Abstract
Neuropeptide Y (NPY) is implicated in the regulation of blood pressure (BP) and NPY pathways in the hypothalamus are sensitive to dietary fat. We evaluated the potential effect of a functional variant rs16147 located in the NPY gene promoter region on the association between 2-year diet intervention and change in multiple BP measures in the randomized Pounds Lost Trial. The NPY rs16147 was genotyped in 723 obese adults who were randomly assigned to one of four diets differing in the target percentages of energy derived from fat, protein, and carbohydrate. The changes of four BP phenotypes including systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP) and mean arterial pressure (MAP) during 2-year diet intervention were analyzed. In the total participants and participants with hypertension, we observed significant and consistent interactions between rs16147 genotype and dietary fat intake on changes in multiple BP phenotypes at 2 years (all P for interactions< 0.05). The risk allele (C allele) was associated with a greater reduction of BP phenotypes in response to low-fat diet, while an opposite genetic effect was observed in response to high-fat diet. In addition, the C allele was related to greater changes in 4 BP phenotypes in hypertensive compared to non-hypertensive participants. Our data suggest that NPY rs16147 may modulate the association between dietary fat intake and changes in BP phenotypes, and the C allele exerts a long-term beneficial effect on lowering BP in response to low-fat diet in obese and hypertensive subjects.
Keywords: NPY, genetic variation, dietary fat, gene-diet interaction, blood pressure
Obesity frequently coexists with hypertension and both are important risk factors for cardiovascular disease.1 Many studies have shown that dietary intervention on weight loss resulted in lower blood pressure (BP),2–5 and weight reduction is recommended in major guidelines as primary intervention in the treatment of high BP6. However, the BP lowering effect with weight loss response to dietary intervention exhibited substantial inter-individual variations;5, 7 and accumulating evidence suggests that genetic variants may contribute to such differential responses.8–10.
Neuropeptide Y gene (NPY) is widely expressed in the peripheral and central nervous systems11–12 and involved in diverse physiological functions including BP regulation.13–15 Previous studies showed that plasma NPY levels correlated with the BP levels16 and were elevated in hypertensive patients.17 In addition, NPY levels are modulated by dietary factors, especially, dietary fat.18–20
Recently, a functional single nucleotide polymorphism (SNP) in the promoter region of NPY, rs16147 (C-399T), was found to be related to risks for early-onset atherosclerosis21 and ischemic stroke,22–23 and showed allele-specific effects on NPY gene expression and NPY peptide level.21, 24–25 However, no study has examined the effect of this functional genotype and its interaction with dietary factors on BP.
In this study, we aimed to investigate whether NPY rs16147 genotype modulated the effects of weight-loss diet varying in macronutrients on changes of BP phenotypes in a 2-year randomized intervention trial. In addition to systolic blood pressure (SBP), diastolic blood pressure (DBP), we also assessed pulse pressure (PP, the difference between SBP and DBP), a measure of central arterial stiffness and a predictor of cardiovascular mortality, and mean arterial pressure (MAP), a weighted average of SBP and DBP. Both PP and MAP are predictive of hypertension and cardiovascular disease.26–27
Methods
Study Population
The Pounds Lost Trial was conducted from October 2004 through December 2007 at two sites: Harvard School of Public Health and Brigham & Women’s Hospital in Boston, Massachusetts; the Pennington Biomedical Research Center of Louisiana State University System, Baton Rouge, Louisiana. The design and sample collection have been described previously in detail.28 Briefly, the study population was composed of 811 overweight or obese participants aged 30 to 70 years and had a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) of 25 to 40. Major criteria for exclusion were the presence of diabetes treated with oral medications or insulin, or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation as assessed by interview and questionnaire. Individuals with type 2 diabetes controlled with diet, or with hypertension or hyperlipidemia treated with diet or drugs, were eligible to participate. The participants were randomly assigned to one of four diets constituting a two-by-two factorial design; the target percentages of energy derived from fat, protein, and carbohydrate in the four diets were 20, 15 and 65%; 20, 25 and 55%; 40, 15, and 45%; 40, 25 and 35%. After 2 years, 645 (80% of total population) participants completed the trial. The study was approved by the human subjects committee at each institution and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Measurements
Body weight and waist circumference were measured in the morning before breakfast on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months. Dietary intake was assessed in a random sample of 50% of the participants, by a review of the 5-day diet record at baseline and by 24-hour recall during a telephone interview on 3 nonconsecutive days at 6 months and at 2 years.28 Biomarkers of nutrient intake were used to validate self-reported adherence to macronutrient targets as follows: HDL cholesterol for carbohydrate, urinary nitrogen excretion for protein, and respiratory quotient for fat.28–29 Blood pressure was measured on two days at baseline and at 6, 12, and 24 months, by automated device (Omron HeathCare, IntelliSense Professional Digital Blood Pressure Monitor, HEM907XL), by methods established in other large NIH trials.30 The calibration was evaluated at regular intervals using a mercury manometer. PP was calculated as SBP minus DBP; and MAP was determined by using the formula: 1/3SBP + 2/3DBP.26
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood Kit (Qiagen, Chatsworth, CA). SNP NPY rs16147 was genotyped successfully in 723 total participants with available DNA samples using the OpenArray™ SNP Genotyping System (BioTrove, Woburn, MA). The genotype success rate was 99%. Replicated quality control samples (10%) were included in every genotyping plate with greater than 99% concordance.31
Statistical Analysis
The primary outcomes were changes in 4 BP phenotypes, including SBP, DBP, PP and MAP, during the intervention. Because previous studies have shown that NPY was sensitive to dietary fat,18–20 we therefore compared low-fat (20%) versus high-fat (40%) diets in the primary analysis, and compared average-protein (15%) versus high-protein (25%) diets in secondary analysis. We also performed stratified analysis according to baseline hypertension status. The Hardy-Weinberg equilibrium and comparison of categorical variables were assessed with χ2 test. Differences in continuous variables at baseline were tested using general linear models, with adjustment for age, sex and ethnicity. The main effects of genotype and diet intervention on 2-year changes of BP phenotypes were analyzed using general linear regression models, with adjustment for covariates including age, sex, ethnicity, baseline BMI, baseline value for respective BP phenotypes, antihypertensive medication use and weight loss. We excluded individuals with missing measures at each time point in the analysis. Moreover, to analyze the potential interactions between genotype and diet intervention, an interaction product term of genotype-diet was included in the model. Linear mixed models, using time as a repeated measurement factor, were used to test genetic associations with the trajectory of changes in outcomes according to diet intervention during the 2 years of follow-up by including genotype-time interaction terms. Additive genetic models were analyzed for genotype, following the previous reports.32–33 As the majority of the study population were white (80%), similar analyses were repeated in white participants. We used Quanto 1.2.4 software (http://hydra.usc.edu/gxe; University of Southern California, LosAngeles, CA) to estimate the detectable interaction effects of genotype by diet intervention under an additive model. The study had 80% power to detect the gene-diet interaction by accounting for 6.9 mmHg change in SBP, 4.6 mmHg change in DBP, 4.0 mmHg change in PP, and 5.2 mmHg change in MAP for hypertensive subjects at 2 years at a significance level of 0.05. All reported P-values were two-sided and a P-value of 0.05 was considered statistically significant. All data were analyzed with SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of Study Population
The distribution of NPY rs16147 genotype was in Hardy-Weinberg equilibrium in the study sample and the different ethnic groups (P>0.10), and the minor allele frequency (MAF) was 0.48 in total participants. The genotype frequencies were significantly different among ethnicity (P=0.005). No significant differences across the genotype were observed in weight, BMI, any of the BP phenotypes, the prevalence of hypertension and biomarkers of adherence (urinary nitrogen, respiratory quotient and HDL cholesterol) at baseline, though the genotype was related to age (Table 1).
Table 1.
Baseline Characteristics of the Study Participants According to NPY rs16147 Genotypes*
| Characteristics | TT (n=203) |
TC (n=341) |
CC (n=179) |
P† |
|---|---|---|---|---|
| Age, yr | 52.5 ± 8.7 | 50.6 ± 9.4 | 49.9 ± 9.3 | 0.0004 |
| Sex, n (%) | 0.657 | |||
| Female | 126 (28.6) | 211 (47.8) | 104 (23.6) | |
| Male | 77 (27.3) | 130 (46.1) | 75 (26.6) | |
| Race or ethnic, n (%) | 0.005 | |||
| White | 146 (25.4) | 274 (47.6) | 155 (27.0) | |
| Black | 44 (39.3) | 53 (47.3) | 15 (13.4) | |
| Hispanic or other | 13 (38.9) | 14 (38.9) | 9 (25.0) | |
| Weight, kg | 93.2 ± 6.2 | 93.2 ± 15.3 | 93.4 ± 15.3 | 0.635 |
| BMI, kg/m2 | 32.8 ± 4.1 | 32.7 ± 3.7 | 32.4 ± 4.0 | 0.328 |
| Waist circumference, cm | 104.0 ± 13.4 | 103.7 ± 12.7 | 103.0 ± 13.2 | 0.237 |
| BP, mmHg | ||||
| SBP | 120.5 ± 13.9 | 119.8 ± 13.3 | 119.0 ± 13.3 | 0.970 |
| DBP | 75.6 ± 9.4 | 75.8 ± 9.6 | 75.7 ± 9.2 | 0.705 |
| PP | 44.9 ± 9.3 | 44.0 ± 8.7 | 43.3 ± 7.6 | 0.611 |
| MAP | 90.6 ± 10.2 | 90.5 ± 10.1 | 90.1 ± 10.2 | 0.824 |
| Hypertension | 80 (39.4) | 129 (37.8) | 55 (30.7) | 0.167 |
| Antihypertensive medication use | 65 (32.0) | 101 (29.6) | 44 (24.6) | 0.265 |
| Biomarkers of adherence | ||||
| Urinary nitrogen, g | 12.3 ± 4.7 | 11.8 ± 4.1 | 12.5 ± 4.5 | 0.804 |
| Respiratory quotient | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.945 |
| HDL cholesterol, mg/dL | 49.5 ± 13.2 | 48.5 ± 13.6 | 48.0 ± 14.2 | 0.579 |
Data are n (%), means ± SD.
P-values were calculated by χ2 test for categorical variables, and multivariate analysis of covariance for continuous variables after adjusted for age, sex and ethnicity. SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: Pulse pressure; MAP: mean arterial pressure.
Effect of NPY rs16147 Genotype on 2-Year Changes in BP Response to Dietary Fat
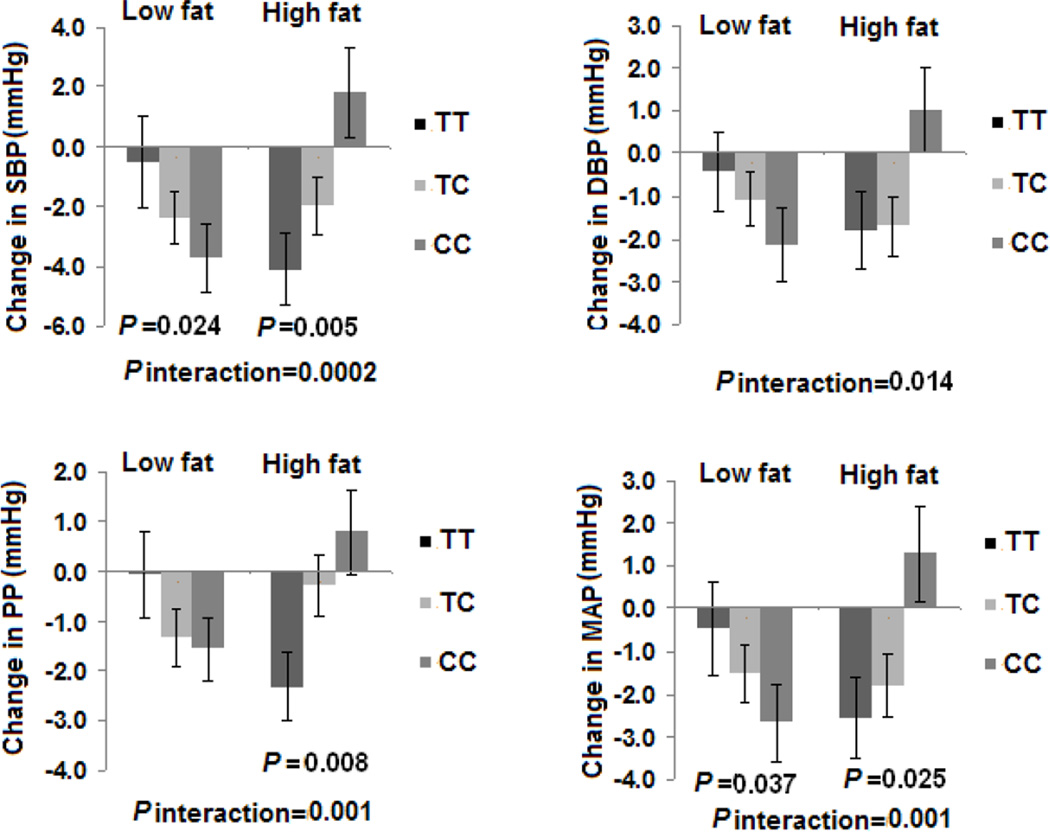
In all the participants, statistically significant and consistent interactions between rs16147 genotype and dietary fat intake were found on changes in SBP, DBP, PP and MAP during 2-year intervention after multivariate adjustment for potential confounders including age, sex, ethnicity, baseline BMI, baseline value for respective BP phenotypes, antihypertensive medication use and weight loss (P for interactions=0.0002, 0.014, 0.001 and 0.001, respectively) (Figure 1). In the adjusted model, the C allele was associated with a greater decrease in 2 BP phenotypes’ (SBP and MAP) responses to low-fat diet intake and an increase in 3 BP phenotypes’ (SBP, PP and MAP) in the high-fat group (all P<0.05) (Figure 1).
Figure 1.
Effects of NPY rs16147 genotype and fat intervention on changes in BP at 2 years in all the participants. SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: Pulse pressure; MAP: mean arterial pressure. Data included 75 and 82 (TT), 127 and 125 (TC), and 74 and 61 (CC) participants at the low fat group and the high fat group at 2 years (total n=544). P values are adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective phenotypes, antihypertensive medication use and weight loss.
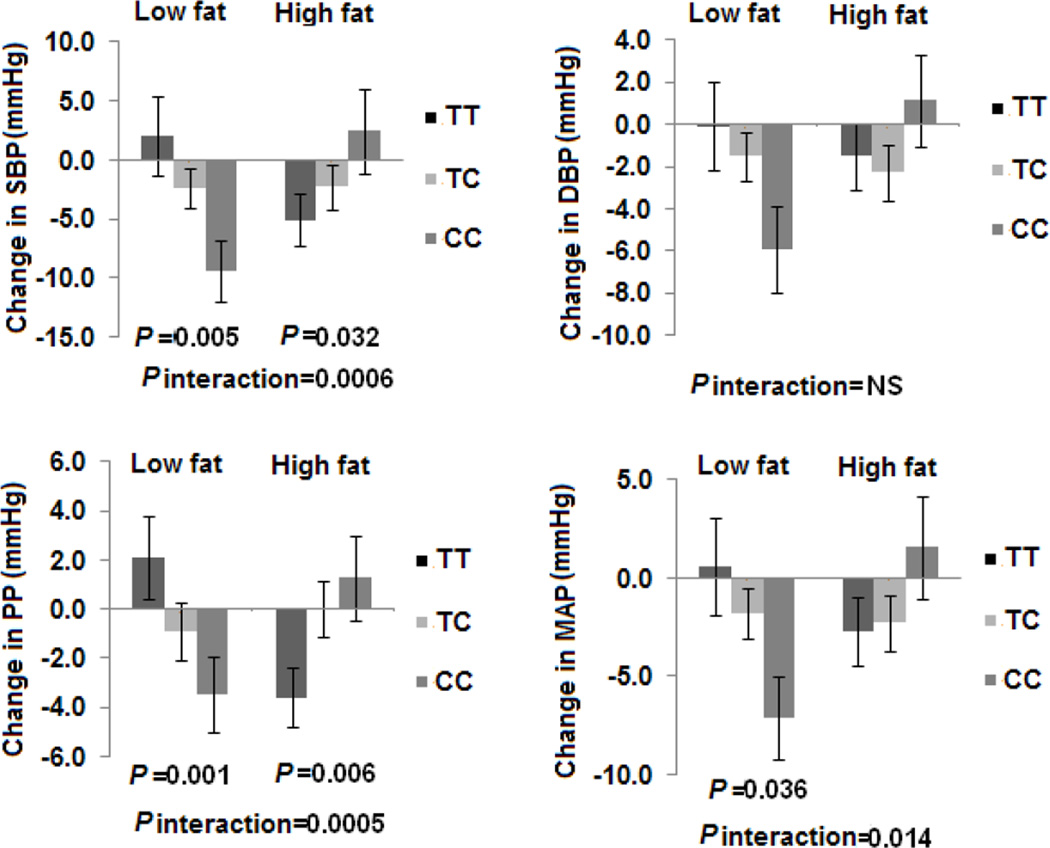
We next performed secondary analysis to test whether NPY rs16147 had different modulation on BP response to dietary fat intake in subgroups with (N=264) and without hypertension (N=459). In participants with hypertension, we observed significant gene-diet interactions on SBP, PP and MAP using the same statistical models (P for interactions=0.0006, 0.0005 and 0.014, respectively) (Figure 2). In the low-fat group, the risk allele (C allele) was associated with greater reduction in 3 BP phenotypes (SBP, PP and MAP). Conversely, the C allele was associated with increase in 2 BP phenotypes (SBP and PP) in the high-fat group. In the non-hypertensive subjects, we did not find any significant interactions between rs16147 genotype and dietary fat intake and genetic effects on any of BP phenotypes (all P>0.05). In addition, the C allele had greater changes in the 4 BP traits in hypertension group compared to non-hypertension group in response to both low-fat diet and high-fat diet (S1). When consuming low-fat diets, the C allele was associated with a 5.1 mmHg greater reduction of SBP in subjects with hypertension, but only with a 0.2 mmHg greater reduction of SBP in non-hypertensive subjects. Similarly less reduction of DBP, PP and MAP was found in the non-hypertension group compared to the hypertension group.
Figure 2.
Effects of NPY rs16147 genotype and fat intervention on changes in BP at 2 years in hypertension participants. SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: Pulse pressure; MAP: mean arterial pressure. Data included 29 and 33 (TT), 51 and 51 (TC), and 18 and 21 (CC) participants at the low fat group and the high fat group at 2 years (total n=203). P values are adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective phenotypes, antihypertensive medication use and weight loss.
At 2 years, no significant genetic effects and interactions were observed on changes in body weight (all P>0.05, S1 and S-Figure 1).
We did not find significant interactions between this NPY variant and dietary protein intake. Similar results were found when the analyses were restricted to the white participants.
The Trajectory of Changes in BP Phenotypes by the NPY rs16147 Genotype in Response to Dietary Fat
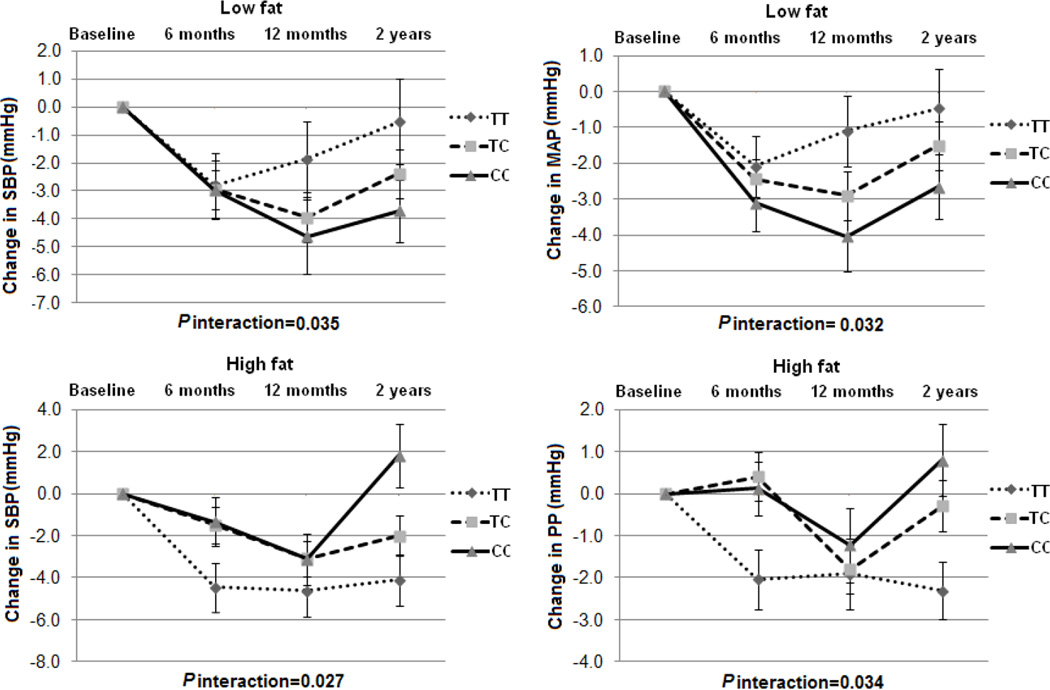
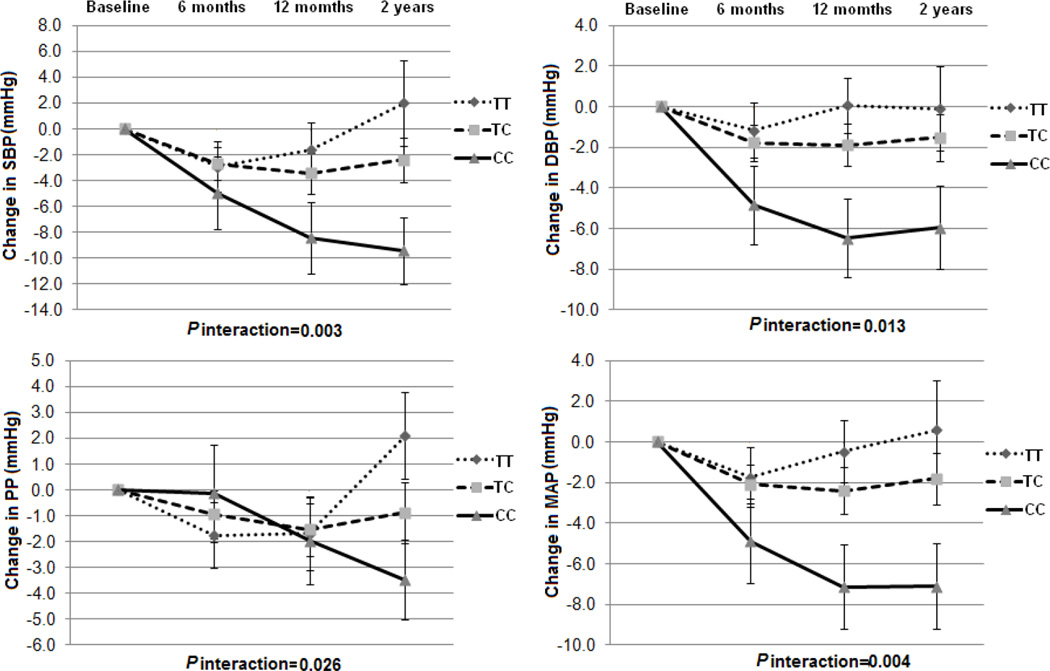
We further examined the dynamic pattern of changes in BP phenotypes by NPY genotype during 2-year intervention. In all the participants, we observed significant genotype-time interactions on changes in SBP and MAP response to low-fat diet (P for interactions=0.035 and 0.032), and changes in SBP and PP response to high-fat diet (P for interactions=0.027 and 0.034) (Figure 3). In the participants with hypertension, we observed significant and consistent genotype-time interactions on changes in all the 4 BP phenotypes in the low-fat group (P for interactions=0.003, 0.013, 0.026 and 0.004, respectively), but not in the high-fat group (Figure 4). In addition, in the low-fat group, the C allele was associated with sustained improvement in 2 BP phenotypes (SBP and MAP) in all the participants and 4 BP phenotypes in the participants with hypertension across 2-year intervention (Figure 3 and 4). Consistent with the findings presented above, the genotype-time interactions on changes in BP were not significant in the non-hypertensive subjects. No significant genotype-time interaction on weight change in each subgroup was found over time (S-Figure2). The similar dynamic patterns of the genotype effects on BP phenotypes were observed in the white population.
Figure 3.
Changes in BP in the low-fat and high-fat diet intervention group according to NPY rs16147 genotype from baseline to 6 months, 12 months and 2 years in all the participants. SBP: systolic blood pressure; PP: Pulse pressure; MAP: mean arterial pressure. Data included 358 and 365, 319 and 323, 290 and 292, and 276 and 268 in the low-fat and high-fat diet group at baseline, 6 months, 12 months and 2 years. P values are adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective phenotypes, antihypertensive medication use and weight loss.
Figure 4.
Changes in BP in the low fat diet intervention group according to NPY rs16147 genotype from baseline to 6 months, 12 months and 2 years in hypertensive participants. SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: Pulse pressure; MAP: mean arterial pressure. Data included 127, 118, 111 and 98 at baseline, 6 months, 12 months and 2 years. P values are adjustment for age, sex, ethnicity, baseline BMI, baseline values for respective phenotypes, antihypertensive medication use and weight loss.
Discussion
In the 2-year randomized weight-loss intervention trial, we observed significant and consistent interactions between the NPY rs16147 SNP and dietary fat intake in relation to changes in multiple BP phenotypes. Interestingly, such gene-diet interactions only appeared in hypertensive patients, but not in non-hypertensive subjects. Carriers of the C allele exhibited significantly greater reduction in BP phenotypes when consuming low-fat diet but showed greater increase in BP phenotypes in response to high-fat diet intake at 2 years.
To the best of our knowledge, this is the first study examining the interactions between NPY genetic variant and dietary fat intake in relation to long-term changes of BP phenotypes. Our findings are in concordance with the functional roles of NPY in the regulation of BP.13, 34 The SNP rs16147 (C-485T) is a functional variant located in the NPY gene promoter region and in linkage disequilibrium (LD) with other NPY SNPs, which were associated with early-onset coronary artery disease in previous study.21 Consistent evidence has shown an allele-specific effect of rs16147 on NPY expression and the SNP accounts for the majority of the variation in expression in vivo,24–25 which was correlated with plasma NPY peptide levels.25 The NPY SNP has been previously related to several BP related conditions, such as early-onset atherosclerosis21 and ischemic stroke.22–23 Findings from the present study provide further support for the significant association between the NPY genetic variant and BP regulation. Therefore, we can speculate that rs16147 polymorphism might affect the transcription efficiency and expression of NPY gene, resulting in changes either in systemic or local levels of NPY, which can induce vasoconstriction and stimulate vascular smooth muscle cell proliferation and angiogenesis, as well as stimulate sympathetic nervous system activity, contributing to increase BP level.34–35
Several previous studies have demonstrated that NPY pathways in the hypothalamus were responsive to the amounts of fat present in the ingested diet.36–37 In animal studies, long-term feeding of high-fat diets led to decreased NPY levels in hypothalamus,18–19 whereas feeding of low-fat diets led to an increased NPY gene expression in hypothalamus.20 These studies provide fundamental evidence for the potential mechanisms underlying the observed gene-dietary fat interaction in relation to changes in BP. Our results showed that carriers of the C allele exhibited opposite effects on BP changes in response to low- and high-dietary fat intakes, which are in line with recently-proposed hypothesis of ‘differential susceptibility’.20, 36 The hypothesis suggests that vulnerability genes or risk alleles may function more like plasticity genes, thereby rendering some individuals more responsive to environmental influences than others where genetic risk is either attenuated by a favorable environment or amplified by an adverse environment.20, 36, 38 Consistent with this idea, our data supported that the risk allele (C allele) may act as either a protective or a detrimental factor, depending on the differential exposure to dietary fat intake.
Another intriguing finding from the present study is that the gene-diet interaction was only observed in participants with hypertension, and the risk allele had remarkably greater changes in 4 BP phenotypes in hypertensive patients than those without hypertension in response to low-fat or high-fat diet interventions. The results suggest that the initial BP levels might affect the NPY genetic modulation on subsequent BP changes by a diet intervention. These findings indicate that low-fat diets might benefit more on those with the C allele and high BP. In contrast, when consuming high-fat diet, carriers of the C allele with hypertension had more unfavorable affect on BP.
There are several potential limitations that warrant consideration. First, we only analyzed one SNP rs16147. However, this SNP is a functional variant with high frequency and in LD with several previously reported variants related to cardiovascular disease.21 Our sample size rendered us insufficient power to test gene-diet interactions for rare variants. Thus, we did not include low-frequency SNPs such as rs16139 which has also been previously reported.39–40 In addition, although our study is thus far the largest and longest diet intervention trial on changes in BP, the size may be relatively small to detect small genetic effects or gene-diet interactions. Furthermore, it is difficult to tease out which macronutrient is responsible for the observed interactions because diet fat is correlated with other macronutrients such as carbohydrates. Finally, as the majority of the participants in the present study are whites and of a specific BMI range, the generalizability of our findings to other minority groups, or the general population with normal range of body weight need to be further verified.
In conclusion, we found that genetic variation within the NPY promoter region might modulate long-term changes of BP in response to weight-loss diet intervention varying in fat among overweight or obese subjects. Individuals with the C allele showed a greater reduction in BP in response to low-fat diet but more increase in BP in response to high-fat diet; and such interactions only presented in hypertensive subjects. These findings may provide novel information on the development of effective diet intervention in lowering BP levels in high-risk populations.
Perspectives
Neuropeptide Y (NPY) is implicated in the regulation of blood pressure (BP) and NPY pathways in the hypothalamus are sensitive to dietary fat. A functional variant rs16147 located in the promoter region of NPY gene was found to influence NPY gene expression, NPY levels, BP and cardiovascular risk. We evaluated the potential effect of variant rs16147 on the association between 2-year weight-loss diet intervention and changes in multiple BP measures in 723 obese patients from the randomized Pounds Lost Trial. Our results indicate that individuals with the C allele (the risk allele for high BP) might significantly benefit from low-fat diet intake in long-term reduction of BP phenotypes; and such benefits were more evident in hypertensive subjects. These findings may provide novel information to the development of personalized, more effective diet intervention based on genetic background in prevention of hypertension.
Supplementary Material
Novelty and Significance.
This is the first study to date to assess the interactions between a functional variant in Neuropeptide Y (NPY) gene and popular weight-loss diets on changes in multiple BP measures in the largest and longest randomized intervention trial. We found the individuals with the risk allele of variant rs16467 might obtain more benefits from low-fat diet intake in long-term reduction of BP phenotypes; and such benefits were more evident in hypertensive subjects. These novel findings provide supportive evidence on the development of personalized diet intervention in prevention of hypertension based on genetic background.
Acknowledgment
We are particularly grateful to all participants in the trial for their dedication and contribution to the research.
Sources of Funding
This study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981), the General Clinical Research Center (RR-02635), the Boston Obesity Nutrition Research Center (DK46200) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718). Dr. Lu Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). Dr. Xiaomin Zhang was supported by the National Natural Science Foundation of China (NNSFC 30972453) and Program for New Century Excellent Talents in University (NCET-10-0420).
Footnotes
Disclosures
None.
References
- 1.MacMahon S, Cutler J, Brittain E, Higgins M. Obesity and hypertension: Epidemiological and clinical issues. Eur Heart J. 1987;8(Suppl B):57–70. doi: 10.1093/eurheartj/8.suppl_b.57. [DOI] [PubMed] [Google Scholar]
- 2.Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: A systematic review. Hypertension. 2005;45:1035–1041. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 4.Horvath K, Jeitler K, Siering U, Stich AK, Skipka G, Gratzer TW, Siebenhofer A. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch Intern Med. 2008;168:571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 5.Siebenhofer A, Jeitler K, Berghold A, Waltering A, Hemkens LG, Semlitsch T, Pachler C, Strametz R, Horvath K. Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database Syst Rev. 2011;9 doi: 10.1002/14651858.CD008274.pub2. CD008274. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Okura T, Tanaka K, Nakanishi T, Lee DJ, Nakata Y, Wee SW, Shimokata H. Effects of obesity phenotype on coronary heart disease risk factors in response to weight loss. Obes Res. 2002;10:757–766. doi: 10.1038/oby.2002.103. [DOI] [PubMed] [Google Scholar]
- 8.Kelly TN, Rice TK, Gu D, Hixson JE, Chen J, Liu D, Jaquish CE, Bazzano LA, Hu D, Ma J, Gu CC, Huang J, Hamm LL, He J. Novel genetic variants in the alpha-adducin and guanine nucleotide binding protein beta-polypeptide 3 genes and salt sensitivity of blood pressure. Am J Hypertens. 2009;22:985–992. doi: 10.1038/ajh.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufficy L, Naumovski N, Ng X, Blades B, Yates Z, Travers C, Lewis P, Sturm J, Veysey M, Roach PD, Lucock MD. G80a reduced folate carrier snp influences the absorption and cellular translocation of dietary folate and its association with blood pressure in an elderly population. Life Sci. 2006;79:957–966. doi: 10.1016/j.lfs.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Schorr U, Beige J, Ringel J, Turan S, Kreutz R, Distler A, Sharma AM. Hpa ii polymorphism of the atrial natriuretic peptide gene and the blood pressure response to salt intake in normotensive men. J Hypertens. 1997;15:715–718. doi: 10.1097/00004872-199715070-00002. [DOI] [PubMed] [Google Scholar]
- 11.Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM. Neuropeptide y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 12.Gray TS, Morley JE. Neuropeptide y: Anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- 13.Michel MC, Rascher W. Neuropeptide y: A possible role in hypertension? J Hypertens. 1995;13:385–395. [PubMed] [Google Scholar]
- 14.Coelho EF, Ferrari MF, Maximino JR, Fior-Chadi DR. Change in the expression of npy receptor subtypes y1 and y2 in central and peripheral neurons related to the control of blood pressure in rats following experimental hypertension. Neuropeptides. 2004;38:77–82. doi: 10.1016/j.npep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Michalkiewicz M, Zhao G, Jia Z, Michalkiewicz T, Racadio MJ. Central neuropeptide y signaling ameliorates n(omega)-nitro-l-arginine methyl ester hypertension in the rat through a y1 receptor mechanism. Hypertension. 2005;45:780–785. doi: 10.1161/01.HYP.0000153953.69799.f2. [DOI] [PubMed] [Google Scholar]
- 16.Odar-Cederlof I, Ericsson F, Theodorsson E, Kjellstrand CM. Is neuropeptide y a contributor to volume-induced hypertension? Am J Kidney Dis. 1998;31:803–808. doi: 10.1016/s0272-6386(98)70049-6. [DOI] [PubMed] [Google Scholar]
- 17.Wocial B, Ignatowska-Switalska H, Pruszczyk P, Jedrusik P, Januszewicz A, Lapinski M, Januszewicz W, Zukowska-Grojec Z. Plasma neuropeptide y and catecholamines in women and men with essential hypertension. Blood Press. 1995;4:143–147. doi: 10.3109/08037059509077586. [DOI] [PubMed] [Google Scholar]
- 18.Stricker-Krongrad A, Cumin F, Burlet C, Beck B. Hypothalamic neuropeptide y and plasma leptin after long-term high-fat feeding in the rat. Neurosci Lett. 1998;254:157–160. doi: 10.1016/s0304-3940(98)00678-8. [DOI] [PubMed] [Google Scholar]
- 19.Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: Long-term effects on blood pressure, brain neuropeptide y, and adiposity markers. Am J Physiol Endocrinol Metab. 2005;288:E1236–E1243. doi: 10.1152/ajpendo.00505.2004. [DOI] [PubMed] [Google Scholar]
- 20.Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 21.Shah SH, Freedman NJ, Zhang L, Crosslin DR, Stone DH, Haynes C, Johnson J, Nelson S, Wang L, Connelly JJ, Muehlbauer M, Ginsburg GS, Crossman DC, Jones CJ, Vance J, Sketch MH, Granger CB, Newgard CB, Gregory SG, Goldschmidt-Clermont PJ, Kraus WE, Hauser ER. Neuropeptide y gene polymorphisms confer risk of early-onset atherosclerosis. PLoS Genet. 2009;5:e1000318. doi: 10.1371/journal.pgen.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim NS, Oh SM, Ko MM, Cha MH, Kang BK, Bang OS. Association of the c-399t promoter polymorphism of neuropeptide y with susceptibility to ischemic stroke. Clin Biochem. 2009;42:1699–1704. doi: 10.1016/j.clinbiochem.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Yu JT, Yu NN, Gao SS, Song JH, Ma T, Wang ND, Tang YC, Zhang N, Tan L. Neuropeptide y polymorphisms and ischemic stroke in chinese population. Clin Chim Acta. 2010;411:242–245. doi: 10.1016/j.cca.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Buckland PR, Hoogendoorn B, Guy CA, Coleman SL, Smith SK, Buxbaum JD, Haroutunian V, O'Donovan MC. A high proportion of polymorphisms in the promoters of brain expressed genes influences transcriptional activity. Biochim Biophys Acta. 2004;1690:238–249. doi: 10.1016/j.bbadis.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human npy expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 27.Wain LV, Verwoert GC, O'Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dorr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimaki T, Kuhnel B, Lopez LM, Polasek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Volker U, Volzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sober S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O'Donnell CJ, Salomaa V, d'Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco MF, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kahonen M, Viikari J, Doring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, Konig IR, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, Mitchell GF, Smith NL, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, Laranjo NM, Sacks FM, Smith SR. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: Results from the pounds lost trial. Am J Clin Nutr. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, carbohydrate intake on blood pressure and serum lipids: Results of the omniheart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 31.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: The preventing overweight using novel dietary strategies (pounds lost) trial. Circulation. 2011;124:563–571. doi: 10.1161/CIRCULATIONAHA.111.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sommer WH, Lidstrom J, Sun H, Passer D, Eskay R, Parker SC, Witt SH, Zimmermann US, Nieratschker V, Rietschel M, Margulies EH, Palkovits M, Laucht M, Heilig M. Human npy promoter variation rs16147:T>c as a moderator of prefrontal npy gene expression and negative affect. Hum Mutat. 2010;31:E1594–E1608. doi: 10.1002/humu.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witt SH, Buchmann AF, Blomeyer D, Nieratschker V, Treutlein J, Esser G, Schmidt MH, Bidlingmaier M, Wiedemann K, Rietschel M, Laucht M, Wust S, Zimmermann US. An interaction between a neuropeptide y gene polymorphism and early adversity modulates endocrine stress responses. Psychoneuroendocrinology. 2011;36:1010–1020. doi: 10.1016/j.psyneuen.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Baltatzi M, Hatzitolios A, Tziomalos K, Iliadis F, Zamboulis C. Neuropeptide y and alpha-melanocyte-stimulating hormone: Interaction in obesity and possible role in the development of hypertension. Int J Clin Pract. 2008;62:1432–1440. doi: 10.1111/j.1742-1241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 35.Pons J, Lee EW, Li L, Kitlinska J. Neuropeptide y: Multiple receptors and multiple roles in cardiovascular diseases. Curr Opin Investig Drugs. 2004;5:957–962. [PubMed] [Google Scholar]
- 36.Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baltazi M, Katsiki N, Savopoulos C, Iliadis F, Koliakos G, Hatzitolios AI. Plasma neuropeptide y (npy) and alpha-melanocyte stimulating hormone (a-msh) levels in patients with or without hypertension and/or obesity: A pilot study. Am J Cardiovasc Dis. 2011;1:48–59. [PMC free article] [PubMed] [Google Scholar]
- 38.Ronald L, Simons MKL, Steven R. H. Beach, Gene H. Brody, Robert A. Philibert and Frederick X. Gibbons. Social environment, genes, and aggression : Evidence supporting the differential susceptibility perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karvonen MK, Valkonen VP, Lakka TA, Salonen R, Koulu M, Pesonen U, Tuomainen TP, Kauhanen J, Nyyssonen K, Lakka HM, Uusitupa MI, Salonen JT. Leucine7 to proline7 polymorphism in the preproneuropeptide y is associated with the progression of carotid atherosclerosis, blood pressure and serum lipids in finnish men. Atherosclerosis. 2001;159:145–151. doi: 10.1016/s0021-9150(01)00468-3. [DOI] [PubMed] [Google Scholar]
- 40.Ilveskoski E, Viiri LE, Mikkelsson J, Porsti I, Lehtimaki T, Karhunen PJ. Neuropeptide y signal peptide pro7 substitution protects against coronary artery atherosclerosis: The helsinki sudden death study. Atherosclerosis. 2008;199:445–450. doi: 10.1016/j.atherosclerosis.2007.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.