Abstract
Study Objectives:
Spinal cord injury (SCI) is associated with 2-5 times greater prevalence of sleep disordered breathing (SDB) than the general population. The contribution of SCI on sleep and breathing at different levels of injury using two scoring methods has not been assessed. The objectives of this study were to characterize the sleep disturbances in the SCI population and the associated physiological abnormalities using quantitative polysomnography and to determine the contribution of SCI level on the SDB mechanism.
Methods:
We studied 26 consecutive patients with SCI (8 females; age 42.5 ± 15.5 years; BMI 25.9 ± 4.9 kg/m2; 15 cervical and 11 thoracic levels) by spirometry, a battery of questionnaires and by attended polysomnography with flow and pharyngeal pressure measurements. Inclusion criteria for SCI: chronic SCI (> 6 months post injury), level T6 and above and not on mechanical ventilation. Ventilation, end-tidal CO2 (PETCO2), variability in minute ventilation (VI-CV) and upper airway resistance (RUA) were monitored during wakefulness and NREM sleep in all subjects. Each subject completed brief history and exam, Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Berlin questionnaire (BQ) and fatigue severity scale (FSS). Sleep studies were scored twice, first using standard 2007 American Academy of Sleep Medicine (AASM) criteria and second using new 2012 recommended AASM criteria.
Results:
Mean PSQI was increased to 10.3 ± 3.7 in SCI patients and 92% had poor sleep quality. Mean ESS was increased 10.4 ± 4.4 in SCI patients and excessive daytime sleepiness (ESS ≥ 10) was present in 59% of the patients. Daytime fatigue (FSS > 20) was reported in 96% of SCI, while only 46% had high-risk score of SDB on BQ. Forced vital capacity (FVC) in SCI was reduced to 70.5% predicted in supine compared to 78.5% predicted in upright positions (p < 0.05). Likewise forced expiratory volume in first second (FEV1) was 64.9% predicted in supine compared to 74.7% predicted in upright positions (p < 0.05). Mean AHI in SCI patients was 29.3 ± 25.0 vs. 20.0 ± 22.8 events/h using the new and conventional AASM scoring criteria, respectively (p < 0.001). SCI patients had SDB (AHI > 5 events/h) in 77% of the cases using the new AASM scoring criteria compared to 65% using standard conventional criteria (p < 0.05). In cervical SCI, VI decreased from 7.2 ± 1.6 to 5.5 ± 1.3 L/min, whereas PETCO2 and VI-CV, increased during sleep compared to thoracic SCI.
Conclusion:
The majority of SCI survivors have symptomatic SDB and poor sleep that may be missed if not carefully assessed. Decreased VI and increased PETCO2 during sleep in patients with cervical SCI relative to thoracic SCI suggests that sleep related hypoventilation may contribute to the pathogenesis SDB in patients with chronic cervical SCI.
Citation:
Sankari A; Bascom A; Oomman S; Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 2014;10(1):65-72.
Keywords: Sleep, spinal cord injury, tetraplegia, central apnea
Spinal cord injury (SCI) affects a large number of adults worldwide, with an estimated incidence rate of 15 to 40 cases per million populations. The prevalence of SCI in the United States is estimated to exceed 250,000 people, with approximately 11,000 new cases of SCI each year.1 Most SCIs occur during the second or third decade of life, leading to a major impact on young individuals in the community.2 Respiratory complications secondary to ineffective cough, poor airway clearance, infections and respiratory failure are major causes of morbidity and mortality in patients with SCI, particularly at cervical levels.3 In a longitudinal study from Norway, respiratory failure and ineffective mucous clearance were the main complications after SCI and respiratory causes of death were increased twofold as expected for their age.4 While advances in acute care have resulted in improved survival for the first year after SCI, there is minimal change in survival when the injury becomes chronic after few years.4
BRIEF SUMMARY
Current Knowledge/Study Rationale: High prevalence of sleep disordered breathing (SDB) has been reported in many previous studies and usually classified under the rubric “obstructive sleep apnea.” Most studies utilized unattended, limited recordings lacking the precision to characterize the type of events reliably or the ability in identifying the mechanism.
Study Impact: Cervical SCI patients manifest as central SDB and hypoventilate during sleep more commonly than thoracic injuries, which require special consideration in diagnosis and treatment. Limited sleep study recordings (using type IV devices) may fail to accurately detect and classify SDB in patients with SCI. We propose that all SCI patients should undergo full polysomnography studies and be scored using new AASM criteria to better detect SDB.
Patients with SCI are also at increased risk of sleep disordered breathing (SDB), with a prevalence ranging from 27% to 62%.5–13 A longitudinal study in the first year after cervical SCI found that 60% of patients with cervical SCI developed SDB within 2 weeks of injury, peaked at 13 weeks and returned back to 60% after a one-year follow-up.13 It is of note that both SDB and chronic SCI are associated with increased adverse cardiovascular consequences.14,15 Hence it is plausible that SDB may contribute to increased cardiovascular mortality in SCI patients.16 Unfortunately, SDB in SCI patients remains underdiagnosed and undertreated. Furthermore, there are insufficient data on the type of SDB, mechanism of disease, and predictors for the increased prevalence and the relationship to level of injury.
The purposes of this study were (1) to characterize the sleep disturbances in the SCI population using standardized questionnaires and the associated physiological abnormalities using quantitative polysomnography with pharyngeal pressure catheter; (2) to determine the contribution of SCI level on the SDB mechanism (obstructive vs. central); and (3) to determine the magnitude of the fall in ventilation during sleep in chronic SCI patients. To this end, we measured pressure and ventilatory parameters during wake and sleep in addition to apneahypopnea index (AHI). Results of this study have previously been reported in the form of abstracts.17
METHODS
Subjects
The Human Investigation Committee of Wayne State University and the VA Medical Center approved the experimental protocol. An informed written consent was obtained and subjects had a screening polysomnography. We studied adults (> 18 years old) with chronic SCI if they fit the inclusion and exclusion criteria.
Inclusion criteria were as follows: non-ventilator dependent subjects with chronic SCI (> 6 months post-injury), American Spinal Injury Association grade A, B, C, or D, spanning the spectrum from cervical to thoracic levels (T6 and above).
Participants were excluded from the study for any of the following: (1) < 18 years of age; (2) pregnant or lactating females; (3) currently ventilator dependent or with tracheostomy tube in place; (4) history of cardiac disease including heart failure, peripheral vascular disease or stroke; (5) history of head trauma resulting in neurological symptoms or loss of consciousness; (6) advanced lung, liver or chronic kidney disease; (7) extreme obesity, defined for this protocol as BMI > 38 kg/m2 (to avoid the effect of morbid obesity on pulmonary mechanics and ventilatory control); or (8) other illness that would interfere with completion of the study in the investigators' judgment.
The participants were recruited from the local and regional spinal cord injury care centers including the Detroit VA Medical Center and the Rehabilitation Institute of Michigan. Additional mailings were sent local electronic database of patients with International Classification of Disease (ICD-9) codes corresponding to paraplegia or quadriplegia (344.0 or 344.1). Letters were sent to area physicians soliciting referrals of appropriate patients. In addition, SCI patients were contacted through publications on the internet by contacting SCI support groups.
Measurements
Every subject who agreed to enroll had brief history and exam and completed the following questionnaires: Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Berlin questionnaire and fatigue severity scale (FSS). All subjects had baseline spirometry (forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and FEV1/ FVC ratio) and respiratory muscle forces (maximal inspiratory and expiratory pressure MIP and MEP, respectively) in upright and supine position to assess positional effect on respiratory function. In addition to standard polysomnography including EEG and chin EMG, nasal airflow was measured by a pneumotachometer (Hans Rudolph, inc., Model 3700A, Shawnee, KS) connected to a tight-fitting nasal mask. Tidal volume (VT) was obtained by integrating the pneumotachograph flow signal. End-tidal carbon dioxide (PETCO2) was measured with a gas analyzer (VacuMed, Model 17515, Ventura, CA). Supraglottic pressure was measured with a pressure tipped catheter (Millar Instruments, Houston, TX), positioned in the hypopharynx.
Data Analysis
The best effort of the baseline spirometry and respiratory muscle forces were reported in each participant (in both absolute and % of predicted) on the same night of the sleep study, which were then summarized as mean ± SD. Baseline wake and sleep monitoring: sleep, ventilation and noninvasive blood pressure were monitored in each subject to assess the effect of SCI during wakefulness and sleep. Periods of 2 min from wakefulness and stable NREM sleep were measured to assess baseline ventilation (VI, VT, FB, TI, TE, PETCO2, O2Sat), and minute ventilation coefficient of variation (VI-CV), which were then summarized as mean ± SD. VI-CV was calculated for VI by the following formula: CV% = (SD/mean)*100.
Standard polysomnography (PSG) was performed according to AASM standards and respiratory events were scored by the 2007 standard (the only scoring criterion accepted by Medicare) criterion18 and by the 2012 AASM recommended scoring criterion.19 The standard scoring criterion for respiratory hypopnea was ≥ 30% reduction in nasal flow signal for ≥ 10 sec associated with ≥ 4% desaturation from pre-event baseline. In 2012, the recommended scoring criterion for respiratory events as hypopnea was modified to 30% reduction in nasal flow for ≥ 10 sec associated with ≥ 3% desaturation from pre-event baseline or arousal.19
Supraglottic pressure was used to differentiate the central from obstructive apneas by identifying the absence or presence of effort as described previously.20 Sleep disordered breathing was identified if the calculated AHI was ≥ 5 events/h of sleep. Central SDB was defined as AHI ≥ 5 events/h of sleep and central apnea index (CAI) > 5 events/h sleep. Cheyne-Stokes respiration (CSR) was defined as ≥ 3 consecutive cycles of cyclical crescendo and decrescendo change in breathing amplitude and at least one of the following: (1) ≥ 5 central apneas or hypopneas/h of sleep, (2) cyclical crescendo and decrescendo change in breathing lasting ≥ 10 consecutive minutes.19 The cycle duration ≥ 40 sec was not implemented in the definition of CSR, as SCI patients did not have heart failure. Periodic breathing (PB) was defined as cyclical increases in the rate and depth of breathing (hyperpnea) alternating with either a reduction by 50% (hypopnea) or complete cessation (apnea) of nasal airflow and respiratory effort lasting ≥ 10 seconds.21,22 We excluded obstructive patterns when defining periodic breathing. For hypopneas, we identified periodic breathing if there was decreased effort by supraglottic pressure and belts.
Statistical Analysis
All data were assessed for normal distribution. An unpaired t-test was used to compare the mean values of all demographic parameters. A paired t-test or adequate nonparametric test such as Wilcoxon signed rank test (when normal distribution failed) was used to compare the group values of each ventilatory data between wake and sleep (VI, VT, FB, TI, TE, PETCO2, O2Sat, and VI-CV). Paired t-test was used to compare the mean values of PSG data scored using conventional AASM scoring criteria and new recommended criteria. Chi-square analysis was used to compare the proportions of SDB in SCI (defined as AHI > 5) using conventional AASM scoring criteria and new recommended criteria. Analysis of variance (ANOVA) was used for subgroups comparisons of thoracic vs. cervical effect on the ventilatory data between wake and sleep. To assess the relationship between the self-reported questionnaires (ESS, PSQI, and FSS) and objectively measured tests (AHI, sleep efficiency, and arousal index) a Spearman correlation analysis was used. Multiple linear regression models were used to identify if ESS, PSQI, and FSS or NC can be independent predictors of AHI.
RESULTS
We studied 26 chronic SCI patients including 15 with cervical (C4-C7) and 11 with thoracic (T2-T6) levels who had similar demographics. Poor sleep quality and fatigue were noted, as evidenced by a high mean PSQI score 10.3 ± 3.7 in 26 SCI patients; 92% had poor sleep quality (PSQI score > 5 indicates poor nocturnal sleep). The mean score of the sleepiness scale (ESS) was 10.4 ± 4.4 in SCI patients and daytime sleepiness (ESS ≥ 10) was present in 59% of the patients. Daytime fatigue (FSS > 20) was reported in 96% those with SCI, while 46% had high-risk scores for OSA on Berlin questionnaire. Table 1 summarizes the demographics, sleep quality, and daytime symptoms in these participants using the PSQI, ESS, FSS, and Berlin questionnaires.
Table 1.
SCI Patient characteristics and sleep quality
Baseline spirometry on the day of the assessment revealed that forced vital capacity (FVC) in SCI was 70.5% predicted in supine compared to 78.5% predicted in upright positions (Figure 1; p < 0.05). Likewise, FEV1 was 64.9% predicted in supine compared to 74.7% predicted in upright positions (p < 0.05). Mean maximal inspiratory pressure (MIP) was 83.6% and 85.9% predicted in supine and upright positions, respectively (p = NS). Mean maximal expiratory pressure (MEP) was 42.2% and 47.5% predicted in supine and upright positions, respectively (p < 0.05). Table 2 summarizes the upright and supine pulmonary function and respiratory muscle strengths measures in both cervical and thoracic SCI categories. It is of note that the effect of body position on PFTs was different in thoracic SCI versus cervical SCI. FEV1/FVC and MEP decreased in the supine position in cervical SCI but not in thoracic SCI patients. Despite significant drops in FVC, FEV1, and FEV1/FVC from upright to supine positions (6.4 ± 12.1, 14.3 ± 13.2, and 5.7% ± 9.5%, respectively; p < 0.05), there was no correlation between positional FVC change and measured maximal inspiratory pressure in either position.
Figure 1. Mean values of (% predicted) forced vial capacity (FVC), FEV1, and FEV1/FVC of 26 SCI participants during upright (gray bars) and supine (white bars) positions.
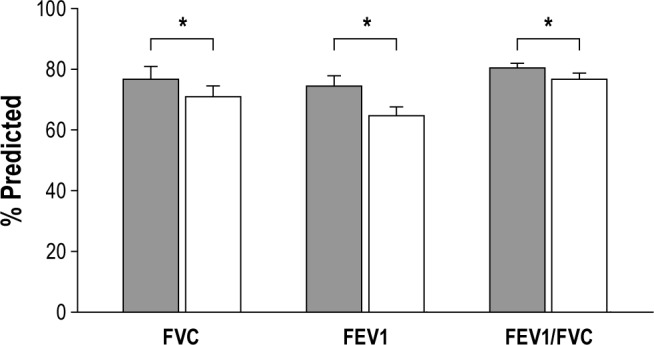
*p < 0.05.
Table 2.
Baseline pulmonary function and muscle forces
Table 3 summarizes the characteristics of sleep and polysomnography data. In-laboratory polysomnography studies (PSG) with pharyngeal catheter and pneumotachometer revealed that 77% of SCI patients had sleep disordered breathing (AHI > 5). Mean AHI in SCI patients was 29.3 ± 25.0 vs. 20.0 ± 22.8 events/h using the new and conventional AASM scoring criteria, respectively (p < 0.001). The majority of SCI patients (77%) had SDB (AHI > 5) using the new AASM scoring criteria compared to 65% using standard conventional criteria (p < 0.05). It is of note that 9 of 15 (60%) cervical SCI patients demonstrated evidence of central SDB manifesting as central apnea or periodic breathing. Four of the 9 (44%) had Cheyne-Stokes respiration pattern (CSR) as shown in Figure 2. In sub-analysis conducted after excluding individuals who were using opioids (6 cervical and 3 thoracic), it was found that 89% of cervical and 50% of thoracic SCI cases had SDB, with AHI ≥ 5 events/ hour. One-third of those with cervical SCI vs. 13% of those with thoracic SCI had central sleep apnea with CAI > 5/h (Table 4).
Table 3.
Characteristics of sleep and polysomnography data
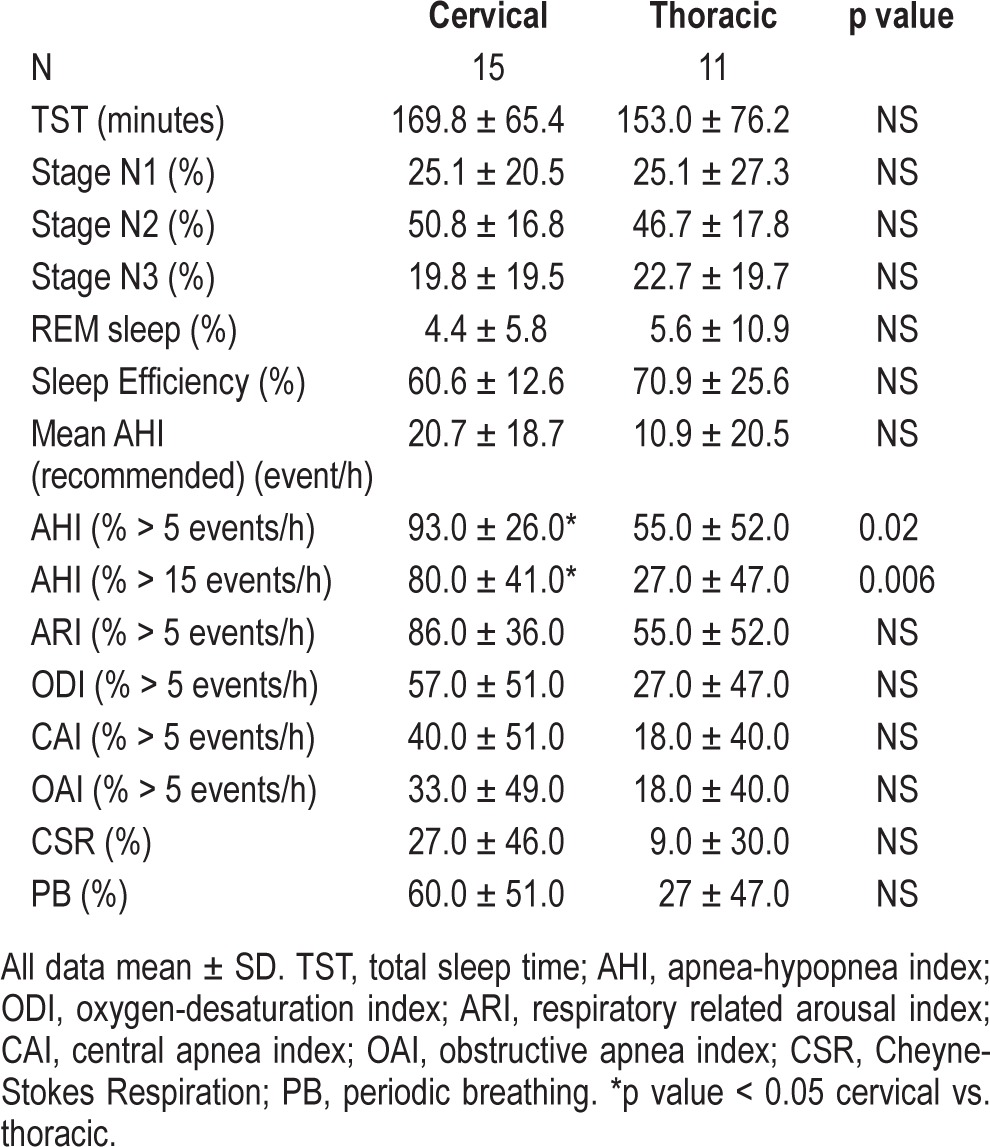
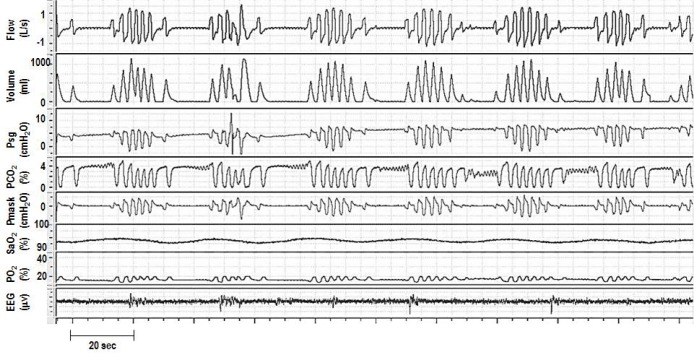
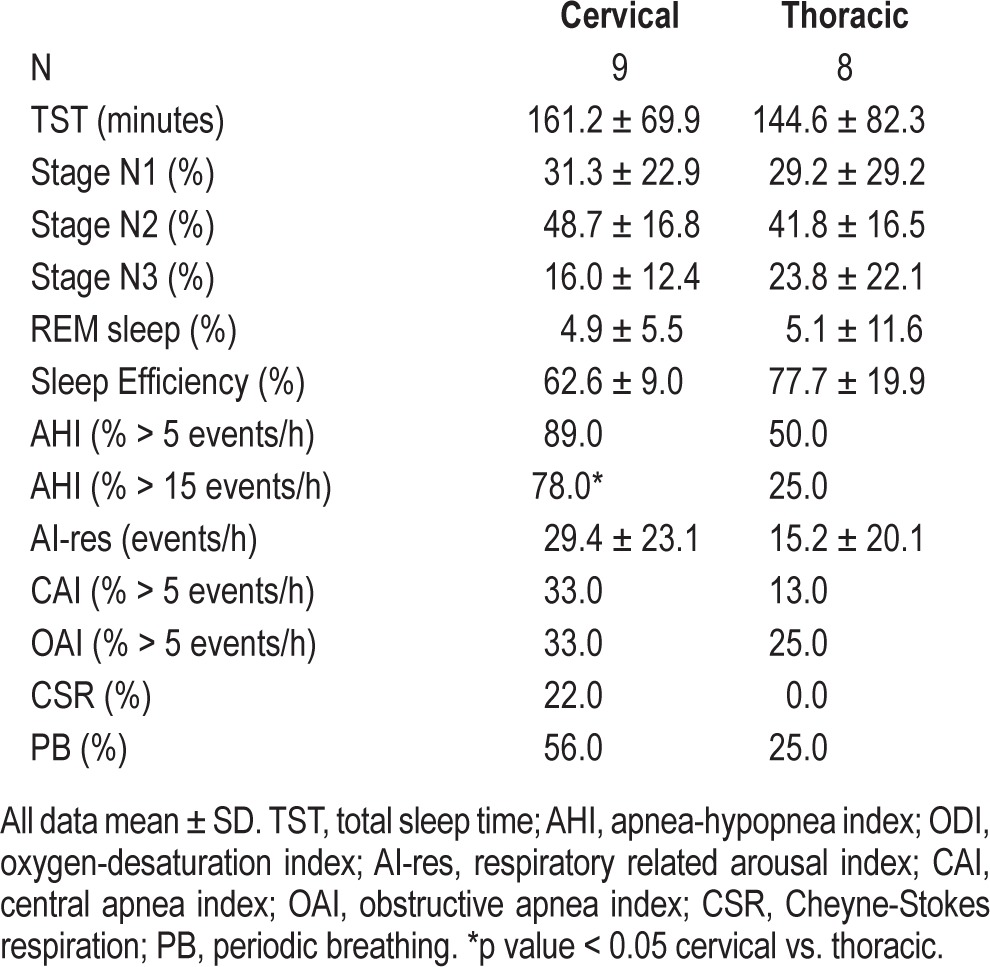
Figure 2. Polygraph from a cervical SCI patient (C6 ASIA C) during stage N2 sleep with Cheyne-Stokes respiration (crescendodecrescendo pattern) and breathing instability associated with fluctuations in end tidal CO2 (PETCO2) and O2 saturation.
Note the occurrence of arousals during hyperpneas (maximum tidal volume, which is a common feature in Cheyne-Stokes respiration). Psg, supraglottic pressure; Pmask, mask pressure.
Table 4.
Characteristics of sleep and polysomnography data after excluding patients using opioids
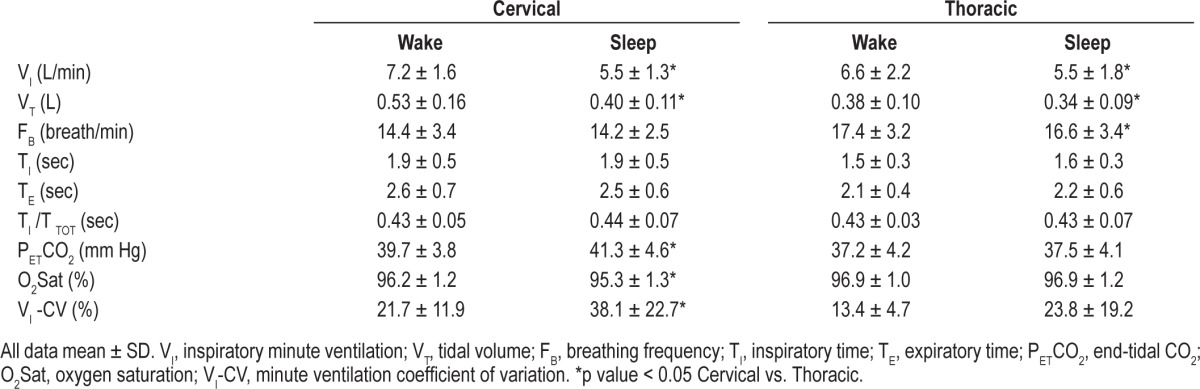
The sleep state was associated with significant changes in ventilation and gas exchange. Median minute ventilation decreased from 7.2 L/min during wakefulness to 5.5 L/min during stable NREM sleep (p < 0.05) as shown in Figure 3. In addition, patients with cervical SCI demonstrated substantial respiratory variability and hypoventilation after transitioning to sleep as evidenced by increased coefficient of variation of VI, lower SpO2 and higher PETCO2 relative to thoracic SCI patients (Figure 4). Baseline ventilation and oxygen saturation were similar between cervical and thoracic SCI patients during wakefulness (Table 5).
Figure 3. Group values of minute ventilation of 26 SCI participants during wake (gray bars) and sleep (white bars).

*p < 0.05.
Figure 4. Group values of PETCO2 (upper panel) and minute ventilation coefficient of variation (lower panel) of 26 SCI participants (15 cervical and 11 thoracic) during wake (gray bars) and sleep (white bars).
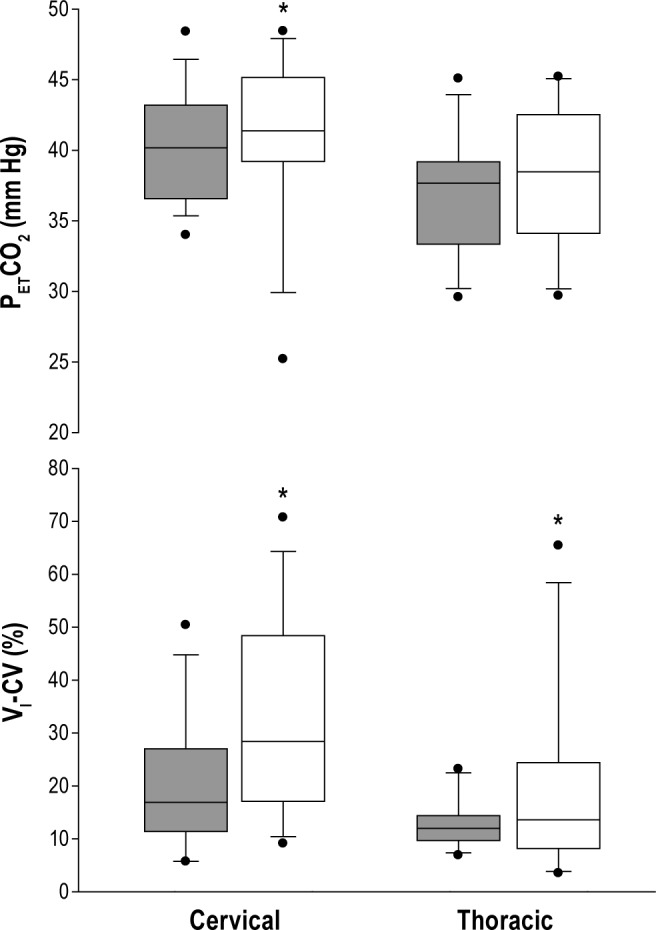
*p < 0.05.
Table 5.
Ventilatory parameters (N + 26)
Both AHI and respiratory related arousal index correlated positively with daytime sleepiness (ESS score) (r = 0.40 and 0.39, respectively; p < 0.05) but not with PSQI or FSS (p = NS). AHI also correlated with neck circumference and age (r = 0.52 and 0.43, respectively; p < 0.05), but not with BMI (p = NS). Using multiple linear regression model to predict AHI from the following correlating variables (ESS, NC, and age), ESS score was the only independent predictor of AHI (p < 0.05).
DISCUSSION
The major findings of this study were that (1) 77% of SCI survivors have symptomatic SDB and poor sleep quality better detected by new AASM scoring criteria; (2) the level of SCI (cervical versus thoracic) affected the prevalence of SDB and type of respiratory events (more central SDB noted in cervical SCI) and PFT findings; (3) decreased ventilation (VT) in the cervical level (VT dropped 21%) compared to thoracic (11% drop) between wakefulness and sleep indicates the importance of hypoventilation during sleep in the mechanism of SDB in chronic cervical SCI.
This is the first study to assess sleep disordered breathing and ventilation changes comparing two different levels of SCI (cervical vs. thoracic). Ventilation decreased significantly more in those with cervical SCI than those with thoracic SCI, as evidenced by the greater drop in tidal volumes and the rise in end-tidal CO2 seen in cervical versus thoracic SCI cases indicating the occurrence of alveolar hypoventilation during sleep.
We noted that sleep quality was very poor in the majority of SCI patients regardless of their level of injury or severity of sleep disordered breathing. SCI patients screening revealed excessive daytime fatigue and sleepiness. We considered several possibilities to explain poor sleep quality and daytime function in patients with SCI. Medication use may be implicated; however, less than one-third of those with SCI were receiving narcotic medications and there was no correlation between daytime symptoms and medication use. Melatonin deficiency has also been reported in patients with SCI; this may affect sleep quality and daytime function. In a small sample of SCI individuals it was found that melatonin level does not increase at night in cervical SCI (tetraplegia) compared to those with thoracic SCI and controls.23 However, melatonin deficiency does not explain poor sleep in thoracic SCI, as melatonin levels increased similar to the able body group. Accordingly, we attribute poor daytime function to increased indices of SDB rather than non-respiratory factors.
Our study revealed a higher prevalence of SDB (defined by AHI > 5; 93% of cervical and 55% of thoracic SCI) than what has been reported in the literature. However, screening tools were inadequate to identify patients with SDB. For example, the Berlin questionnaire suggested that only 50% of SCI patients (thoracic and cervical equally) were at high risk for sleep apnea. High prevalence of OSA has been reported in many previous studies—up to 60%, particularly in the tetraplegic population.4–13 However, SDB in SCI patients is usually classified under the rubric “obstructive sleep apnea.” Most studies utilized unattended, limited recordings lacking the precision to characterize the type of events reliably. In fact, the most significant finding was that more than 90% of cervical SCI patients demonstrated SDB, with the majority demonstrating central SDB not explained by daytime hypoventilation, cardiac dysfunction or use of narcotics. Subsequently, more than half of patients with cervical SCI had oxygen desaturation index above five per hour of sleep. Overall, the pattern of SDB differed in the two groups: SDB in thoracic SCI patients may represent “garden variety” sleep apnea/hypopnea syndrome, whereas SDB in cervical SCI patients appears to be centrally mediated, representing an adverse effect of the withdrawal of the wakefulness stimulus to breathe. In contrast to the thoracic SCI group, the majority of events (apnea and hypopnea) observed in the cervical SCI group were central and one in four had Cheyne-Stokes respiration pattern without evidence of heart failure. This unique observation may have significant implications regarding the diagnosis of SDB in patients with SCI.
The fundamental cause of central SDB is the loss of the wakefulness drive to breathe this could be a direct result of hypoventilation (hypercapnic type) or a consequence of post-hyperventilation hypocapnia (hypocapnic type) below a highly sensitive “apneic threshold.”24–27 Central apnea rarely occurs as isolated events but as cycles of apnea or hypopnea alternating with hyperpnea, a reflection of the negative feedback closed-loop cycle that characterize ventilatory control; this is often described using the engineering concept of “loop gain,”28,29 which is the net ventilatory change for a given perturbation, combining the response of the ventilatory system to changing PETCO2, (the controller), and the effectiveness of the lung/respiratory system in lowering PETCO2 in response to hyperventilation (the plant). Changes in either parameter would change the requisite hypocapnia to reach central apnea. In addition, sleep state instability, manifested by recurrent arousals, might trigger or exacerbate breathing instability due to rapid fluctuation in chemoreflex sensitivity or upper airway patency.30,31
Repetitive arousals may also contribute to the breathing instability by augmenting the ventilatory response to a given perturbation and periodic breathing.30 Sleep fragmentation might be very relevant to tetraplegic patients, who are already known to have increased occurrences of spasticity, pain and periodic leg movements.12 As a result of these alterations between sleep onset and arousal, repetitive perturbations in ventilatory control can occur throughout sleep. Subtle autonomic arousals, without EEG changes may also contribute to the genesis of unstable breathing and poor sleep quality.30
Our study corroborated previous studies documenting decreased VC, especially in the supine position, indicative of a restrictive defect in SCI patients. Interestingly, there was no significant difference between the two groups. Patients with cervical SCI only demonstrated reduced FEV1/FVC ratio in the supine relative to the upright position. The etiology of this finding is unclear but may be due to a combination of decreased lung volumes and the interruption of the sympathetic innervation of the lung by cervical spine injury, leaving the parasympathetic innervation unopposed.32 Our findings are consistent with the study by Dicpinigaitis et al.,32 which revealed a muscarinic receptor bronchial hyperresponsiveness in cervical SCI patients, as evidenced by the presence of methacholine hyperresponsiveness that is reversible with inhaled ipratropium bromide. The combination of decreased expiratory flow and decreased MEP may contribute to retained secretions and contribute to the development of episodic hypoxia and sleep fragmentation.
We found that FVC and FEV1 were decreased and became lower in the supine position compared to the upright position, which may play a role in predisposing SCI patients for sleep disordered breathing by more collapsible airway and hypoventilation under smaller lung volumes in supine position. Contrary to our findings, previous studies have reported that FVC and FEV1 in tetraplegia patients tended to be larger in supine than seated positions.33,34 Our study measured forced, not slow, vital capacity in upright and supine positions on the day of the sleep study. The changes in spirometric measurements in SCI patients were found previously to be dependent on injury level in complete injuries.34 Incomplete SCI had less effect on FVC in cervical levels, which may explain the higher FVC values in our study, as most injuries were incomplete and low cervical.
Methodological Considerations
Several considerations may influence the interpretation of the findings. First, our design does not allow us to address the prevalence of SDB in patients with SCI. However, we included all consecutive subjects who qualified for enrolment independent of the presence of sleep symptoms. Second, the use of invasive measurements and the small sample size may have affected sleep continuity and generalizability of the findings. Third, our findings do not allow us to isolate the independent effect of SCI on ventilation given the concomitant use of medications and the presence of comorbid conditions. Fourth, although the cervical and thoracic SCI body mass indexes were not statistically different, thoracic SCI individuals were more overweight, which could play a role in the increased obstructive SDB disorders compared to cervical group.
Clinical Implications
Our findings have significant implications regarding the diagnosis and treatment of sleep apnea in patients with SCI: (1) Positive screening questionnaires in our study may lack the discriminatory power to inform further diagnostic evaluation and may be superfluous given the high prevalence of SDB in this population; (2) Many studies in chronic SCI patients have used type III or IV devices, which have not been validated beyond the diagnosis of OSA in patients with a high index of suspicion. Conventional “qualitative” polysomnography may also fail to detect hypopneas if oxyhemoglobin desaturation is mild, or if arousal threshold is elevated, a potential consequence of narcotics administration. In fact, the diagnosis of SDB may be missed if type IV devices using ODI are used for the diagnosis. We propose that all SCI patients should undergo full polysomnography either as an in-lab (type I) or home polysomnography (type II) studies. Limited recordings may fail to accurately detect and classify SDB in patients with SCI; (3) Nearly all SCI patients have poor sleep quality (increased PSQI) and daytime fatigue (increased FSS), but these do not predict SDB severity. Excessive daytime sleepiness, measured by ESS score, is the only independent predictor of SDB severity; (4) Cervical SCI patients manifest as central SDB more commonly than thoracic injuries, which require special consideration in diagnosis and treatment; (5) Therapeutic methods that target hypoventilation during sleep in cervical SCI may play important role in the treatment of SDB.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was funded by the Department of Veterans Affairs (Award Number I01BX007080 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Nicole Nickert, Sukanya Pranathiageswaran, Lola Adekanmbi, and Anita D'Souza for technical assistance. Author contributions to the study: conception and design: Abdulghani Sankari and M. Safwan Badr; analysis and interpretation: Abdulghani Sankari, Amy Bascom, Sowmini Oomman, and M. Safwan Badr; drafting the manuscript for important intellectual content: Abdulghani Sankari and M. Safwan Badr.
REFERENCES
- 1.Jackson AB, Dijkers M, DeVivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil. 2004;85:1740–8. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62–8. doi: 10.1038/sc.1995.16. [DOI] [PubMed] [Google Scholar]
- 3.Lu K, Lee TC, Liang CL, Chen HJ. Delayed apnea in patients with mid- to lower cervical spinal cord injury. Spine. 2000;25:1332–8. doi: 10.1097/00007632-200006010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hagen EM, Eide GE, Rekand T, et al. A 50-year follow-up of the incidence of traumatic spinal cord injuries in Western Norway. Spinal Cord. 2010;48:313–8. doi: 10.1038/sc.2009.133. [DOI] [PubMed] [Google Scholar]
- 5.Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil. 2007;88:333–7. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Short DJ, Stradling JR, Williams SJ. Prevalence of sleep apnoea in patients over 40 years of age with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1992;55:1032–6. doi: 10.1136/jnnp.55.11.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEvoy RD, Mykytyn I, Sajkov D, et al. Sleep apnoea in patients with quadriplegia. Thorax. 1995;50:613–9. doi: 10.1136/thx.50.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biering-Sorensen F, Biering-Sorensen M, Hilden J. Reproducibility of Nordic Sleep Questionnaire in spinal cord injured. Paraplegia. 1994;32:780–6. doi: 10.1038/sc.1994.124. [DOI] [PubMed] [Google Scholar]
- 9.Burns SP, Little JW, Hussey JD, Lyman P, Lakshminarayanan S. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil. 2000;81:1334–9. doi: 10.1053/apmr.2000.9398. [DOI] [PubMed] [Google Scholar]
- 10.Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33:308–15. doi: 10.1038/sc.1995.70. [DOI] [PubMed] [Google Scholar]
- 11.Star AM, Osterman AL. Sleep apnea syndrome after spinal cord injury. Report of a case and literature review. Spine. 1988;13:116–7. doi: 10.1097/00007632-198801000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Stockhammer E, Tobon A, Michel F, et al. Characteristics of sleep apnea syndrome in tetraplegic patients. Spinal Cord. 2002:286–94. doi: 10.1038/sj.sc.3101301. [DOI] [PubMed] [Google Scholar]
- 13.Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil. 2005;86:1193–9. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 16.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 17.Oomman S, Sankri-Tarbichi AG, Bascom-Latin A, Badr MS. Sleep disorders in chronic spinal cord injury, a unique phenotype of sleep-disordered breathing. Am J Respir Crit Care Med. 2011:A3691–A3691. (American Thoracic Society International Conference Meetings Abstracts) [Google Scholar]
- 18.Iber C, Israel S, Chesson A, Jr, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 19.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasmnet.org. [Google Scholar]
- 20.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:369–77. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowat AM, Wardlaw JM, Dennis MS. Abnormal breathing patterns in stroke: relationship with location of acute stroke lesion and prior cerebrovascular disease. J Neurol Neurosurg Psychiatry. 2007;78:277–9. doi: 10.1136/jnnp.2006.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 23.Verheggen RJ, Jones H, Nyakayiru J, et al. Complete absence of evening melatonin increase in tetraplegics. FASEB J. 2012;26:3059–64. doi: 10.1096/fj.12-205401. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey JA, Skatrud JB. A sleep-induced apneic threshold and its consequences. Am Rev Respir Dis. 1986;133:1163–70. doi: 10.1164/arrd.1986.133.6.1163. [DOI] [PubMed] [Google Scholar]
- 25.Badr MS. Central sleep apnea. Primary Care. 2005;32:361. doi: 10.1016/j.pop.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol. 2000;89:192–9. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 27.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 28.Khoo MC. Using loop gain to assess ventilatory control in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1044–5. doi: 10.1164/ajrccm.163.5.ed1101c. [DOI] [PubMed] [Google Scholar]
- 29.Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Transactions on Biomedical Engineering. 2002;49:206–16. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- 30.Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol. 1996;80:1475–84. doi: 10.1152/jappl.1996.80.5.1475. [DOI] [PubMed] [Google Scholar]
- 31.Longobardo G, Evangelisti CJ, Cherniack NS. Effects of neural drives on breathing in the awake state in humans. Respir Physiol. 2002;129:317–33. doi: 10.1016/s0034-5687(01)00325-5. [DOI] [PubMed] [Google Scholar]
- 32.Dicpinigaitis PC, Spungen AM, Bauman WA, Absgarten A, Almenoff PL. Bronchial hyperresponsiveness after cervical spinal cord injury. Chest. 1994;105:1073–6. doi: 10.1378/chest.105.4.1073. [DOI] [PubMed] [Google Scholar]
- 33.Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90:405–11. doi: 10.1152/jappl.2001.90.2.405. [DOI] [PubMed] [Google Scholar]
- 34.Linn WS, Spungen AM, Gong H, Jr, Adkins RH, Bauman WA, Waters RL. Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord. 2001;39:263–8. doi: 10.1038/sj.sc.3101155. [DOI] [PubMed] [Google Scholar]