Abstract
Assortative mating is measured as a phenotypic or genotypic correlation between mates. Although biologists typically view assortative mating in terms of mate preference for similar partners, correlations between mates can also arise from phenotypic spatial structure arising from spatial isolation or habitat preferences. Here, we test whether diet-assortative mating within an ecologically variable population of threespine stickleback results from small-scale geographic isolation or microhabitat preference. We find evidence for assortative mating in the form of a positive correlation between mated pairs’ diets (measured using stable isotopes). Stable isotopes reveal diet differences between different nesting areas and among individuals using different nest habitat within a nesting area. This spatial segregation of diet types should generate some assortative mating, but is insufficient to explain the observed assortment strength. Significant male–female isotope correlations remain after controlling for spatial variables. We therefore conclude that sticklebacks’ diet-assortative mating arises from additional behavioral preference. More generally, our results illustrate the point that spatial segregation can only drive appreciable levels of phenotypic assortative mating when environment-phenotype correlations are parallel and strong in both sexes. Consequently, intraspecific assortative mating may typically entail mating preferences rather than just spatial cosegregation of phenotypes.
Keywords: Diet variation, Gasterosteus aculeatus, habitat choice, spatial cosegregation, stable isotopes
Assortative mating occurs when there is a phenotypic or genotypic correlation between mates (Wright 1921). Such correlations may drive deviations from Hardy–Weinberg equilibrium, inflate the genetic variance of a population (Lynch and Walsh 1998), and promote reproductive isolation between populations (Felsenstein 1981; Coyne and Orr 2004). The resulting deviations from Hardy–Weinberg equilibrium can also create statistical biases in quantitative genetics and association mapping studies (Gimelfarb 1986; Falconer and Mackay1994; Redden and Allison 2006). Assortative mating is therefore of interest when studying the genetic structure of populations or speciation.
Despite its importance for population genetics, the mechanistic basis of assortative mating is often unknown. Assortative mating may arise from multiple mechanisms including (1) mating preference for phenotypically similar individuals (Andersson 1994), (2) directional sexual selection on both sexes (Crespi 1989), or (3) spatial or temporal structure of phenotypes during the breeding season (Rice 1987). The first two of these mechanisms require the expression of mating preferences for specific phenotypic traits. In contrast, spatially generated assortative mating does not require mating preferences; instead, assortment arises incidentally because phenotypically similar individuals are more likely to encounter each other. Consequently, if biologists are to understand the role of mate preferences in driving assortative mating, they must also evaluate the alternative hypothesis that assortative mating is an artifact of spatial heterogeneity.
Two major types of spatial heterogeneity can generate trait correlations between mated pairs. First, dispersal barriers or isolation by distance may simultaneously promote phenotypic spatial divergence, and constrain individuals to mate locally. Phenotypic divergence between geographically distinct subpopulations may be due to adaptive genetic differences or phenotypic plasticity. As long as individuals are more likely to mate with spatially proximate individuals, this spatial phenotypic structure will lead to assortative mating even in the absence of behavioral preferences for mates with specific traits.
Second, divergent phenotypes may be spatially well mixed, but exhibit different behavioral microhabitat preferences, such that individuals are more likely to encounter similar individuals when mating, but be well mixed at all other times in their life history. Assortative mating is again a result of reduced encounter rate between individuals of different phenotypes, but at a fine spatial scale that might not be immediately obvious. Behavioral choices may indeed play an important role in assortative mating by habitat choice, but individuals choose their breeding location rather than their partner’s phenotype.
The role of discrete habitats in assortative mating has been recognized for some time. For example, ecological divergence can contribute to reproductive isolation when habitat preferences lead to spatial segregation of mating pairs, as occurs when divergent host races of phytophagous insects mate on their host plants (Caillaud and Via 2000; Berlocher and Feder 2002). More generally, assortative mating can occur whenever male and female phenotypes influence the choice of breeding site or time. If both male and female morphology are correlated with breeding location, a correlation between the morphologies of mated pairs may occur even in the absence of mate preferences. Such habitat-induced correlations are obvious when they involve mating in discrete habitat types, but may be overlooked when they arise through fine-scale microhabitat partitioning. Despite hundreds of empirical studies of within-population assortative mating in animals (Jiang, Bolnick, and Kirkpatrick, unpubl. ms.), very few, if any, distinguish between spatial structure and preference in generating assortment.
Here, we describe a test of whether spatial cosegregation underlies assortative mating in an ecologically variable but phenotypically unimodal (single-species) population of threespine stickleback (Gasterosteus aculeatus). Snowberg and Bolnick (2008) documented assortative mating with respect to diet (measured by stable isotopes) in one population of phenotypically variable stickleback. We confirm this previous finding in another stickleback population, and test whether this assortment arises from spatial structure between phenotypes and breeding location or microhabitat. Based on our results, we offer some general insights as to how readily spatial structure can generate intraspecific assortative mating.
STUDY SYSTEM
Threespine stickleback are a model system in ecology, evolution, and behavior. Many studies have focused on reproductive isolation between sympatric benthic/limnetic species pairs (Boughman 2001; Rafferty and Boughman 2006; Kozak and Boughman 2009; Kozak et al. 2011), divergent lake/stream (Raeymaekers et al. 2010; Eizaguirre et al. 2010), marine/freshwater (Bell 1982; McKinnon et al. 2004), and marine/marine ecotypes (Kitano et al. 2009; Kume et al. 2010). Recently, Snowberg and Bolnick (2008) also found evidence for assortative mating within populations, in which mated male/female pairs were more ecologically similar than expected by chance. Within any given lake population of stickleback, individuals differ in their propensity to eat benthic macroinvertebrates or pelagic zooplankton, as revealed by stomach content analyses, isotopic measures of diet, and direct observation of foraging microhabitat use (Araujo et al. 2008; Bolnick and Paull 2009; Matthews et al. 2010; Snowberg, Bolnick, and Hendrix, unpubl. data). Individual diet is also consistently associated with trophic morphology (Snowberg, Bolnick, and Hendrix, unpubl. data). Snowberg and Bolnick (2008) found a correlation between male and female isotopes from mated pairs, indicating assortative mating with respect to diet (which is reflected in isotope ratios, see below). However, it is unknown whether this assortative mating is a result of mate preferences for diet-derived cues, morphological traits correlated with diet, or because diet types are spatially segregated across a lake or among adjoining microhabitats. Specifically, the previously observed assortative mating could be a result of spatial isolation: across-lake gradients in stickleback diets, such that ecologically divergent individuals are less likely to encounter each other during the breeding season. Or, assortative mating could reflect microhabitat preferences: individuals with divergent diets may be spatially well mixed and frequently encounter each other, but select subtly different nest sites when mating. This microhabitat partitioning may reduce interbreeding among diet strategies even when they are in close spatial proximity.
There are a number of prior studies suggesting that ecological difference may be associated with nesting habitat. In a few lakes, stickleback exists as sympatric species pairs (benthic and limnetic species), which exhibit strong ecological, morphological, and genetic differences that are sustained by assortative mating (Schluter and McPhail 1992). Benthic and limnetic stickleback differ in their nest location and characteristics, with limnetic males nesting in open, shallower areas and benthic males nesting in dense vegetation at deeper depths within the littoral zone (McPhail 1994). Females also differ in their habitat use, making encounters with males of their own species more likely for benthic and limnetic stickleback (Vamosi and Schluter 1999). Benthic-like and limnetic-like populations from allopatric solitary lakes also differ in their nest location in a manner similar to the species pairs (Vines and Schluter 2006). Given this background information, we posit that ecologically divergent individuals within a solitary population may tend to select different microhabitats for mating, leading to assortative mating. Consistent with this possibility of assortment via spatial structure, we have found some morphological, isotopic, and dietary differences among sites within a given lake, although most (~90%) trait variance occurs within rather than among collection sites within a lake (Snowberg, Bolnick, and Paull, unpubl. data). Even within a site, individuals caught in adjoining traps (meters apart) tend to exhibit significantly different isotopes, suggesting micro-scale habitat choice or assortative shoaling that could generate spatially driven assortative mating. Finally, experimental transplants show that individuals can actively choose habitats based on their phenotype, facilitating adaptive divergence (Bolnick et al. 2009).
The primary goals of this study are to determine if (1) there is assortative mating within a single population of stickleback, as indicated by a correlation between the diets of mated individuals, (2) there is spatial isolation between individuals with different isotopes (diets), (3) there is microhabitat segregation by diet, and (4) geographic and microhabitat segregation is sufficient to explain the observed assortative mating. If not, we must invoke additional sources of nonrandom mating (most likely mate preferences) to explain assortative mating within populations. In addition, we test for divergence in male trophic morphology across microhabitats, which would suggest that males with different phenotypes (including diet) select different nest microhabitats (matching habitat choice; Edelaar et al. 2008), instead of nest location dictating individuals’ diets. We then generalize our results beyond stickleback by identifying the conditions required for spatial segregation of phenotypes to yield empirically reasonable levels of assortative mating within populations.
Methods
USING STABLE ISOTOPES TO STUDY ASSORTATIVE MATING
Stable isotopes are commonly used to study diet variation (Tieszen et al. 1983; Newsome et al. 2007). We take advantage of three facts to study diet-assortative mating in stickleback: (1) stable isotopes reveal individuals’ past diets (see below), (2) females’ isotopes are passed down to their eggs, and (3) females deposit their eggs in males’ nests, which the male then guards until after hatching. Thus, if males and females mate assortatively with respect to diet, we should see a corresponding correlation between male and egg isotopes. Because males guard nests, we are able to record the mating location and habitat, and study correlations between diet and habitat without directly observing mating, which is not practical in stickleback.
Carbon and nitrogen isotope ratios provide complementary information on fishes’ diets. Limnetic and benthic primary producers (phytoplankton and periphyton, respectively) fix C12 and C13 isotopes in different ratios (France 1995; Post 2002). These ratios are preserved in consumers’ tissues with only slight fractionation. As a result, the ratio of these isotopes (δ13C) provides a measure of how much benthic or limnetic carbon an individual uses (Matthews et al. 2010). Nitrogen provides a complementary measure of trophic position, because the ratio of N14 to N15 (δ15N) displays a stepwise enrichment at each higher trophic level (Hobson and Clark 1992a). Stable isotopes turn over slowly in tissues, integrating diet over the course of weeks to months (Hobson and Clark 1992b; Hobson 1993). Consequently, stable isotope differences among individuals may be used to infer sustained among-individual diet differences.
Female’s isotopes ratios are correlated with the isotopes of their eggs (Gray 2001; Snowberg and Bolnick 2008). We confirmed this relationship for the studied population by capturing 33 gravid females, and analyzing the stable isotopes of their liver tissues and mature eggs (see below for isotope methods). The positive correlation between female and egg isotopes (δ13C r = 0.976; δ15N r = 0.799) allows us to use eggs’ isotopes as a proxy for female diet.
SAMPLE COLLECTION
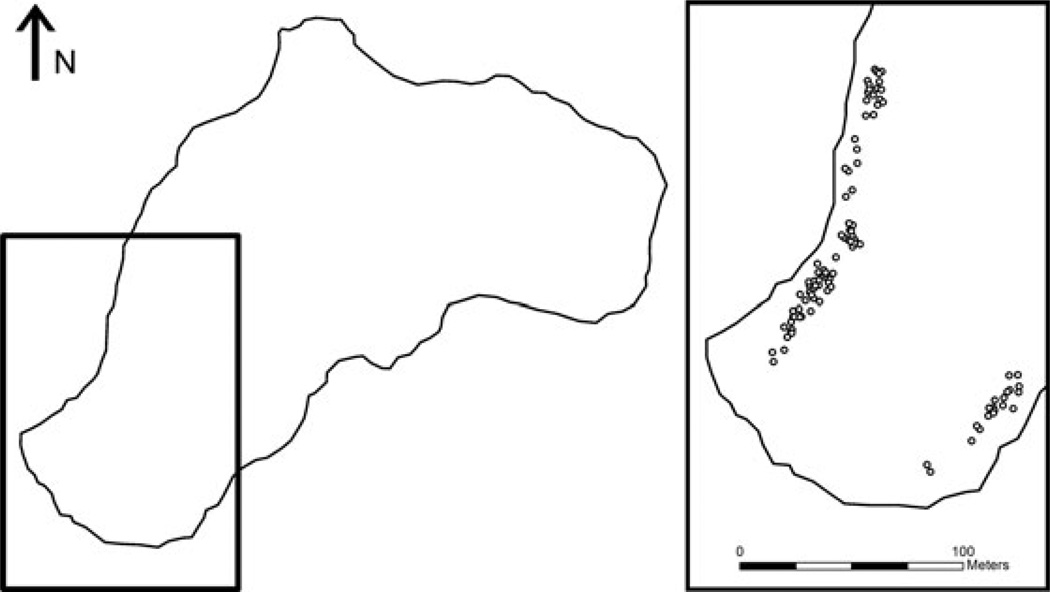
This study took place in Burnt-Out Lake, British Columbia (Fig. 1). Burnt-Out Lake is a small (approximately 8 ha) lake in the Browns Bay watershed on Vancouver Island. No other lakes in the watershed contain stickleback, minimizing the potential for immigration to inflate our estimates of assortment. Our previous study of diet assortment in stickleback (Snowberg and Bolnick 2008) took place in a lake from a separate watershed, 5 kilometers away from Burnt-Out Lake. While small lakes are less subject to disruptive selection on diet (Bolnick and Lau 2008) and therefore may show weaker assortment, the choice of a smaller lake allowed us to survey a large proportion of the lake for nesting activity.
Figure 1.
Stickleback nests in Burnt-Out Lake were located in two discrete nesting colonies on opposite shores. The area separating these colonies was searched for nests but consisted of marshy habitat and woody debris where stickleback did not appear to be nesting.
Over a one-week period in late June 2008, we collected males and their eggs from 102 geo-referenced nests (see below for information about spatial distribution). Nest guarding males and the eggs from their nest were collected by snorkelers with dip nets. Snorkelers searched along shorelines in all suitable nesting habitat in the lake and collected all observed nests containing eggs. Nest guarding males in this lake have bright red nuptial coloration that helped in locating breeding males. Males also display stereotyped nest care activities (e.g. fanning) that facilitated finding nests containing eggs. Nests containing larval stickleback were not collected. While our collection of nests may not have been complete, it was not biased toward collecting in a particular habitat or depth range. Nests were collected between 0.2–2.1 m in depth. Visibility in the lake was high and no nests were observed deeper than our collection range. Gravid females were collected opportunistically using minnow traps and dip nets from the nesting areas.
We used a Trimble GeoXT GPS accurate to approximately 1 m horizontal distance to record the nest location in UTM. Before collecting a nest, we measured nest depth to within 10 cm, and photographed each nest to categorize vegetation cover (among dense vegetation = 1, in open area = 0) or large logs (directly under a submerged log = 1, not sheltered = 0). These microhabitat variables were chosen based on previous studies of association between stickleback nesting habitat and diet/morphology (McPhail 1994; Vines and Schluter 2006) and were supported as being biologically relevant to nesting stickleback by observations in the field: males nesting among dense vegetation or sheltered by a large log were less likely to swim away from their nest when approached by a snorkeler (L. K. Snowberg, pers. obs.) Nest substrate was uniform throughout the study area and was therefore not included in analysis. We cannot rule out the possibility that other nest characteristics differed because between males. We did not include nest structure as a covariate it represents an extended phenotype of the male, rather than a habitat feature.
We collected liver samples for stable isotope analysis from all nesting males. For each male we randomly selected 1–2 eggs from a single clutch per nest (eggs from a clutch tend to be adhered into a clump and are at the same developmental stage) for isotope analysis. We also collected liver samples and eggs from gravid females. Samples were oven dried at 50°C for 72 hours. Approximately 125 µg of each sample was packed in tin capsules and shipped to the UC Davis Stable Isotope Facility for analysis. The facility analyzes δ13C and δ15N isotopes using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK).
Individuals can differ in isotope ratios for either of two reasons: (1) if their diets are the same but their prey have different isotope ratios; or (2) if they have different diets. Consequently, across-lake gradients in environmental isotope ratios can lead to across-lake gradients in stickleback isotopes, even if stickleback diets are constant. If individuals mate locally, these across-lake isotope gradients could create a misleading male–female correlation in isotopes, which is not matched by male–female correlations in diet. Using basal primary consumers of benthic and limnetic producers (snails and mussels, respectively), we can account for the isotopic variation among alternate resources to calculate an index of percent benthic carbon and relative trophic position for each individual fish (Post 2002). We collected snails (N = 21) and mussels (N = 27) with GPS location data from throughout the nesting areas. We used the equations in Post (2002) to convert carbon stable isotope ratios into percent benthic carbon. This procedure simply calculates where the fish carbon isotopes are relative to end-points defined by the carbon isotopes of snails (primary consumer assumed to represent 100% benthic carbon) and mussels (primary consumer assumed to represent 0% benthic carbon). The percent benthic carbon value for an individual is used along with the nitrogen stable isotope ratio to calculate relative trophic position (Post 2002). Relative trophic position is calculated as the extent to which δ15N is enriched above the value expected given baseline benthic and limnetic δ15N and the proportional contribution of those sources to the fish’s diet (the estimated percent benthic carbon).
A limitation of the Post (2002) method is that stickleback sometimes exhibit δ13C values outside the range of mussel and snail δ13C (Snowberg and Bolnick, unpubl. data). As a result, percent benthic carbon may be negative or exceed 100%, leading to nonsensical estimates of relative trophic position. Here, for the few individuals whose carbon transgressed the baseline endpoints (16/102 males), we calculated relative trophic position by setting percent benthic carbon to the closest endpoint (0 or 100%). In practice, this has a negligible effect on the calculated trophic position because the nitrogen stable isotopes of mussels and snails in this population are nearly identical. Thus the vast majority of variation in trophic position is a direct reflection on the nitrogen stable isotopes of each fish (r = 0.966 between δ15N and trophic position) rather than any correlated effect arising from their carbon isotopes.
The strength of assortative mating by diet is calculated by first obtaining the correlation between nesting male and egg isotopes (rm*e), and the correlation between gravid female and egg isotopes (rf*e). The strength of assortative mating (r̂m*f) is the estimated correlation between male and female isotopes, calculated from the preceding two correlations as r̂m*f = rm*e/rf*e (Snowberg and Bolnick 2008). The standard error of rm*f is calculated using the relationship
We analyzed correlations between male and egg isotopes (rm*e) separately for trophic position and percent benthic carbon. Unlike in our previous study of assortative mating by diet (Snowberg and Bolnick 2008), carbon and nitrogen isotopes were not correlated in this population. Consequently, rather than testing for a male/egg correlation using an isotopic principal component axis (as we did previously), we separately tested each isotope measure for correlations between male liver tissue isotopes and the isotopes of eggs from the males nest. These correlations were calculated for both the whole lake and each shore individually. Because, we lacked precise location data for gravid females (to assign local mussel or snail baselines), we calculated female-egg correlations using raw isotope values rather than percent benthic carbon or trophic position. The conversion to percent benthic carbon and trophic position are linear transformations of raw isotope ratios, so the lack of baseline data should not affect the female-egg correlation. All statistical analyses were carried out in R (R Development Core Team 2011).
UNDERSTANDING THE ROLE OF SPATIAL COSEGREGATION IN ASSORTATIVE MATING
To determine whether spatial structure explains diet-assortative mating, we tested whether shared male-habitat and female-habitat correlations explain the observed male–female isotope correlation. Finally, we tested whether assortment occurs that cannot be explained by the measured habitat variables, by obtaining residuals of male and egg isotopes with respect to spatial and habitat variables, and testing for a correlation between these residuals.
Nests were found along two opposite shorelines of the lake (Fig. 1). We collected 81 nests from the northwest (NW) shore and 21 nests from the southeast (SE) shore. The difference in number of nests collected reflects a smaller nesting colony on the SE shore. The area along the shoreline between nest clusters consisted of marshy vegetation and no nests were found in this area. We therefore treat the shores as discrete clusters of nests. It is approximately 100 m across the lake between these clusters and stickleback were observed swimming in the open water between nest shores. Stickleback must come to shallow water to nest or lay eggs (Wooten 1976).
There was no indication of spatial trends in isotopes along a given shoreline for either stickleback or mussels and snails (used as isotopic baseline). We therefore used shore as a categorical variable in further analysis of spatial effects. Nitrogen isotopes differed significantly between shores for mussels (NW shore = 2.61 ± 0.13, SE shore = 2.97 ± 0.08, [mean ± SE], P = 0.019) but not snails (NW shore = 3.23 ± 0.14, SE shore = 3.00 ± 0.19, P = 0.38). Similarily, carbon isotopes differed significantly between shores for mussels (NW shore = −31.27 ± 0.11, SE shore = −31.54 ± 0.07, P = 0.048) but not for snails (NW shore = −26.22 ± 0.53, SE shore = −27.88 ± 0.77, P = 0.10). Consequently, we used mean mussel isotopes for each shore seperately and a lakewide mean for snails to calculate trophic position and percent benthic carbon according to the methods of Post (2002) as described above. Analyses were also done with raw nitrogen and carbon isotope data and produced equivalent results throughout all analyses. In addition, using mean isotopes for each shore seperately for both snails and mussles did not change our conclusions.
We calculated the strength of male-female correlation (assortative mating) expected to arise from the observed habitat cosegregation for each measured habitat variable. We measured the correlations between each habitat variable and male and egg isotopes (rh*m and rh*e). We then calculated the expected correlation between male and egg isotopes, based on the male-habitat and egg-habitat correlation (r̂m*e = rh*m*rh*e). This formula is a simple application of standard partial correlations, in which an indirect phenotypic correlation between males and females arises from phenotype-habitat correlations within each sex. Note that the egg-habitat correlation could be influenced both by female habitat preference and by female preference for spatially structured males. Therefore, the spatially predicted male-egg isotope correlation may be an overestimate of the actual role of spatial segregation in assortative mating, because our calculation may be influenced by female preferences for spatially structured male traits. We compared the predicted assortment-by-space, to the observed level of assortative mating.
Finally, we tested whether assortative mating remains after statistically removing the effect of both space and microhabitat. We generated separate linear models of the relationships between male and egg isotopes and measured habitat variables (shore, depth, vegetation, and wood cover). We then tested for a correlation between the residuals of these models for males and the eggs collected from their nest. A significant correlation between the residuals indicates that there is assortative mating on isotopes, above and beyond any correlations arising from habitat cosegregation.
RELATIONSHIP BETWEEN NEST HABITAT AND MALE DIET AND MORPHOLOGY
The spatial structure in male isotopes, evaluated above, might also be reflected in male trophic morphology. We measured morphological features typically associated with diet in stickleback (as described in Snowberg and Bolnick 2008) for all males. In both species-pair lakes and within solitary lakes, fish with larger gapes, deeper bodies, and fewer, shorter gill rakers are more efficient at using benthic prey, whereas the opposite is true for limnetic prey (Schluter 1995; Robinson 2000). For each nesting male collected we measured standard length and gape width using digital calipers (accurate to 0.01 mm). We counted the number of gill rakers on the right side of the gill arch and measured the longest gill raker using an ocular micrometer. Gape width and gill raker length are highly correlated with standard length so the residuals of these measures on standard length were used in analyses.
We used general linear models to test whether nest habitat (depth and vegetative and wood cover) depend on male diet and morphology. Nest depth, vegetative cover, and wood cover were used as dependent variables, to reflect our hypothesis that male morphology influenced their choice of nest habitat. Our fish were sampled within weeks of the onset of the breeding season, and it is unlikely that males’ nesting habitat could appreciably influence their morphology, via phenotypic plasticity, over such a short-time scale. Similarly, nest habitat is unlikely to have had enough time to influence male isotopes. We evaluated three separate models with nest habitat characters as functions of male percent benthic carbon, trophic position, standard length, gill raker number, and residual gill raker length and gape width. We used the function step AIC in the MASS package of R (Venables and Ripley 2002) to chose the best fit model. For depth we fit a linear model starting with all morphology and isotope data and allowing both removal and adding of variables. For vegetative and wood cover we used the same procedure to fit a binomial GLM.
Results
ASSORTATIVE MATING
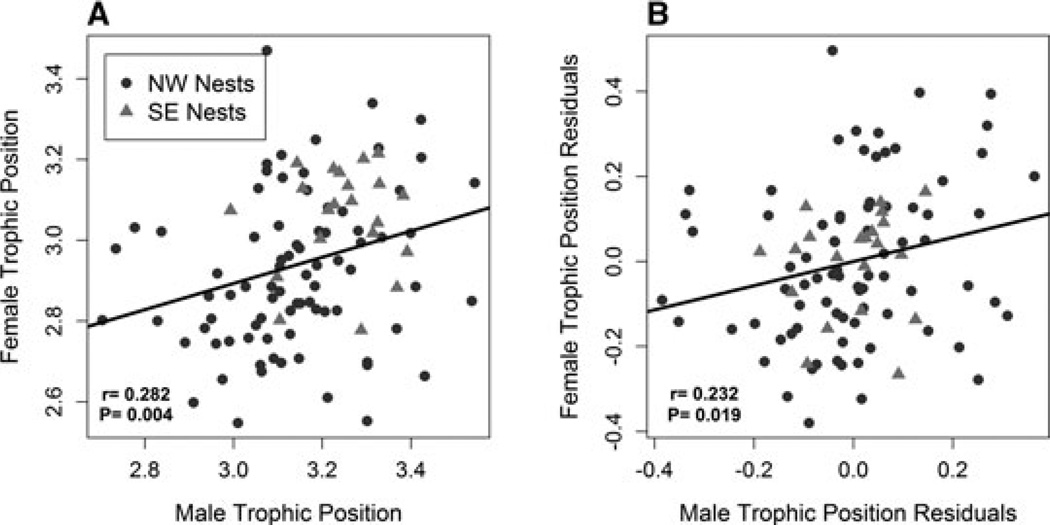
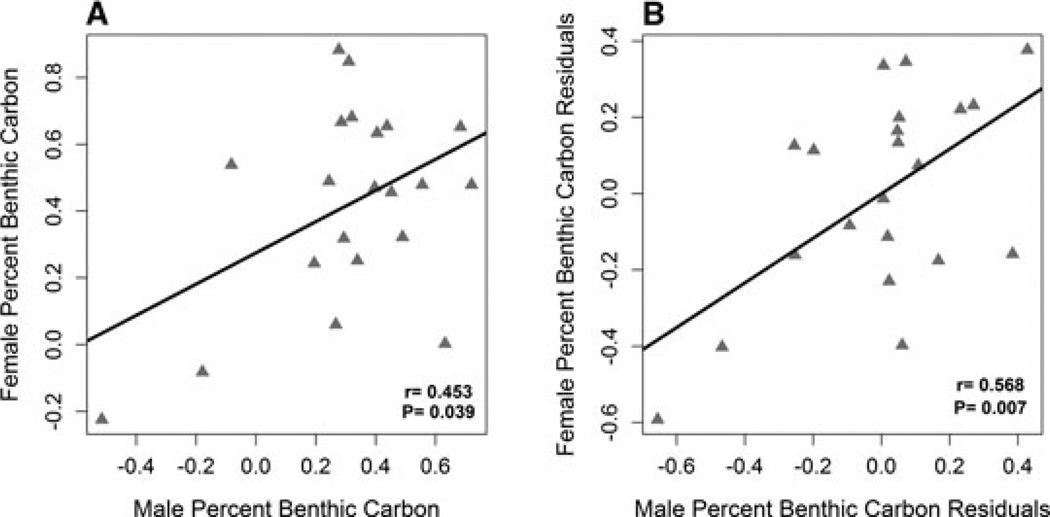
There was significant assortative mating by trophic position lakewide (rm*e = 0.282, P = 0.004, Fig. 2A). For percent benthic carbon, only the SE shore showed a significant correlation (rm*e = 0.453, P = 0.039, Fig. 3A). Egg isotopes were strongly correlated with female liver isotopes (Nitrogen: rf*e = 0.799, P < 0.0001, Carbon: rf*e = 0.976, P < 0.0001), supporting the use of eggs as a proxy for female liver isotopes. Using the correlation between female liver and egg isotopes we estimate correlation between male and female trophic position is r̂m*f = 0.353 (SE = 0.0513). The estimated correlation between male and female percent benthic carbon (on the SE shore) is r̂m*f = 0.464 (SE = 0.0966).
Figure 2.
Male and female trophic position measured using male liver isotopes and female egg isotopes were significantly correlated (rm*e = 0.282 P = 0.004; A). The residuals of the relationships between male and female isotopes with measured habitat variables were also significantly correlated (r = 0.232 P = 0.019) suggesting assortative mating is not simply explained by habitat (B).
Figure 3.
Male and female percent benthic carbon measured using male liver isotopes and female egg isotopes were significantly correlated in nests collected on the southeast shore (rm*e = 0.453 P = 0.039; A). The residuals of the relationships between male and female isotopes with measured habitat variables were also significantly correlated (r = 0.568, P = 0.007) suggesting assortative mating is not simply explained by habitat (B).
THE ROLE OF SPATIAL COSEGREGATION IN ASSORTATIVE MATING
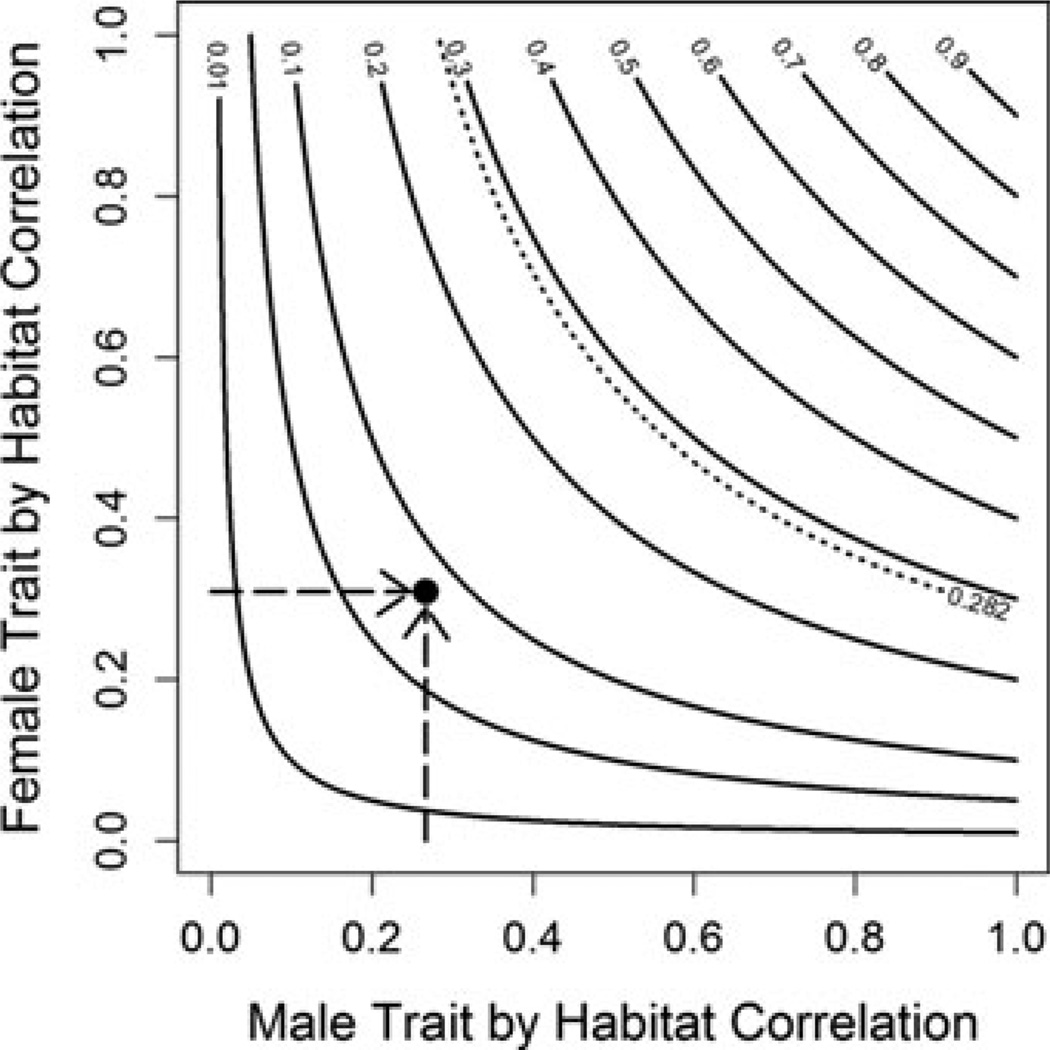
In univariate correlations, male trophic position was significantly correlated with shore, nest depth, and vegetation cover. Egg (female) trophic position was only significantly correlated with shore (Table 1A). However, the observed assortative mating for trophic position (rm*e = 0.282, 95% confidence interval: 0.091–0.452) is stronger than could be explained by spatial cosegregation of diet measures with any of the measured habitat features (Table 1A). For example, cosegregation by shore only would lead to a rm*e of 0.083 (see Fig. 4). Thus, spatial cosegregation of diet strategies can generate some weak assortative mating (r < 0.1), but is insufficient to explain the observed assortment.
Table 1.
Univariate correlations between diet and nest habitat for males(rm*h) and eggs (re*h) and the predicted correlation between males and females (rm*e) that would result from spatial cosegregation, calculated as rm*h * re*h.
| A. Trophic position: observed rm*e = 0.282 (95% CI: 0.091–0.452) | |||||
|---|---|---|---|---|---|
| Habitat feature | rm*h | Pm*h | re*h | Pe*h | rm*e |
| Shore | 0.267 | 0.007 | 0.309 | 0.002 | 0.083 |
| Nest depth | 0.267 | 0.007 | −0.132 | 0.19 | −0.035 |
| Vegetation cover | 0.241 | 0.015 | 0.089 | 0.37 | 0.021 |
| Wood cover | 0.109 | 0.28 | 0.041 | 0.69 | 0.004 |
| B. Percent benthic carbon: observed rm*e = 0.453 (95% CI: 0.027–0.740) | |||||
|---|---|---|---|---|---|
| Habitat feature | rm*h | Pm*h | re*h | Pe*h | rm*e |
| Nest depth | −0.112 | 0.63 | −0.413 | 0.063 | 0.041 |
| Vegetation cover | 0.167 | 0.47 | −0.208 | 0.37 | −0.034 |
| Wood cover | 0.321 | 0.16 | −0.199 | 0.39 | −0.064 |
Figure 4.
The strength of assortative mating via spatial cosegregation depends on the simultaneous strength of male-habitat and female-habitat correlations. The indirect partial correlation between males and females, rm*f is the product of the partial correlations between each sex and a habitat variable (rm*h and rf*h), shown by contour lines. Dashed lines representative data from this study: the measured correlations between male and female tropic position and shore (both significant) lead to a predicted strength of assortment of 0.083. This value is significantly less than the measured correlation of 0.282.
The measured habitat variables are not independent, so we also carried out a test for assortative mating that accounted for multivariate microhabitat data. Both male and egg isotopes were separately modeled as functions of multiple habitat variables; if there is assortative mating above and beyond the effect of habitat, the residuals of these models should be correlated. In a linear model, male trophic position was significantly associated with nest depth (P = 0.0054) and shore (P = 0.00032; Table 2A). Egg trophic position (a proxy for females) was also correlated with shore (P = 0.0038; Table 2B). Both males and eggs had higher trophic position on the SE shore. Residuals of these models were calculated to control for patterns of association between diet and nest habitat and location. Significant male/egg correlations remained after accounting for these spatial effects, as demonstrated by correlations between residual isotope scores (Trophic postion: r = 0.232, P = 0.019, Fig. 2B).
Table 2.
Model results of trophic position as a function of nest habitat. Correlations between the residuals of these models provide an estimate of assortative mating independent of habitat isolation.
| A. Males | Estimate | Std. error | t value | P |
|---|---|---|---|---|
| (Intercept) | 2.972 | 0.0468 | 63.552 | <0.0001 |
| Nest Depth | 0.156 | 0.0547 | 2.850 | 0.0054 |
| Vegetation Cover | 0.0596 | 0.0328 | 1.814 | 0.073 |
| Wood Cover | 0.0412 | 0.0347 | 1.189 | 0.24 |
| Shore | 0.139 | 0.0373 | 3.734 | 0.00032 |
| B. Females | Estimate | Std. error | t value | P |
| (Intercept) | 2.952 | 0.0570 | 51.809 | <0.0001 |
| Nest depth | −0.0783 | 0.0666 | −1.175 | 0.24 |
| Vegetation cover | 0.0647 | 0.0399 | 1.621 | 0.11 |
| Wood cover | 0.0192 | 0.0416 | 0.461 | 0.65 |
| Shore | 0.135 | 0.0453 | 2.971 | 0.0038 |
Predicted correlations between male and female percent benthic carbon due to univariate spatial cosegregation were all negative and outside the confidence interval of the correlation on the southeast shoreline (rm*e = 0.453, 95% confidence interval: 0.027–0.740), except for depth (Table 1B). Both males and females showed a negative but nonsignificant correlation between depth and percent benthic carbon, with a predicted correlation between male and female diet of 0.0406 (Table 1B). The partial correlation between male and female percent benthic carbon and nest depth fell within the 95% confidence interval of the observed correlation (Table 1B). It is worth noting, however, that the observed benthic carbon assortment on the southeast shoreline has wide confidence intervals due to small sample size on that shoreline. Because the estimate of rm*e fell within the 95% confidence interval it is necessary to further explore whether nest depth alone explains the observed level of assortment. We found the residuals of the relationships between percent benthic carbon and nest depth for male and eggs were significantly correlated (r = 0.450, P = 0.041), implying that assortment remains even after we remove the covariance between diet and nest depth. This result is consistent with our finding that the assortative mating expected from spatial cosegregation is less than we actually observe. We therefore conclude that none of the measured habitat variables can, by itself, account for the observed correlation between male and female percent benthic carbon.
For percent benthic carbon a multivariate model was made using only the nests located on the SE shore as this was the only location showing assortative mating by carbon isotopes (Table 3). No factors were significant in these models for male or female percent benthic carbon. Significant male/egg correlations remained after removing spatial effects using residuals of these models (percent benthic carbon on the SE shore: r = 0.568, P = 0.007, Fig. 3B). In addition, we performed post-hoc tests to explore why the pattern of assortment by percent benthic carbon differed between the NW and SE shore. We used linear models of lakewide percent benthic carbon for males and eggs with habitat variables, including a shore∗habitat interaction term. We found that there is a significant shore∗depth interaction for egg percent benthic carbon (P = 0.048), with more benthic females tending to lay eggs shallower on the SE shore (where assortative mating was observed) and tending to lay eggs deeper on the NW shore. The significance of assortment on the SE shore relies heavily on the nest with the lowest percent benthic carbon for both male and egg (Fig. 3). This point has high leverage and a Cook’s Distance > 1. Removal of this point makes the relationship between male and female percent benthic carbon non-significant (percent benthic carbon on the SE shore: r = 0.192, P = 0.42). However, careful examination of the data reveals no obvious bias or error in the collection of the isotope data and so it is inappropriate to remove this data point from our analysis. Note that this outlier does not influence the habitat-corrected assortative mating with respect to trophic position, described above.
Table 3.
Model results of percent benthic carbon as a function of nest habitat for the southeast shore. Correlations between the residuals of these models provide an estimate of assortative mating independent of habitat isolation.
| A. Males | Estimate | Std. error | t value | P |
|---|---|---|---|---|
| (Intercept) | 0.417 | 0.180 | 2.318 | 0.033 |
| Nest depth | −0.326 | 0.279 | −1.169 | 0.26 |
| Vegetation cover | 0.180 | 0.156 | 1.156 | 0.26 |
| Wood cover | 0.209 | 0.137 | 1.533 | 0.14 |
| B. Females | Estimate | Std. error | t value | P |
| (Intercept) | 0.749 | 0.185 | 4.039 | 0.00085 |
| Nest depth | −0.449 | 0.287 | −1.564 | 0.14 |
| Vegetation cover | −0.00273 | 0.160 | −0.017 | 0.99 |
| Wood cover | −0.0975 | 0.141 | −0.693 | 0.50 |
RELATIONSHIP BETWEEN NEST HABITAT AND MALE DIET AND MORPHOLOGY
Based on AIC model selection, we found that males tended to have deeper nests when they exhibited more limnetic carbon, higher trophic position, and nested on the NW shore (Table 4A). There was a marginal tendency for males with shorter residual gill raker length to nest deeper. Males with higher trophic position tended to nest in denser vegetation, and there was a marginal effect of gape width and shore (Table 4B). Finally, larger males tend to nest closer to wood cover (Table 4C). These results suggest that males with different diet and phenotype partition nest habitats more finely than previously suspected, as the depth gradient over which these traits differ spans only 2 vertical meters. Thus, there are systematic ecological and morphological differences between fish that are often just a few horizontal meters apart.
Table 4.
Best-fit model results for nest habitat related to male isotopes and morphology. All models started with all factors included (percent benthic carbon, trophic position, standard length, gape width residual, and gill raker length residual). All factors from the best fit model are included.
|
A. Nest depth |
Estimate | Std. error | t value | P |
|---|---|---|---|---|
| (Intercept) | −0.870 | 0.554 | −1.572 | 0.12 |
| Percent benthic carbon |
−0.191 | 0.0822 | −2.328 | 0.022 |
| Trophic position |
0.568 | 0.174 | 3.269 | 0.0015 |
| Gape width residual |
−0.906 | 0.507 | −1.786 | 0.077 |
| Raker length residual |
−0.552 | 0.250 | −2.206 | 0.030 |
| Shore | −0.248 | 0.0669 | −3.704 | 0.00036 |
|
B. Vegetative cover |
Estimate | Std. error | z value | P |
| (Intercept) | −14.469 | 4.976 | −2.908 | 0.0036 |
| Trophic position | 4.468 | 1.574 | 2.838 | 0.0045 |
| Gape width residual |
−7.803 | 4.278 | −1.824 | 0.068 |
| Shore | −1.080 | 0.590 | −1.829 | 0.067 |
|
C. Wood cover (Intercept) |
Estimate −7.812 |
Std. error 3.041 |
z value −2.569 |
P 0.010 |
| Standard length |
0.151 | 0.0686 | 2.207 | 0.027 |
Discussion
Assortment is measured as a correlation between male and female traits, but like any correlation there is no implication that the focal trait actually causes the assortment (e.g. that individuals exhibit a preference for phenotypically similar mates). It is therefore important to disentangle the various processes that may generate assortative mating, to understand the causes of reproductive isolation among individuals, between populations, and the resulting genetic structure. Two major alternative processes are plausible—individuals may either tend to encounter similar phenotypes when searching for a mate (spatial or habitat isolation), or they may select similar phenotypes from among the pool of individuals that they encounter (mate preferences). We show that, at least in stickleback, phenotypic divergence across space and microhabitats does occur within even small lakes, and can generate weak assortative mating. However, although nest habitat and location are associated with diet and morphology, these associations are not sufficient to explain the level of assortative mating observed in this population. Our results therefore suggest that mate preference rather than spatial isolation is key to generating assortative mating by diet in this population. We explore the levels of assortative mating that can be created by cosegregation without direct mate preference and conclude that our results are likely quite general across taxa.
Spatial isolation of individuals with different diets could take two general forms. First, individuals found in isolated locations might feed on different prey and rarely interbreed due to reduced encounter rates. In the context of our study, this could appear as across-lake spatial gradients in diets or isotope signatures that generate assortative mating because ecologically divergent individuals never encounter each other. We did find evidence that individuals nesting on different shores have significantly different isotope signatures. Whether we analyze the raw data (δ13C and δ15N) or their spatially adjusted equivalents (percent benthic carbon and trophic position), males and females differ in isotopes in the same direction between shores. Thus, some of the signal of assortative mating on a whole-lake level may be due to across-lake phenotypic differentiation. The causal basis of this differentiation is not clear. Are individuals nesting on different shores constrained to have different diets, or do individuals with different prey preferences opt to settle in different locations? The latter explanation seems more likely. If individuals randomly choose a shore to nest on and subsequently acquire different diets, males (which are constrained to remain near their nest) should exhibit greater between-shore isotope differences than females, which may move freely between shores. Quite the contrary, females exhibit greater between-shore isotope differences than the less mobile males. We therefore posit that individuals choose that shore they nest on based on already established prey or habitat preferences. Bolnick et al. (2009) previously showed that habitat preference can reduce stickleback dispersal between habitats, facilitating adaptive divergence.
A second general form of spatial isolation arises if the various phenotypes are spatially well mixed and frequently encounter each other, but prefer subtly different microhabitats when searching for mates. In the context of stickleback, this could represent differences in nest-site selection by males and females. In the benthic/limnetic stickleback species pairs and in benthic-like lakes and limnetic-like lakes, limnetic diet is associated with nesting in open, shallower areas, and benthic diet with nesting in dense vegetation at deeper depths within the littoral zone (McPhail 1994; Vines and Schluter 2006). Interestingly, we found the opposite trend for most traits: males and females with stereotypically “limnetic” traits (lower percent benthic carbon, higher trophic position, Matthews et al. 2010; and smaller gape width, Robinson 2000) tended to nest or lay eggs in deeper nests. Also, males guarding nests in dense vegetation had smaller gapes (a limnetic trait) and higher trophic position. The one exception was that individuals with shorter gill raker length (a benthic trait; Robinson 2000) used deeper nests. The atypical results are not simply due to differences between shores, because the trends hold within a given shore. Nests tended to be deeper on the NW shore, where fish tended to have higher percent benthic carbon and lower trophic position. Wood cover was only significantly correlated with male standard length, with larger males (a benthic trait) guarding nests that were shielded by large logs. Although this type of cover has not been analyzed in previous studies of nesting habitat, it should provide shelter in a manner similar to nesting among dense vegetation favored by larger (Kraak et al. 2000) or benthic males (McPhail 1994). In this population, the associations between habitat and ecotype primarily go against a priori predictions derived from the benthic/limnetic species pairs.
Correlations between male and female isotopes generated by spatial cosegregation are too weak to explain the observed correlations between males and females. For nitrogen isotopes, all patterns of spatial cosegregation are too weak to explain the observed correlation between male and female trophic position. In contrast, carbon stable isotopes were correlated with depth for both sexes on the southeast shore. Based on this cosegregation, we calculated a predicted male–female correlation that was substantially less than the observed male–female correlation, but was within its 95% confidence interval. However, the confidence interval for this correlation is quite broad because of the small sample size on the SE shore. Analyses of isotope residuals provide clearer evidence that cosegregation is insufficient to explain assortative carbon assortative mating. Individuals with more benthic isotope residuals (for a given nest depth) tend to mate with individuals with more benthic isotope residuals (also controlling for nest depth).
Despite being too weak to generate appreciable assortative mating, the fine-scale phenotype-environment correlations found here (e.g. over a depth gradient of approximately two meters) are themselves quite noteworthy. Most studies of habitat choice have dealt with either discrete habitats or discrete phenotypes, but this study suggests animals may partition habitats much more finely than typically appreciated. Such partitioning can generate biased encounter rates between phenotypes. Biased encounter rates are a major component of models of speciation by habitat isolation, such as is seen in host races of phytophagous insects. The role of spatial separation is quite obvious in such cases, where plant hosts represent discrete and distinct entities. However, phenotypes may also be distributed nonrandomly across more subtle environmental gradients, as we document here for three-spine stickleback. If these fine-scale correlations between phenotypic traits and continuous environmental variables are very common, subtle microhabitat differentiation might commonly play a small role in generating assortative mating.
The strength of assortative mating observed in this study was similar to that observed in our previous study (Snowberg and Bolnick 2008), and thus suggests that assortative mating by diet may be quite general in stickleback. In the previous study, we used a principal component axis to summarize isotope signatures (δ13C and δ15N were correlated) and found a male-egg correlation of rm*e = 0.348 and an estimated male–female correlation of r̂m*f = 0.507 (Snowberg and Bolnick 2008). In contrast, the present study examined each isotope separately, as the isotopes were uncorrelated. For trophic position the correlation between the isotopes of male liver and eggs in their nest was rm*e = 0.282. Given the female-egg correlation, we estimate that the strength of assortative mating (male–female correlation) is r̂m*f = 0.353. For percent benthic carbon assortative mating only occurred along one shore and the male-egg correlation was rm*e = 0.453, yielding an estimated male–female correlation of r̂m*f = 0.464. These numbers imply that diet-assortative mating may be slightly weaker than previously observed in Mohun Lake, although direct comparison is complicated by differences in the spatial extent of sampling and differences in C–N correlations between studies. These observed differences in the strength of assortment raise the intriguing possibility that assortative mating varies among populations, and the corresponding question of what ecological or evolutionary forces explain such variation in the strength of assortment.
The suggestion that assortment varies between populations is further supported by our finding of carbon assortment on only one shore of Burnt-Out Lake. It is unclear why assortment by percent benthic carbon only existed for one shore and showed no pattern on the other shore. There was a significant depth-shore interaction for egg percent benthic carbon. On the SE shore, where carbon assortment was observed, both males and females tended to nest (or lay eggs) deeper when they had a more limnetic isotope signature. This mutual correlation between isotopes and depth could be due to spatial cosegregation, or because more limnetic females preferred to mate with more limnetic males, who happened to nest deeper. In contrast on the NW shore females showed a nonsignificant opposite trend, laying eggs shallower when they had a more limnetic isotope signature. Some caution is necessary in interpreting the difference in carbon assortment between shores because the significance of assortment on the SE shore is dependent on an outlier data point: a nest with low benthic carbon for both the male and female. However, there is no reason to believe the data from this nest represent anything beyond an assortative mating between a male and female with unusual diet for their nesting area. It therefore is not proper to disregard this data in our conclusions. In fact, matings between individuals who are phenotypic outliers will be informative to our understanding of assortment, as these individuals should have lower encounter rates with phenotypically similar individuals. Interestingly, rare phenotypes theoretically should become less choosy due to increased search costs (Real 1990; Crowley et al. 1991). Changes in choosiness due to differences in cost have been experimentally demonstrated in stickleback (Bakker and Milinski 1991; Milinski and Bakker 1992).
To generalize our results, it is helpful to ask how strong spatial segregation must be to generate plausible levels of assortative mating. Using standard principles of partial correlation, an indirect phenotypic correlation between males and females, via their joint association with a habitat variable, is equal to the product of the partial correlation between each sex’s trait values and the habitat variable (Fig. 4). Thus, assortative mating via spatial cosegregation requires both sexes to exhibit reasonably strong trait-environment correlations. Assortative mating for a given average trait-environment correlation is strongest when the two sexes show equal trait-environment correlations, and the strength of assortment will still be weaker than either trait-environment correlation alone. To illustrate this point, a recent meta-analysis of over 1000 empirical estimates of male–female trait correlations within populations found an average strength of assortment of 0.28 (Jiang, Bolnick, and Kirkpatrick, unpubl. ms.). To achieve this typical level of assortment via cosegregation, both sexes must exhibit trait-environment correlations of 0.53. Such correlations are probably quite reasonable in some insect host races with discrete habitats (host plants), and when assortment arises from isolation by distance, but may be unusually strong for more subtle quantitative environmental variation within a single population, as studied here. Consequently, we anticipate that the results presented here for stickleback may be generally applicable to a wide range of species.
After controlling for the relationships between isotopes of both males and females with all measured microhabitat features and spatial differences, we find that there is a significant correlation between the residual variations. We therefore conclude that assortative mating with respect to both carbon source and trophic position are not merely the result of spatial structure of individual diet strategies during the breeding season. This conclusion must be accompanied with a caveat that it remains possible that males and females cosegregate along some unmeasured environmental gradient that reduces their encounter rates with different phenotypes. However, we measured the environmental variables most commonly seen to differ between benthic and limnetic species pairs (McPhail 1994; Vines and Schluter 2006) and our general results suggest that unmeasured environment-phenotype correlations must be quite strong to produce the strength of assortative mating observed here. Therefore, we feel fairly confident in concluding that while spatial structure may contribute weakly to assortative mating, there must be some active mate preferences generating diet-assortative mating.
This evidence for mate preferences is inferential, having been arrived at by process of elimination. However, there is some additional evidence supporting this conclusion. Most notably, individuals directly assess the diet of conspecifics (including potential mates) through olfaction. In one study, individual stickleback experimentally fed a particular prey type subsequently preferred to associate with shoals of individuals who have fed on that same prey (Ward et al. 2004). Olfactory cues were necessary and sufficient to generate such diet-assortative shoaling. Such associations could generate assortative mating in diet-variable natural populations. To test this possibility, we conducted a laboratory mate-choice study extending the results of Ward et al. (2004) to the context of mating. Males and females were experimentally fed either benthic or limnetic prey (chironomid larvae or Daphnia). Females were then used in mate-choice, choosing between unfamiliar same- and different-diet males, as well as no-choice mate trials. We found that gravid females associated preferentially with nesting males who had been fed the same experimental diet rather than a different diet than the female when only olfactory cues were present (P = 0.009; Snowberg and Bolnick, unpubl. ms.). Gravid females also progressed further through courtship with nesting males fed the same diet than a different diet (P = 0.017; Snowberg and Bolnick, unpubl. ms). This suggests gravid females may use diet cues directly in assessing potential mates, consistent with our inference based on the data presented here. In addition, olfactory cues are used in other contexts of stickleback mating, such as disassortative mating by MHC (Reusch et al. 2001) and assortment between benthic and limnetic stickleback species (Rafferty and Boughman 2006; Kozak et al. 2011). Another potential contributor to assortative mating in stickleback is phenotype matching of mates based on morphological traits that are correlated with diet. We are unable to measure a female’s morphology using eggs collected from a nest and so this possibility would require observing matings or using other methods to find the female associated with each nest. These mechanisms of assortment are not mutually exclusive and may form a complex web of traits contributing to reproductive isolation.
In conclusion, we find that individuals can exhibit phenotype-environment correlations. When males and females exhibit parallel phenotype-environment correlations (as occurs for some traits in stickleback), then positive assortative mating can result. However, we find that these phenotype-environment correlations must be quite strong in both sexes (r > 0.5) to generate weak assortative mating comparable to values typically seen within populations. We therefore suggest that spatial or habitat segregation will be important in situations where trait-environment correlations are very strong, such as in insect host races, but may be less important in other settings. In either case, it will be generally important to control for both coarse spatial structure and fine-scaled habitat structure in future studies of assortative mating.
ACKNOWLEDGMENTS
Will Stutz, Marcio Araújo, and Rose Carlson assisted with fieldwork. Chris Smith assisted with preparation of isotope samples. Mark Kirk-patrick provided valuable feedback on statistical analysis. Will Stutz and Christine Parent provided comments on earlier drafts of this manuscript. This work was supported by a David and Lucille Packard Foundation fellowship to DIB. LKS was supported by a NSF GRF.
LITERATURE CITED
- Andersson M. Sexual selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- Araújo MS, Guimarães PR, Jr., Svanbäck R, Pinheiro A, Guimarães P, dos Reis SF, Bolnick DI. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology. 2008;89:1981–1993. doi: 10.1890/07-0630.1. [DOI] [PubMed] [Google Scholar]
- Bakker TC, Milinski M. Sequential female choice and the previous male effect in sticklebacks. Behav. Ecol. Sociobiol. 1991;29:205–210. [Google Scholar]
- Bell MA. Differentiation of adjacent stream populations of threespine stickleback. Evolution. 1982;36:189–199. doi: 10.1111/j.1558-5646.1982.tb05023.x. [DOI] [PubMed] [Google Scholar]
- Berlocher SH, Feder JL. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu. Rev. Entomol. 2002;47:773–815. doi: 10.1146/annurev.ento.47.091201.145312. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Lau OL. Widespread disruptive selection in natural populations of sticklebacks in post-glacial lakes. Am. Nat. 2008;172:1–11. doi: 10.1086/587805. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Paull JE. Diet similarity declines with morphological distance between conspecific individuals. Evol. Ecol. Res. 2009;11:1217–1233. [Google Scholar]
- Bolnick DI, Snowberg LK, Patenia C, Stutz WE, Ingram T, Lau OL. Pheontype-dependent native habitat preference facilitates divergence between parapatric lake and stream stickleback. Evolution. 2009;63:2004–2016. doi: 10.1111/j.1558-5646.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- Boughman JW. Divergent natural selection enhances reproductive isolation in sticklebacks. Nature. 2001;411:944–947. doi: 10.1038/35082064. [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Via S. Specialized feeding behavior influences both ecological specialization and assortative mating in sympatric host races of pea aphids. Am. Nat. 2000;156:606–621. doi: 10.1086/316991. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- Crespi BJ. Causes of assortative mating in arthropods. Anim. Behav. 1989;38:980–1000. [Google Scholar]
- Crowley PH, Travers SE, Linton MC, Cohn SL, Sih AS, Sargent CR. Mate density, predation risk and the seasonal sequence of mate choices: a dynamic game. Am. Nat. 1991;137:567–596. [Google Scholar]
- Edelaar P, Siepielski AM, Clobert J. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution. 2008;62:2462–2472. doi: 10.1111/j.1558-5646.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- Eizaguirre C, Lenz TL, Sommerfeld RD, Harrod C, Kalbe M, Milinksi M. Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three-spine stickleback ecotypes. Evol. Ecol. 2010;25:605–622. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th edition. San Francisco: Benjamin Cummings; 1994. [Google Scholar]
- Felsenstein J. Skepticism toward Santa Rosalia, or why are there so few kinds of animals? Evolution. 1981;35:124–138. doi: 10.1111/j.1558-5646.1981.tb04864.x. [DOI] [PubMed] [Google Scholar]
- France R. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr. 1995;40:1310–1313. [Google Scholar]
- Gimelfarb A. Is offspring-midparent regression affected by assortative mating of parents? Genet. Res. 1986;47:71–75. doi: 10.1017/s0016672300024538. [DOI] [PubMed] [Google Scholar]
- Gray J. Ontogeny and dietary specialization in brown trout (Salmo trutta L.) from Loch Ness, Scotland, examined using stable isotopes of carbon and nitrogen. Ecol. Freshw. Fish. 2001;10:168–176. [Google Scholar]
- Hobson KA. Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar. Ecol. Prog. Ser. 1993;95:7–18. [Google Scholar]
- Hobson KA, Clark RG. Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor. 1992a;94:189–197. [Google Scholar]
- Hobson KA, Clark RG. Assessing avian diets using stable isotopes I: turnover in δ13C in tissues. Condor. 1992b;94:181–188. [Google Scholar]
- Kitano J, Ross JA, Mori S, Kume M, Jones FC, Chan YF, Absher DM, Grimwood J, Schmutz J, Myers RM, Kingsley DM, Peichel CL. A role for a neo-sex chromosome in stickleback speciation. Nature. 2009;461:1079–1083. doi: 10.1038/nature08441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak GM, Boughman JW. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 2009;20:1282–1288. [Google Scholar]
- Kozak GM, Head ML, Boughman JW. Sexual imprinting on ecologically divergent traits leads to sexual isolation in sticklebacks. P Roy Soc. B-Biol. Sci. 2011;278:2604–2610. doi: 10.1098/rspb.2010.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraak SBM, Bakker TCM, Hočevar S. Stickleback males, especially large and red ones, are more likely to nest concealed in macrophytes. Behaviour. 2000;137:907–919. [Google Scholar]
- Kume M, Kitano J, Mori S, Shibuya T. Ecological divergence and habitat isolation between two migratory forms of Japanese threespine stickleback (Gasterosteus aculeatus . J. Evol. Biol. 2010;23:1436–1446. doi: 10.1111/j.1420-9101.2010.02009.x. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Matthews B, Marchinko KB, Bolnick DI, Mazumder A. Specialization of trophic position and habitat use by stickleback in an adaptive radiation. Ecology. 2010;91:1025–1034. doi: 10.1890/09-0235.1. [DOI] [PubMed] [Google Scholar]
- McKinnon JS, Mori S, Blackman BK, David L, Kingley DM, Jamison L, Chou J, Schluter D. Evidence for ecology’s role in speciation. Nature. 2004;429:294–298. doi: 10.1038/nature02556. [DOI] [PubMed] [Google Scholar]
- McPhail JD. Speciation and the evolution of reproductive isolation in the sticklebacks (Gasterosteus) in south-western British Columbia. In: Bell MA, Foster SA, editors. The evolutionary biology of the threespine stickleback. Oxford: Oxford Univ. Press; 1994. pp. 399–437. [Google Scholar]
- Milinski M, Bakker TC. Costs influence sequential mate choice in sticklebacks, Gasterosteus aculeatus . P Roy Soc Lond. B Biol. 1992;250:229–233. [Google Scholar]
- Newsome SC, Martinez del Rio C, Phillips DL, Bearhop S. A niche for isotope ecology. Front Ecol. Environ. 2007;5:429–436. [Google Scholar]
- Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria; 2011. R:A language and environment for statistical computing. ISBN 3–900051-07–0, URL http://www.R-project.org/ [Google Scholar]
- Raeymaekers JAM, Boisjoly M, Delaire L, Berner D, Räsänen K, Hendry AP. Testing for mating isolation between ecotypes: laboratory experiments with lake, stream andy hybrid stickleback. J. Evol. Biol. 2010;23:2694–2708. doi: 10.1111/j.1420-9101.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- Rafferty N, Boughman JW. Olfactory mate recognition in a sympatric species pair of threespine sticklebacks. Behav. Ecol. 2006;17:965–970. [Google Scholar]
- Real L. Search theory and mate choice. I. Models of single-sex discrimination. Am. Nat. 1990;136:376–405. [Google Scholar]
- Redden D, Allison D. The effect of assortative mating upon genetic association studies: spurious associations and population substructure in the absence of admixture. Behav. Genet. 2006;36:678–686. doi: 10.1007/s10519-006-9060-0. [DOI] [PubMed] [Google Scholar]
- Reusch BTH, Häberli MA, Aeschlimann PB, Milinski M. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 2001;414:300–302. doi: 10.1038/35104547. [DOI] [PubMed] [Google Scholar]
- Rice WR. Speciation via habitat specialization : the evolution of reproductive isolation as a correlated character. Evol. Ecol. 1987;1:301–314. [Google Scholar]
- Robinson BW. Trade offs in habitat-specific foraging efficiency and the nascent adaptive divergence of sticklebacks in lakes. Behaviour. 2000;137:865–888. [Google Scholar]
- Schluter D. Adaptive radiation in sticklebacks: trade-offs in feeding performance and growth. Ecology. 1995;76:82–90. [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. Am. Nat. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Snowberg LK, Bolnick DI. Assortative mating by diet in a phenotypically unimodal but ecologically variable population of stickleback. Am. Nat. 2008;172:733–739. doi: 10.1086/591692. [DOI] [PubMed] [Google Scholar]
- Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. Fractiona-tion and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia. 1983;57:32–37. doi: 10.1007/BF00379558. [DOI] [PubMed] [Google Scholar]
- Vamosi SM, Schluter D. Sexual selection against hybrids between sympatric stickleback species: evidence from a field experiment. Evolution. 1999;53:874–879. doi: 10.1111/j.1558-5646.1999.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th edition. New York: Springer; 2002. [Google Scholar]
- Vines TH, Schluter D. Strong assortative mating between allopatric sticklebacks as a by-product of adaptation to different environments. P. Roy Soc. B-Biol. Sci. 2006;273:911–916. doi: 10.1098/rspb.2005.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJW, Hart PJB, Krause J. The effects of habitat- and diet-based cues on association preferences in three-spined sticklebacks. Behav. Ecol. 2004;15:925–929. [Google Scholar]
- Wooten RJ. The biology of the sticklebacks. London: Academic Press; 1976. [Google Scholar]
- Wright S. Systems of mating. III. Assortative mating based on somatic resemblance. Genetics. 1921;6:144–161. doi: 10.1093/genetics/6.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]