Abstract
Study Objectives:
To determine the effects of dronabinol on quantitative electroencephalogram (EEG) markers of the sleep process, including power distribution and ultradian cycling in 15 patients with obstructive sleep apnea (OSA).
Methods:
EEG (C4-A1) relative power (% total) in the delta, theta, alpha, and sigma bands was quantified by fast Fourier transformation (FFT) over 28-second intervals. An activation ratio (AR = [alpha + sigma] / [delta + theta]) also was computed for each interval. To assess ultradian rhythms, the best-fitting cosine wave was determined for AR and each frequency band in each polysomnogram (PSG).
Results:
Fifteen subjects were included in the analysis. Dronabinol was associated with significantly increased theta power (p = 0.002). During the first half of the night, dronabinol decreased sigma power (p = 0.03) and AR (p = 0.03), and increased theta power (p = 0.0006).
At increasing dronabinol doses, ultradian rhythms accounted for a greater fraction of EEG power variance in the delta band (p = 0.04) and AR (p = 0.03). Females had higher amplitude ultradian rhythms than males (theta: p = 0.01; sigma: p = 0.01). Decreasing AHI was associated with increasing ultradian rhythm amplitudes (sigma: p < 0.001; AR: p = 0.02). At the end of treatment, lower relative power in the theta band (p = 0.02) and lower AHI (p = 0.05) correlated with a greater decrease in sleepiness from baseline.
Conclusions:
This exploratory study demonstrates that in individuals with OSA, dronabinol treatment may yield a shift in EEG power toward delta and theta frequencies and a strengthening of ultradian rhythms in the sleep EEG.
Citation:
Farabi SS; Prasad B; Quinn L; Carley DW. Impact of dronabinol on quantitative electroencephalogram (qEEG) measures of sleep in obstructive sleep apnea syndrome. J Clin Sleep Med 2014;10(1):49-56.
Keywords: Dronabinol, sleep, quantitative EEG, ultradian rhythms, OSA
Obstructive sleep apnea (OSA) is associated with increased sleep fragmentation, decreased sleep efficiency and reduced slow wave sleep.1,2 These changes in the sleep process undermine sleep quality and are hypothesized to be an important source of excessive daytime sleepiness, the dominant symptom of OSA. Still, despite decades of investigation, the mechanisms linking sleep, disordered breathing, daytime sleepiness, and cognitive dysfunction in OSA remain poorly defined. This knowledge gap has hindered efforts to develop effective OSA drug treatments. We previously demonstrated that oral dronabinol decreased apnea hypopnea index (AHI) in subjects with OSA.3 Interestingly, although overall sleep stage percentages did not change with treatment, daytime sleepiness decreased significantly. Here, we hypothesized that simple sleep stage distributions may be insensitive to important aspects of sleep architecture contributing to improved alertness with dronabinol treatment. In comparison to visually assigned sleep stages, quantitative changes in EEG power spectra may provide more sensitive markers of sleep depth, structure, and continuity. Further, cannabinoid drugs such as dronabinol have been shown to alter EEG power spectra both in animals and humans.4,5
Ultradian cycling between light and deep sleep and between NREM and REM sleep is another highly characteristic component of normal sleep that can be disrupted in subjects with OSA and is not directly assessed by sleep stage distributions, sleep bout durations, arousal indexes, sleep stage transition frequencies, and similar statistics. Such disruption may play a role in the excessive daytime sleepiness seen in people with OSA. Effects of cannabinoid drugs on ultradian rhythms during sleep have not been systematically studied.
BRIEF SUMMARY
Current Knowledge/Study Rationale: We previously showed that dronabinol decreased AHI in subjects with obstructive sleep apnea. Although sleep stage distribution was not grossly altered, dronabinol also decreased daytime sleepiness, suggesting that important aspects of the sleep process not well characterized by overnight sleep stage percentages may have been affected by dronabinol.
Study Impact: This exploratory analysis suggests that qEEG measures may be sensitive to restorative aspects of the sleep process improved by oral dronabinol in patients with OSA.
The aims of the present study were to quantify the effects of dronabinol on EEG power distributions and ultradian cycling of EEG power in subjects with OSA.
METHODS
Details of the underlying proof of concept trial have been published previously,3 and are summarized here.
Subjects
Seventeen adults (ages 21 to 64 years) with moderate to severe OSA (AHI ≥ 15) were enrolled. Individuals working night or rotating shifts, taking medications with known effects on sleep architecture, with clinically significant and uncontrolled or progressive medical comorbidity or any other primary sleep disorder were excluded. Individuals treated by positive airway pressure discontinued treatment ≥ 7 days prior to enrolling in the study. Two of the 17 subjects initially enrolled discontinued prior to completing the study and were not included in the analysis. Table 1 summarizes baseline characteristics for the 15 subjects included in the present analysis.
Table 1.
Summary of subject characteristics (mean ± SD)
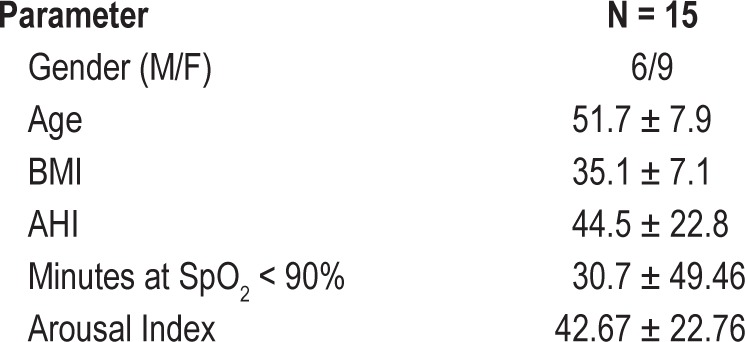
Dose Escalation Protocol
Following a baseline overnight polysomnogram (PSG), which also served as the final screening to document OSA severity, subjects were started on oral dronabinol, 2.5 mg/ day 30 min before bedtime, for 1 week. If this dose was well-tolerated, the dose was increased to 5 mg/day during week 2, and again as tolerated to 10 mg/day during week 3. Repeat PSGs were performed at the end of each treatment week. As outlined in Table 2, a total of 8 subjects were fully escalated and received 10 mg/day dronabinol during the third treatment week. One PSG was technically inadequate at this dose and was not included in the analysis. Five subjects maintained 5 mg/ day dronabinol during the final treatment week, and 2 subjects remained at a dose of 2.5 mg/day throughout. There were no statistically significant differences in baseline characteristics, reported in Table 1, between those subjects that escalated fully and those that did not tolerate escalation.
Table 2.
Summary of dose escalation protocol: values indicate the number of subjects (recordings) at each dose by treatment week, available for final analysis

Power Spectrum Analysis
Polysomnographic data were sampled (256/s) and stored to disk using Alice 5 hardware (Philips Respironics). Fast Fourier transformation (FFT) was applied to contiguous 4-s segments, and periodograms from 7 successive segments were averaged to obtain relative power (% total power) once every 28 s for the delta (≥ 0.5 to 3.5 Hz), theta (> 3.5 to 8 Hz), alpha (> 8 to 12 Hz), and sigma (> 12 to 16 Hz) frequency bands of the central (C4-A1) electroencephalogram (EEG). To determine the balance of high versus low frequency activity in the EEG power spectrum, an activation ratio (AR) was computed as the sum of alpha + sigma power divided by the sum of delta + theta power for each 28-s epoch. Determinations of band definitions and widths, electrode derivations and formation of AR were all fixed prior to data analysis based on common practice reported in the literature. The means and standard deviations of each band and the AR were computed over the entire night and separately for the first and second 4-h recording intervals. Within subjects ANOVA was conducted to determine the effects of treatment dose on relative power in each band as well as on the AR.
Ultradian Analysis
We also investigated the effect of dronabinol on the ultradian structure of EEG power. For this purpose, the best-fitting cosine wave was determined for each frequency band and the AR in each PSG recording. Because the period of any underlying biological ultradian rhythm could not be known in advance and may have differed between individuals and across dronabinol doses, for each PSG recording we successively fitted cosines with periods ranging from 60 to 150 min in 6-min increments by least-squares regression. In each case the cosine with the highest Pearson product-moment correlation coefficient was selected as the best overall fit. Regression analyses were performed to determine the effects of AHI, dose, weight, age, and gender on each parameter (amplitude, period, phase, and R2) of the best-fitting cosine wave for the AR and each EEG band.
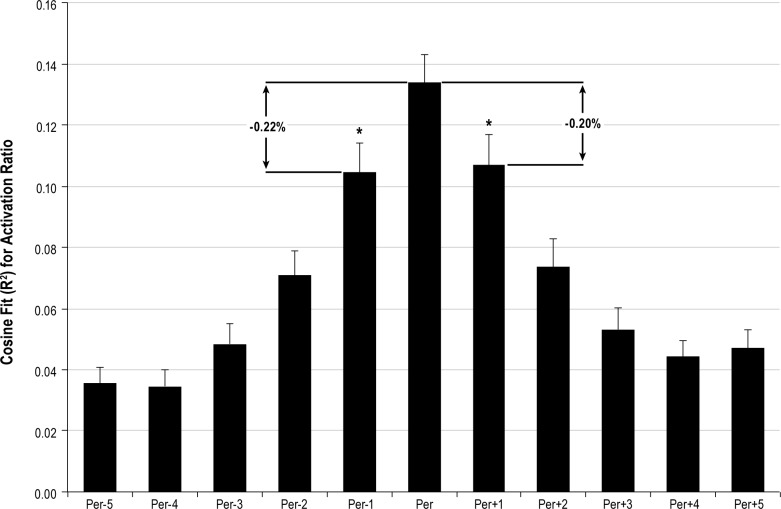
In order to determine the sensitivity of this approach to meaningfully estimate the underlying period of the biological rhythm, we performed a sensitivity analysis. As shown in Figure 1, with respect to the “best fit” cosine wave, shifting the period by 6 min resulted in an average decrease of R2 ≥ 20%, suggesting that the regression procedure realistically identified a meaningful best fit, even when the correlation coefficient was low. In fact, paired t-tests demonstrated statistically significant differences (p < 0.0005) in R2 between the best fit cosine and cosines with periods ± 6 minutes (Figure 1).
Figure 1. Sensitivity of cosine fit to activation ratio (AR) observations for all subjects and all dronabinol doses (N = 60 for best fit period).
Per represents the best fit (highest R2) period for each individual recording. On average, the Pearson correlation coefficient decreased by more than 20% when period was decreased (Per-1) or increased (Per+1) by just 6 minutes from the optimal fit. *p < 0.0005 with respect to best fit period.
RESULTS
Power Spectrum Analysis
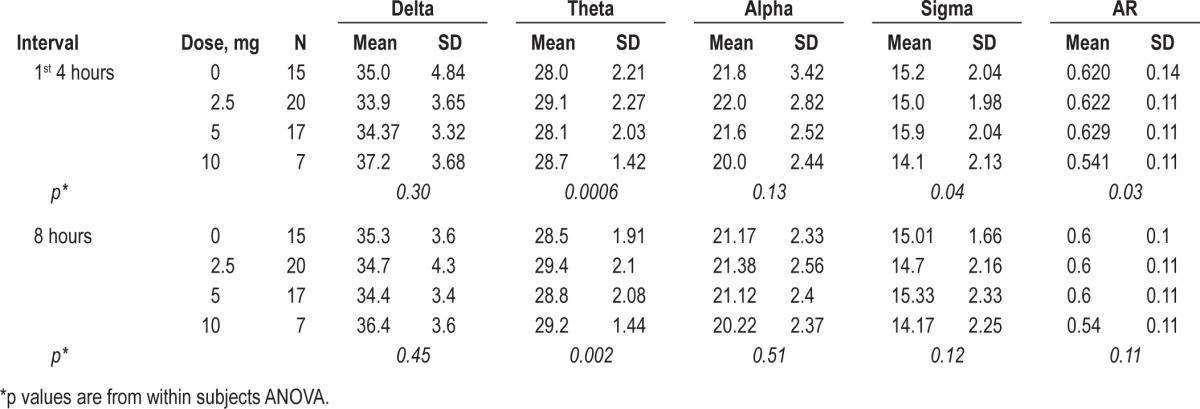
As depicted in Figure 2, within subjects ANOVA revealed significant differences among doses for AR (p = 0.03) in the first 4 h of the night. Figure 3 demonstrates that this effect was driven primarily by dose dependent changes in theta (p = 0.0006) and sigma (p = 0.03) power. The dose-dependent effect remained significant for the full 8-h period for relative theta power (p = 0.002). Similar trends were seen for the full 8-h period for AR and relative sigma power, but these did not reach statistical significance (Table 3).
Figure 2. Dose dependent changes in AR ([alpha + sigma] / [delta + theta]) during the first 4 hours of PSG recording (p = 0.03 by within subjects ANOVA).
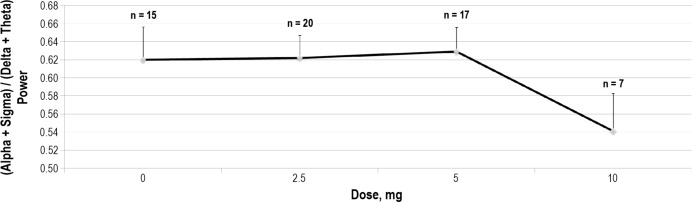
Figure 3. Dose dependent decrease in sigma band relative (% total) power (p = 0.03) and increase in theta band relative power (p = 0.0006).
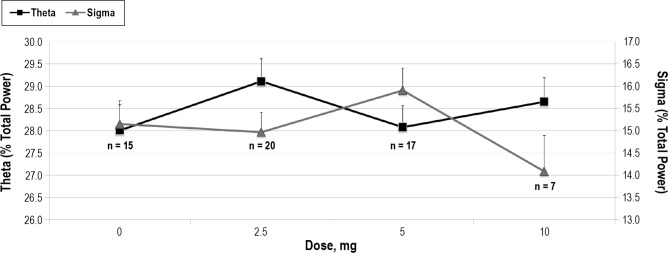
Table 3.
Means and standard deviations by dose for relative power in each frequency band and for the activation ratio (first four hours of the night)
Ultradian Analysis
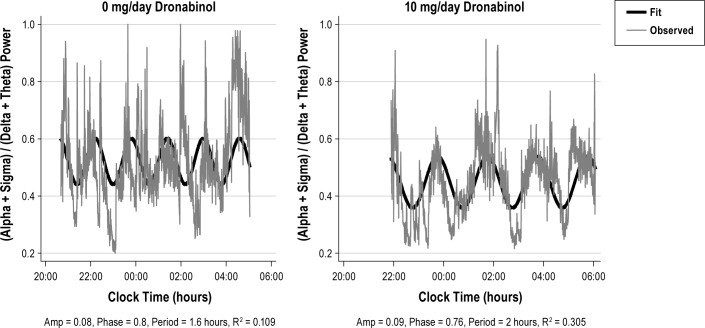
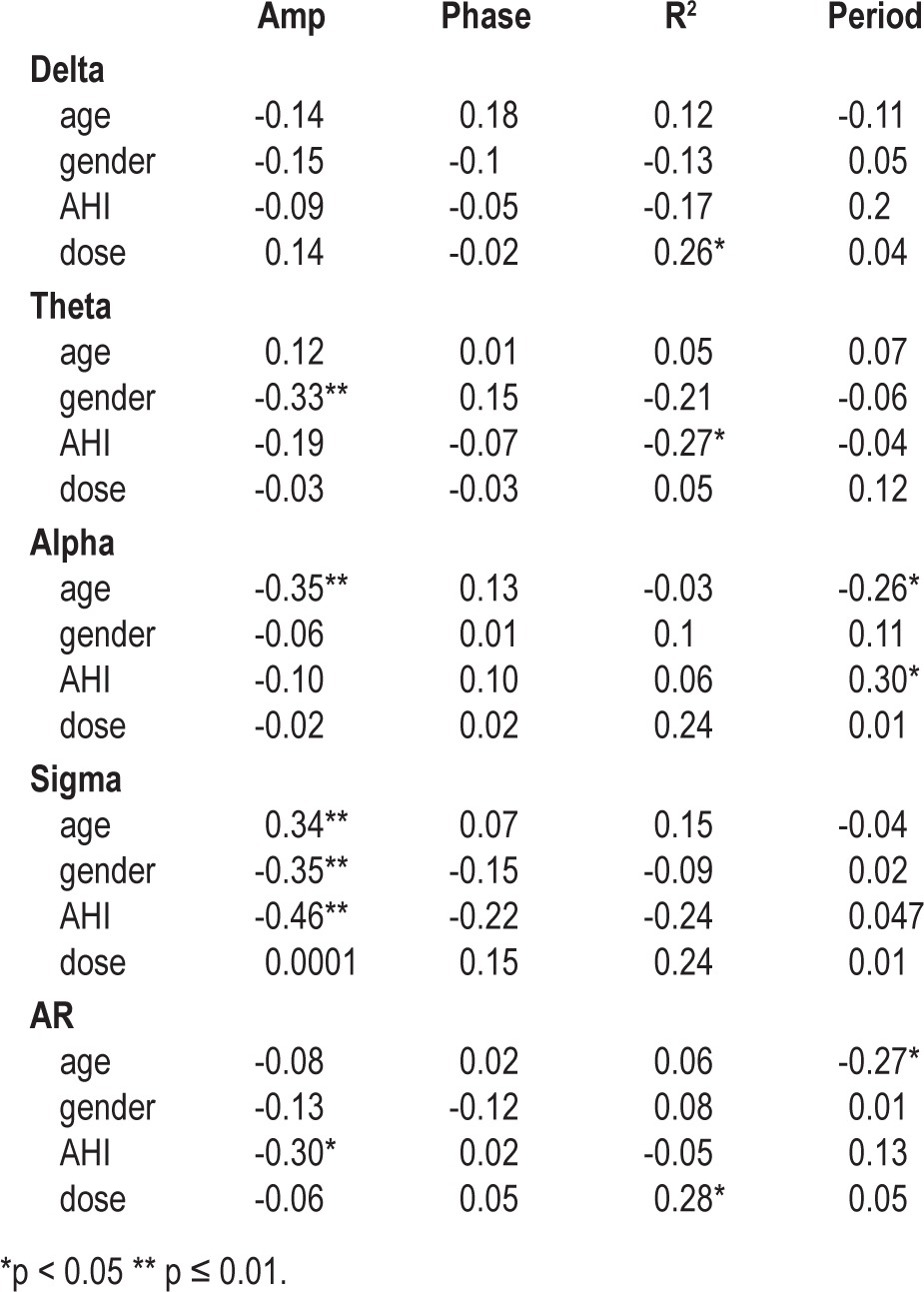
Figure 4 provides an illustration of ultradian fluctuations in AR for a single subject at dronabinol doses of 0 mg (baseline recording) and 10 mg per day. Characteristics for the parameters of ultradian rhythm for each frequency band and the AR are summarized in Table 4. Consistent with Figure 4, the group-wise data show that as the dronabinol dose increased, ultradian fluctuations accounted for an increasing fraction of the overall variance in the AR (p = 0.03). Table 5 shows correlation coefficients between best-fit ultradian cosine parameters (R2, phase, period, and amplitude) and potential explanatory variables including AHI, dose, weight, age, and gender. At increasing dronabinol doses, ultradian rhythms accounted for a higher fraction of total variance in the delta (p = 0.04), alpha (p = 0.06), and sigma (p = 0.07) bands and, as previously noted, the AR (p = 0.03). Females had higher amplitude ultradian rhythms than males in the theta (p = 0.01) and sigma (p = 0.01) bands. Decreasing AHI was associated with increasing amplitude of ultradian rhythms in the sigma frequency band (p < 0.001) and the AR (p = 0.02). In view of the exploratory nature of this analysis, p-values reported in Table 5 were not corrected for multiple comparisons.
Figure 4. Example recordings of AR from a single subject at baseline (left panel) and at a dronabinol dose of 10 mg/day (right panel).
Note that with dronabinol treatment, the ultradian oscillation accounts for a greater fraction of the total variance in AR.
Table 4.
Means and standard deviations by dose for each parameter of the best-fit cosine wave for AR
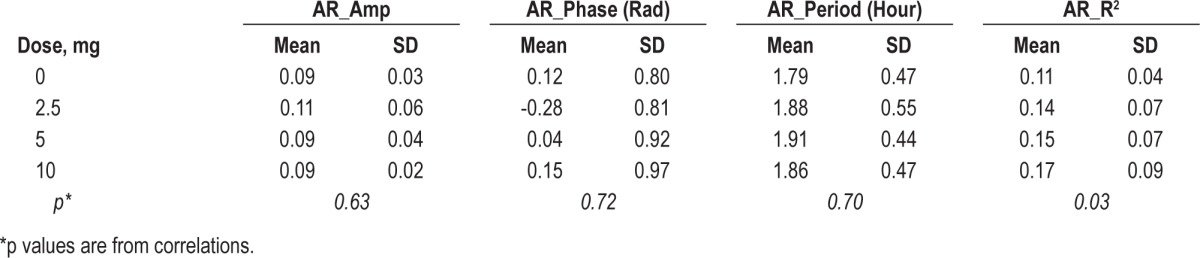
Table 5.
Pearson product-moment correlation matrix among best-fit cosine parameters and potential explanatory variables
Sleep Stage Analysis
Table 6 reports the effects of dronabinol on sleep architecture, based on sleep stage analysis using 30-s epochs. There were no statistically significant changes in the expression of stages 1, 2, slow wave (SWS; stage 3 + stage 4), or REM as a percentage of total sleep time. Similarly, the mean bout durations for stage 1, SWS, and REM remained unchanged, suggesting that sleep fragmentation was not improved by dronabinol. In fact, the mean bout duration for stage 2 was decreased slightly by dronabinol.
Table 6.
Bout durations and sleep stage percentages by dose
DISCUSSION
This exploratory study demonstrates effects of dronabinol on quantitative EEG measures of the sleep process in patients with obstructive sleep apnea and suggests possible mechanisms by which dronabinol reduces daytime sleepiness in this population. Specifically, increasing doses of dronabinol were associated with a shift in EEG power toward delta and theta frequencies and strengthening of ultradian rhythms in the sleep EEG. This suggests that oral dronabinol may have improved restorative aspects of the sleep process, contributing to the observed decrease in daytime sleepiness, despite the absence of changes in overall sleep stage percentages or sleep efficiency.3
Previous investigations have reported a wide variety of cannabinoid effects on sleep architecture in humans. The most consistent finding is a decrease of REM sleep following cannabinoid administration.6–8 Some studies report increased stage 2 sleep with decreased stage 4 sleep,9,10 while others report an increase in stage 4 sleep.11 These inconsistent findings may reflect differences in agents, formulations, routes of administration, concentrations, durations of exposure, ages, populations studied, and other factors. As previously reported and shown in Table 6, we observed no significant changes in overall sleep stage distribution associated with dronabinol administration at doses up to 10 mg/day in our subjects with OSA.3 Table 6 also illustrates that the mean bout durations for SWS and REM sleep were not increased by dronabinol, suggesting that sleep fragmentation, as measured by sleep stage analysis, was not improved. The present analysis revealed, however, that oral dronabinol shifted EEG power toward lower frequencies, reducing the AR (Figures 2, 3; Table 3). Although cannabinoids have been shown to affect sleep EEG power distributions in both animals4,12,13 and healthy humans,5,14 the previously reported effects on theta and sigma band power are inconsistent, and the doses employed were typically higher than in the present study. This is the first report to define the possible impact of oral dronabinol on sleep EEG power distribution in individuals with OSA.
The observed effects were more apparent during the first half of the night after oral administration of dronabinol. A statistically significant decrease in relative sigma power with a concomitant increase in relative theta power was evident during the first 4.5 hours after oral dronabinol dosing. These effects remained statistically significant for relative theta power but were less evident and not statistically significant for relative sigma power when considering the full 8-hour PSG recording period. This temporal pattern could be expected based on the pharmacokinetics of the drug, as the first-compartment plasma elimination half-life of oral dronabinol is 2 to 3 hours,15,16 and with daily dosing, plasma concentrations return to or near to baseline levels within 4 to 5 hours of drug administration.15,17 Although the decrease in relative sigma power and increase in relative theta power were small, the change in activation ratio (AR) at the end of treatment was much larger and may have more biological significance, as AR is a more integrative measure of the EEG power distribution.
Figure 2 suggests the possibility of a minimum effective dose of dronabinol somewhere between 5 mg/day and 10 mg/ day, for reducing AR. However, Figure 3 illustrates that even 2.5 mg/day of dronabinol exerts a significant effect on EEG power in the theta band. This suggests that the net effects of dronabinol on AR may reflect a more complex set of interactions, and not a simple threshold phenomenon. For example, Figure 3 also depicts that the dose effect of dronabinol on theta and sigma power is not monotonic. This may reflect the fact that dronabinol has multiple and potentially interacting sites of action in the central nervous system. Further, the effects of dose and time-on-treatment remain confounded due to the dose escalation study design. In addition, the fact that doses were escalated only in subjects who experienced no ongoing side effects may have introduced a selection bias, such that higher doses were made available only to those subjects who were “less sensitive” to the drug.
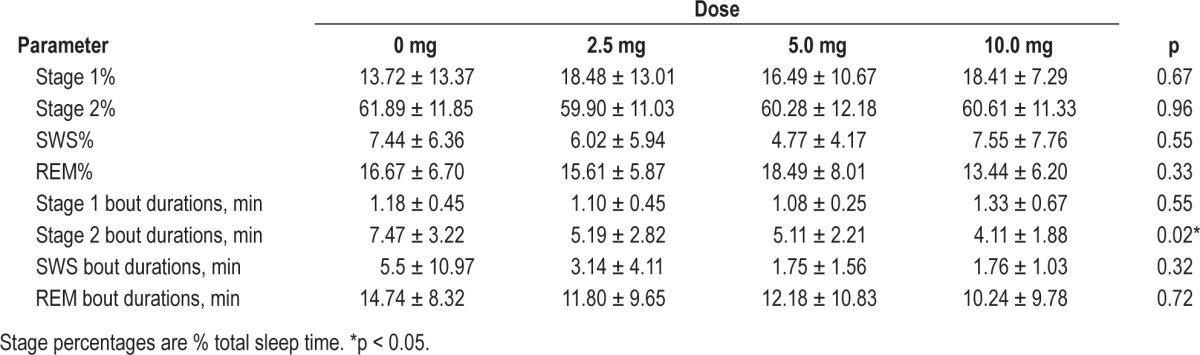
A similar redistribution of sleep EEG power toward lower frequencies has been reported to accompany institution of continuous positive airway pressure treatment for OSA.18 In contrast, hypnotic agents exhibit varying effects on sleep EEG. For example, zolpidem suppresses theta and slow-alpha activity (5-10 Hz) and enhances sigma activity19—effects opposite to those reported here for dronabinol. In contrast, gaboxadol enhances both delta and theta activity,19 similar to the dronabinol effects we observed. To examine whether the observed shift toward lower frequency EEG power may at least partially account for the decreased sleepiness reported by our subjects with OSA,3 we performed a correlation analysis between qEEG measures, AHI, and change in sleepiness from baseline to end of treatment, assessed using the 7-point Stanford Sleepiness Scale. Figure 5 illustrates that after 21 days of dronabinol treatment, relative theta power during sleep was positively associated with change in sleepiness from baseline to end of treatment (r = 0.61; p = 0.02). That is, dronabinol increased relative theta power, and those subjects with the highest theta power at the end of treatment showed the least improvement in sleepiness. Indeed, subjects in whom theta represented > 30% of the total EEG power tended to exhibit increased sleepiness at the end of treatment.
Figure 5. Relationship between relative theta power and change in Stanford Sleepiness Scale (ΔSSS: End of treatment − Baseline) (r = 0.61; p = 0.02).
Sedation is a well-recognized side of effect of cannabinoid use,20,21 and it is possible for this sedation to carry over to the following day. For example, Nicholson et al. reported that healthy subjects experience increased subjective sleepiness the day after evening oral cannabinoid administration.22 Thus, we anticipated that, acting independently, dronabinol might cause sedation/ sleepiness during the daytime with daily dosing. Conversely, we found that subjects reported decreased daytime sleepiness while taking oral dronabinol.3 Although not conclusive, these data are consistent with the possibility that the shift toward lower sleep EEG frequencies, and in particular the observed increase of relative theta power, reflects a direct CNS effect of dronabinol, and therefore a potential biomarker of its sedating properties.
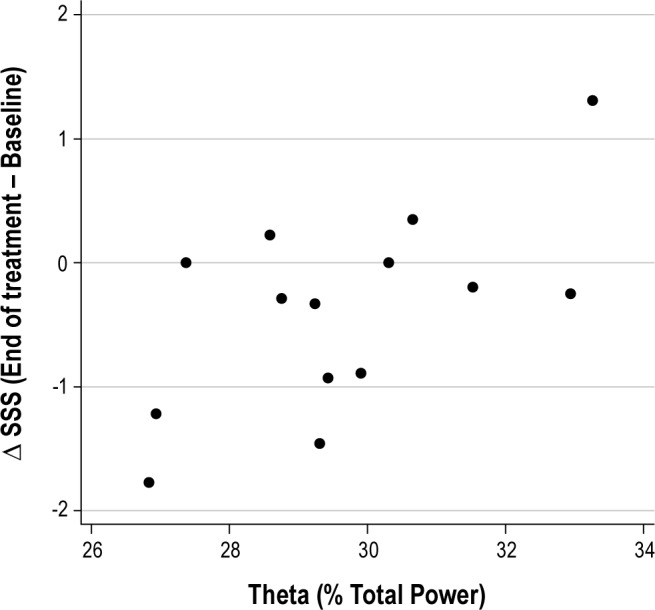
As depicted in Figure 6, AHI at the end of treatment also was significantly related to change in sleepiness (r = 0.54, p < 0.05). In this case, dronabinol treatment consistently decreased AHI,3 and the lower the AHI at the end of treatment, the more sleepiness improved with treatment. As we have previously reported,23,24 the most probable site of dronabinol action for reducing sleep related breathing disorder is cannabinoid receptors in the peripheral nodose ganglia of the vagus nerves. Thus, the balance between potential alerting and sedating effects of dronabinol may reflect a balance between peripheral and central activity of the drug.
Figure 6. Relationship between AHI and ΔSSS (r = 0.54, p < 0.05).
To date, there is virtually no literature regarding the effects of cannabinoids on ultradian rhythms in the sleep EEG. These exploratory results are, to the best of our knowledge, the first demonstration that oral dronabinol improves ultradian cycling in subjects with OSA. Further, this effect was dose-dependent, with ultradian rhythms accounting for a greater fraction of total EEG power at higher dronabinol doses. From the present data, we cannot determine whether this reflects a direct effect of the drug, an indirect effect of reducing AHI,3 or a combination of both effects. It is possible that dronabinol, by decreasing the frequency of sleep state transitions, led to an improvement in ultradian cycling. However this does not appear to have been the case given that dronabinol did not decrease sleep fragmentation assessed by arousal index3 or sleep bout durations (Table 6). It is also possible that the reduction in apneas and hypopneas due to oral dronabinol allowed ultradian cycling to become more evident by reducing an apnea-related masking of ultradian rhythms.
Decreasing AHI was also associated with increasing amplitude of ultradian rhythms (Table 5). This suggests that apneas are not just adding noise to the ultradian rhythms but that apneas are also decreasing the amplitude of ultradian cycling. The potential mechanisms linking disordered breathing events to ultradian rhythm amplitude cannot be determined from the present results. Dronabinol may impact ultradian rhythms by acting directly on sleep homeostatic pathways or indirectly by reducing disordered breathing events. Oral dronabinol may improve breathing stability during sleep by acting peripherally at the nodose ganglia to disinhibit upper airway dilator muscles.3,23 Conversely, by decreasing the rapidity or number of state transitions, dronabinol could have improved the stability of sleep and decreased the occurrence of apneas. Again, this does not seem to be a primary mechanism given the lack of evidence for reduced sleep fragmentation at the dronabinol doses tested. At present, there no published reports documenting improved ultradian sleep structure following institution of any form of treatment for OSA, but this should be further investigated in future clinical trials.
Women exhibited higher amplitude ultradian rhythms in all EEG frequency bands, and this achieved statistical significance for the theta and sigma bands (Table 5). Gender effects on sleep EEG have been previously reported, but the observations are mixed. Fukuda et al. reported that middle-aged and elderly females had larger amounts of spectral power in the delta band than males.25 In contrast, Latta et al. reported that older women have lower delta amplitude than men.26 In another study, adolescent females had lower delta power than males.27 These inconsistent results may arise from varying methods of data collection, participant age, or other factors. This exploratory analysis is the first to report a possible gender difference in the amplitude of ultradian rhythms of the sleep EEG in individuals with sleep apnea. This gender difference did not appear to be influenced by dronabinol administration, as dronabinol dose did not influence the amplitude of ultradian rhythms (Table 5).
We also observed that slower ultradian oscillations (longer periods) were associated with increased sleepiness at the end of the 3-week treatment period: delta (r = 0.74, p = 0.003); theta (r = 0.53, p = 0.05); alpha (r = 0.57, p = 0.04); sigma (r = 0.19, p = 0.51); average period (r = 0.70, p = 0.006). The impact of cannabinoids on ultradian periods of the sleep EEG has not been previously studied, and we found no effect of dronabinol on the period in any frequency band. It seems likely, therefore, that the correlation between ultradian period and sleepiness may reflect some combination of underlying biological factors, rather than a drug effect. Indeed, van Hilten et al. reported that prolonged periods in the ultradian rhythm of sleep EEG in patients with myotonic dystrophy correlated with increased daytime sleepiness.28
It should be noted that the findings of this study and their generalizability are limited by the small sample size. Additionally, subjects followed a dose escalation paradigm in which only 8 of the subjects reached the full dose of 10 mg/ day of dronabinol. Although we cannot rule out the possibility of an important time-on-treatment effect, we were able to identify significant dose effects despite the small sample size. Longer duration, larger clinical studies will be needed to fully delineate both the dose and the time-on-treatment effects of dronabinol in OSA.
In summary, this exploratory study demonstrates that in individuals with OSA, dronabinol treatment yields a shift in EEG power toward delta and theta frequencies and a strengthening of ultradian rhythms in the sleep EEG. In particular, ultradian rhythms became stronger with increasing doses of dronabinol and their amplitudes increased as AHI decreased. These effects suggest that oral dronabinol may have improved restorative aspects of the sleep process, contributing to the observed decrease in daytime sleepiness, despite the absence of changes in overall sleep stage percentages or sleep efficiency previously reported. Larger scale studies will be needed to confirm and elaborate these effects, and to dissect the potentially interacting influences of dose and time-on-treatment.
DISCLOSURE STATEMENT
This study was supported by a grant from Pier Pharmaceuticals and involved investigational use of dronabinol in subjects with obstructive sleep apnea syndrome. Dr. Carley is an inventor on patents and patent applications disclosing the use of dronabinol as a treatment for obstructive sleep apnea. All rights to these patents and applications have been assigned to the University of Illinois. Dr. Carley previously served as a consultant to and member of the board of directors of Pier Pharmaceuticals, which has been acquired by Cortex Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ng AK, Guan C. Impact of obstructive sleep apnea on sleep-wake stage ratio. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4660–3. doi: 10.1109/EMBC.2012.6347006. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi MT, Eiseman NA, Cash SS, Mietus J, Peng CK, Thomas RJ. Probabilistic sleep architecture models in patients with and without sleep apnea. J Sleep Res. 2012;21:330–41. doi: 10.1111/j.1365-2869.2011.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. Front Psychiatry. 2013;4:1. doi: 10.3389/fpsyt.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonamici M, Young GA, Khazan N. Effects of acute δ9-THC administration on EEG and EEG power spectra in the rat. Neuropharmacology. 1982;21:825–9. doi: 10.1016/0028-3908(82)90071-5. [DOI] [PubMed] [Google Scholar]
- 5.Skosnik PD, D'Souza DC, Steinmetz AB, et al. The effect of chronic cannabinoids on broadband EEG neural oscillations in humans. Neuropsychopharmacology. 2012;37:2184–93. doi: 10.1038/npp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975;17:458–66. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 7.Freemon FR. Effects of marihuana on sleeping states. JAMA. 1972;220:1364–5. [PubMed] [Google Scholar]
- 8.Gillin JC, Kotin J, Post R. Sleep during one week of administration of Δ-9 tetrahydrocanabinol to psychiatric patients. J Sleep Res. 1972;1:44. [Google Scholar]
- 9.Pranikoff K, Karacan I, Larson EA, Williams RL, Thornby JI, Hursch CJ. Effects of marijuana smoking on the sleep EEG. Preliminary studies. J Fla Med Assoc. 1973;60:28–31. [PubMed] [Google Scholar]
- 10.Freemon FR. The effect of chronically administered delta-9-tetrahydrocannabinol upon the polygraphically monitored sleep of normal volunteers. Drug Alcohol Depend. 1982;10:345–53. doi: 10.1016/0376-8716(82)90036-9. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19:782–94. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- 12.Willinsky MD, Loizzo A, Longo VG. EEG spectral analysis for the evaluation of the central effects of delta6-tetrahydrocannabinol in rabbits. Psychopharmacologia. 1975;41:123–6. doi: 10.1007/BF00421068. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama N, Kikura-Hanajiri R, Matsumoto N, Huang ZL, Goda Y, Urade Y. Effects of synthetic cannabinoids on electroencephalogram power spectra in rats. Forensic Sci Int. 2012;215:179–83. doi: 10.1016/j.forsciint.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Böcker KBE, Hunault CC, Gerritsen J, Kruidenier M, Mensinga TT, Kenemans JL. Cannabinoid modulations of resting state EEG θ power and working memory are correlated in humans. J Cogn Neurosci. 2010;22:1906–16. doi: 10.1162/jocn.2009.21355. [DOI] [PubMed] [Google Scholar]
- 15.Agurell S, Halldin M, Lindgren JE, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- 16.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Delta(9)-tetrahydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28:545–51. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Schwilke EW, Schwope DM, Karschner EL, et al. Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-carboxy-THC Plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–9. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Chen M, Bian J, He B. [Electroencephalogram spectral power analysis of obstructive sleep apnea syndrome patients before and during continuous positive airway pressure therapy] Zhonghua Jie He He Hu Xi Za Zhi. 2002;25:199–203. [PubMed] [Google Scholar]
- 19.Lundahl J, Deacon S, Maurice D, Staner L. EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol. 2012;26:1081–7. doi: 10.1177/0269881111424457. [DOI] [PubMed] [Google Scholar]
- 20.Noyes R, Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975;15:139–43. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Santos R, Crippa JA, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18:4966–79. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004;24:305–13. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 23.Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep. 2002;25:391–8. [PubMed] [Google Scholar]
- 24.Carley DW, Radulovacki M. Pharmacology of vagal afferent influences on disordered breathing during sleep. Respir Physiol Neurobiol. 2008;164:197–203. doi: 10.1016/j.resp.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda N, Honma H, Kohsaka M, et al. Gender difference of slow wave sleep in middle aged and elderly subjects. Psychiatry Clin Neurosci. 1999;53:151–3. doi: 10.1046/j.1440-1819.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- 26.Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep. 2005;28:1525–34. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 27.Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep. 2005;28:637–43. doi: 10.1093/sleep/28.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Hilte JJ, Kerkhof GA, van Dijk JG, Dunnewold R, Wintzen AR. Disruption of sleep-wake rhythmicity and daytime sleepiness in myotonic dystrophy. J Neurol Sci. 1993;114:68–75. doi: 10.1016/0022-510x(93)90051-y. [DOI] [PubMed] [Google Scholar]