleep EEG spectra: Differences in NREM, REM and individual responses. Life Sci. 1996;58:PL199–205. doi: 10.1016/0024-3205(96)00026-4. [DOI] [PubMed] [Google Scholar]
- 13.Parrino L, Boselli M, Spaggiari MC, Smerieri A, Terzano MG. Multidrug comparison (lorazepam, triazolam, zolpidem, and zopiclone) in situational insomnia: polysomnographic analysis by means of the cyclic alternating pattern. Clin Neuropharmacol. 1997;20:253–63. doi: 10.1097/00002826-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.) [Google Scholar]
- 15.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–9. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez RA, Jacobson AF, de la Gandara J, Goldstein BJ, Steinbook RM. Drug response assessed by the Modified Maudsley Obsessive-Compulsive Inventory. Psychopharmacol Bull. 1989;25:215–8. [PubMed] [Google Scholar]
- 18.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 19.Curcio G, Tempesta D, Scarlata S, et al. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI) Neurol Sci. 2013;34:511–9. doi: 10.1007/s10072-012-1085-y. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Vignatelli L, Plazzi G, Barbato A, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol Sci. 2003;23:295–300. doi: 10.1007/s100720300004. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.McCall WV, Erwin CW, Edinger JD, Krystal AD, Marsh GR. Ambulatory polysomnography: technical aspects and normative values. J Clin Neurophysiol. 1992;9:68–77. [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 25.Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19:478–86. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 26.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 27.Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2002;3:187–99. doi: 10.1016/s1389-9457(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 28.Werth E, Achermann P, Dijk DJ, Borbely AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol. 1997;103:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 29.McCormick L, Nielsen T, Nicolas A, Ptito M, Montplaisir J. Topographical distribution of spindles and K-complexes in normal subjects. Sleep. 1997;20:939–41. doi: 10.1093/sleep/20.11.939. [DOI] [PubMed] [Google Scholar]
- 30.Jobert M, Poiseau E, Jahnig P, Schulz H, Kubicki S. Topographical analysis of sleep spindle activity. Neuropsychobiology. 1992;26:210–7. doi: 10.1159/000118923. [DOI] [PubMed] [Google Scholar]
- 31.Finelli LA, Achermann P, Borbely AA. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 32.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 33.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 34.Alegret M, Junque C, Valldeoriola F, Vendrell P, Marti MJ, Tolosa E. Obsessive-compulsive symptoms in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:394–6. doi: 10.1136/jnnp.70.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferini-Strambi L, Bianchi A, Zucconi M, Oldani A, Castronovo V, Smirne S. The impact of cyclic alternating pattern on heart rate variability during sleep in healthy young adults. Clin Neurophysiol. 2000;111:99–101. doi: 10.1016/s1388-2457(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 37.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–51. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 38.Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–15. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–73. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 40.Ruiter ME, Lichstein KL, Nau SD, Geyer JD. Personality disorder features and insomnia status amongst hypnotic-dependent adults. Sleep Med. 2012;13:1122–9. doi: 10.1016/j.sleep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blom RM, Koeter M, van den Brink W, de Graaf R, Ten Have M, Denys D. Cooccurrence of obsessive-compulsive disorder and substance use disorder in the general population. Addiction. 2011;106:2178–85. doi: 10.1111/j.1360-0443.2011.03559.x. [DOI] [PubMed] [Google Scholar]
- 42.Priest R, Terzano M, Parrino L, Boyer P. Efficacy of zolpidem in insomnia. Eur Psychiatry. 1997;12(Suppl 1):5–14. doi: 10.1016/s0924-9338(97)80015-6. [DOI] [PubMed] [Google Scholar]