Abstract
The Odd-skipped related 1 (Odd1) gene encodes a zinc finger protein homologous to the Drosophila Odd-skipped class transcription factors that play critical roles in embryonic patterning and tissue morphogenesis. We have generated mice carrying a targeted null mutation in the Odd1 gene and show that Odd1 is essential for heart and intermediate mesoderm development. Odd1−/− mutant mouse embryos fail to form atrial septum, display dilated atria with hypoplastic venous valves, and exhibit blood backflow from the heart into systemic veins. In contrast to other transcription factors implicated in atrial septum development, Odd1 mRNA expression is restricted to the central dorsal domain of the atrial myocardium during normal heart development. Moreover, expression patterns of known key regulatory genes of atrial septum development, including Nkx2.5, Pitx2, Tbx5, are unaltered in the developing heart in Odd1−/− mutants compared to that of the wildtype littermates. Furthermore, homozygous Odd1−/− mutant embryos exhibit complete agenesis of adrenal glands, metanephric kidneys, gonads and defects in pericardium formation. Detailed molecular marker analyses show that key regulators of early intermediate mesoderm development, including Lhx1, Pax2, and Wt1, are all down-regulated and nephrogenic mesenchyme undergoes massive apoptosis, resulting in disruption of nephric duct elongation and failure of metanephric induction in the Odd1−/− mutant embryos. These data provide new insights into the molecular mechanisms underlying heart morphogenesis and urogenital development.
Keywords: adrenal gland, atrial septum, heart development, intermediate mesoderm, kidney development, Odd-skipped, Osr1, renal agenesis, venous valve
Introduction
The Drosophila Odd-skipped (odd) gene was initially identified as a pair-rule gene because mutations at this locus cause loss of portions of the odd-numbered segments in the embryo (Nusslein-Volhard and Wieschaus, 1980; Coulter and Wieschaus, 1988). The Odd gene encodes a putative transcription factor containing four C2H2-type zinc finger motifs (Coulter et al., 1990). Gene expression and phenotypic analyses indicate that the Odd protein can either activate or repress the expression of downstream segmentation genes during Drosophila embryogenesis (DiNardo and O’Farrell, 1987; Mullen and DiNardo, 1995; Saulier-Le Drean et al., 1998). Two other Drosophila genes, brother of odd with entrails limited (bowl) and sister of odd and bowl (sob), encoding proteins with highly similar zinc finger domains were isolated by sequence homology (Hart et al., 1996; Wang and Coulter, 1996). Mutations in bowl disrupt terminal segments and gut development during Drosophila embryogenesis (Wang and Coulter, 1996). A genetic screen for genes regulating Drosophila embryonic gut morphogenesis resulted in the identification of another gene with sequence homology to odd, named drumstick (drm), which is essential for proventriculus formation and hindgut elongation (Green et al., 2002). It was demonstrated that the tissue-specifically expressed Drm protein blocks the function of a globally expressed transcription factor Lines by direct protein-protein interaction through the Drm zinc finger domain, which relieves inhibition of Bowl by Lines and leads to nuclear accumulation of the active Bowl protein (Green et al., 2002; Hatini et al., 2005). Recently, it was shown that the Drm-Lines-Bowl pathway links antagonistic Hedgehog and Wingless signaling inputs to regulate epidermal cell differentiation (Hatini et al., 2005). These data indicate that the Odd-skipped family transcription factors play essential roles in Drosophila embryonic development, cell differentiation and tissue morphogenesis.
By searching Expressed Sequence Tags Database for genes homologous to the Drosophila Odd-skipped family zinc finger proteins, So and Danielian (1999) isolated the first vertebrate homolog of odd-skipped and named it odd-skipped related 1 (Osr1, renamed Odd1 - Mouse Genome Informatics database). Odd1 mRNA is expressed throughout the intermediate mesoderm at embryonic day (E) 8.5 during mouse embryogenesis and is activated in many other tissues, including the branchial arches and limb buds in later embryonic stages (So and Danielian, 1999). In mouse embryos lacking both Foxc1 and Foxc2, paraxial mesoderm is respecified to intermediate mesoderm and Odd1 mRNA expression is activated in this respecified tissue (Wilm et al., 2004), suggesting that Odd1 mRNA expression may be important for intermediate mesoderm development. We previously characterized Osr2, another mouse odd-skipped related gene encoding a zinc finger protein with 65% amino acid sequence identity throughout the protein and 98% identity in the zinc finger region with Odd1, and showed that Odd1 and Osr2 displayed distinct expression patterns during mouse embryogenesis (Lan et al., 2001). Recently, a targeted null mutation in the Osr2 gene was generated and characterized. The homozygous Osr2 null mutants exhibited open eyelids and specific defects in secondary palate development but had no phenotypic abnormalities in several tissues including limb and kidney, which normally express both Odd1 and Osr2 mRNAs (Lan et al., 2001; 2004). Here we report that mouse embryos homozygous for a targeted null mutation in Odd1 have specific defects in heart and kidney development. Our data identify Odd1 as a new essential regulator of heart and urogenital development.
Materials and Methods
Generation of Odd1tm1RJ mutant mice
BAC clones containing the Odd1 genomic region were isolated by screening the RPCI-22 129/SvEvTac mouse BAC library (BACPAC Resources, Children’s Hospital of Oakland, Oakland, CA). Two adjacent BamHI fragments containing all three exons of the Odd1 gene were subcloned from a BAC clone into the pBluescript II plasmid vectors (Fig. 1A). A targeting DNA construct, which contains a 2.1 kb 5’ homology arm fused in-frame with a modified bacterial lacZ gene that encodes a nuclearly localized β-galactosidase, a loxP-flanked neo expression cassette, a 5.3 kb 3’ homology arm, and a PGK-DTA expression cassette, was subcloned in the pBluescriptII KS(+) plasmid vector (Fig. 1A). Correct targeting of the Odd1 locus with this construct would result in the replacement of 335 bp of the Odd1 coding region with the lacZ gene, which is expected to produce a functional β-galactosidase fusion protein containing the N-terminal 16 amino acid residues of the Odd1 protein.
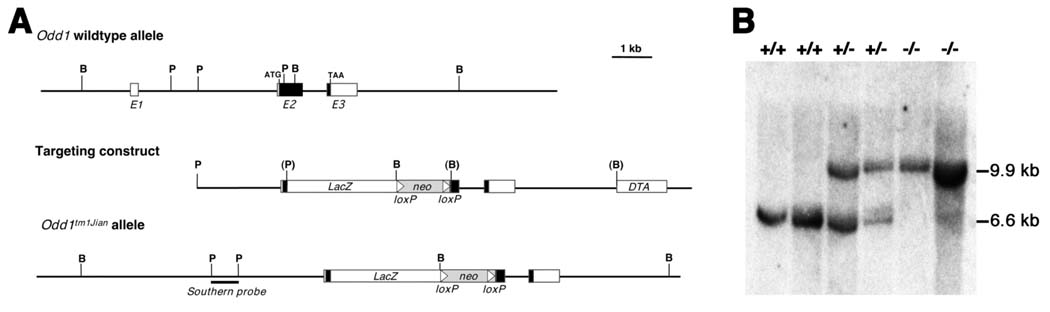
Fig. 1.
Targeted disruption of the mouse Odd1 gene. (A) The Odd1 gene consists of three exons spanning approximately 6 kb of genomic DNA. Boxes indicate exons, with the protein-coding region marked in black. The positions of the translation start (ATG) and stop (TAA) codons are also indicated. Restriction sites are: B, BamHI; P, PstI. The targeting vector used the 2.1 kb PstI fragment containing the intron 1/exon 2 junction as the 5’ arm and the 5.3 kb BamHI fragment located 335 bp downstream as the 3’ arm. A modified bacterial lacZ gene and a loxP-flanked neo expression cassette were inserted in between the arms and a diptheria toxin A (DTA) expression cassette was cloned 3’ to the 3’ arm for negative selection against random integration. (B) Southern hybridization analysis of E11.5 embryonic DNA samples from a litter of F1 heterozygous intercross. The genomic DNA samples were digested with BamHI, separated by electrophoresis through a 0.8% agarose gel, transferred onto a Zetaprobe nylon memberane (BioRad), and hybridized with random prime-labeled probes made from the 700 bp PstI fragment isolated from the Odd1 genomic region 5’ to the targeted region. The 6.6 kb BamHI fragment corresponding to the wildtype allele was detected in wildtype and heterozygous embryos, while the 9.9 kb mutant allele-specific fragment was only detected in heterozygous and homozygous mutants. +/+, wildtype; +/−, heterozygous; −/−, homozygous mutant.
The targeting vector was linearized and electroporated into CJ7 embryonic stem (ES) cells as previously described (Swiatek and Gridley, 1993). G418 resistant ES colonies were screened by Southern hybridization for homologous recombination. Correctly targeted ES clones were injected into blastocysts from C57BL/6J mice and the resultant chimeras bred with either C57BL/6J or ICR females. F1 mice were genotyped by Southern hybridization analysis of tail DNA. Mice and embryos from subsequent generations were genotyped by either Southern hybridization or PCR. PCR with Primer 1 (5’-GTC ATT CAC CCT AAG CCA GAG -3’) and Primer 2 (5’ GAA GCA TCA AGC CAA AGC AGC-3’) amplifies a product of 450 bp from the wild-type Odd1 allele. PCR with Primer 2 and Primer 3 (5’-CTT CTT GAC GAG TTC TTC TGA GG-3’) amplifies a mutant allele-specific product of 760 bp. Heterozygous F1 mice were backcrossed to C57BL/6J and ICR mice, respectively to maintain the mutant mice in two different genetic backgrounds. Heterozygous mice from the backcross generations were intercrossed for analysis of homozygous phenotype.
Histology, in situ hybridization and detection of β-galactosidase activity
Embryos were dissected and DNA was prepared from yolk sac or tail biopsy samples for genotyping by PCR or Southern hybridization. For histology, embryos were fixed in Bouin’s fixative, dehydrated through graded alcohols, embedded in paraffin, sectioned at 7 µm thickness, and stained with hematoxylin and eosin. For in situ hybridization, embryos were fixed overnight at 4°C in 4% paraformaldehyde in PBS. Whole mount and sectioned in situ hybridization were performed as described previously (Lan et al., 2001). Staining for β-galactosidase activity in whole mount embryos and cryostat sections was performed using X-gal as described previously (Hogan et al., 1994). The sections were counterstained with eosin following X-gal staining.
Detection of cell proliferation and apoptosis
For detection of cell proliferation in the intermediate mesoderm, timed pregnant Odd1tm1RJ/+ heterozygous females were injected intraperitoneally on gestational day 9.5 or 10.5 with BrdU (Roche) labeling reagent (3 mg/100g body weight). One hour after injection, embryos were dissected, fixed in Carnoy’s fixative, dehydrated through graded alcohols, embedded in paraffin and sectioned at 5 µm thickness. Immunodetection of BrdU was performed using the BrdU labeling and detection kit (Roche) according to manufacturer’s instructions. Apoptotic cell death in the intermediate mesoderm was assessed by TUNEL labeling of either paraffin sections or cryostat sections using the in situ cell death detection kit (Roche) following manufacturer’s instructions.
For detection of activated Caspase-3, paraffin sections were deparaffinized in xylene and hydrated through an ethanol series to distilled water. Antigen retrieval was carried out by boiling in Trilogy reagent (Cell Margue, hot Springs, AR) for 5 minutes, and peroxidase activity blocked in 3% H2O2 for 20 minutes followed by incubation in Histostain blocking reagent (Zymed laboratories) for 30 minutes. The sections were then incubated overnight with a monoclonal antibody specific for activated Caspase-3 (BD PharMingen) at 500× dilution in PBS containing 0.2% skim milk and 0.3% Triton X-100, followed by incubation with biotinylated anti-rabbit secondary antibody and streptavidin-HRP (Zymed Laboratories), followed by 200× dilution of biotinylated tyramide (Perkin Elmer) in PBS containing 0.05% Tween-20 and detected by Cy3-conjugated tyramide substrate (500× dilution, Perkin Elmer). Washes were performed with PBS containing 0.5% Tween-20 at each step after the primary antibody incubation. Counterstaining of nuclei was carried out with DAPI (1 µg/ml in water) for one minute. The sections were washed with PBS and mounted with 50% glycerol and 20 mg/ml n-propyl gallate for fluorescence microscopy.
Detection of blood flow
Embryos were dissected at E11.5 from timed pregnant Odd1tm1RJ/+ heterozygous females. Yellow casting dye (Connecticut Valley Biological Supply Co. Inc., South Hampton, MA) or Indian ink was injected into the left ventricle of freshly dissected embryos by using pulled glass needles. Injected embryos were immediately fixed in 4% paraformaldehyde overnight, dehydrated in graded methanols, cleared in benzyl benzoate and benzyl alcohol (2:1) and photographed. Genotyping was carried out by allele-specific PCR using yolk sac genomic DNA as templates.
Results
Generation of Odd1tm1RJ mutant mice
To examine the function of the Odd1 gene in mouse embryonic development, we inserted a lacZ gene into the first coding exon of the Odd1 gene through homologous recombination in ES cells (Fig. 1A). The targeted allele, named Odd1tm1RJ, is expected to encode a functional β-galactosidase fusion protein containing the N-terminal 16 amino acid residues of the Odd1 protein and result in loss of Odd1 gene function. Chimeric animals were generated from correctly targeted ES cells and germ line transmission of the targeted Odd1tm1RJ allele was obtained. Southern hybridization analysis confirmed the expected structure of the targeted allele in mutant mice (Fig. 1B). Embryos heterozygous (Odd1+/−) for the Odd1tm1RJ allele were isolated and stained with X-gal to reveal domains of β-galactosidase activity. As shown in Fig. 2, β-galatosidase activity was detected specifically in the intermediate mesoderm cells at E7.5, shortly after they migrated away from the primitive streak (Fig. 2A). At E8.5, β-galactosidase expression is detected throughout the intermediate mesoderm caudal to the cardiac crescent (Fig. 2B). This intermediate mesoderm-specific expression of β-galactosidase activity in the Odd1+/− embryos is identical to the Odd1 mRNA expression pattern at this stage (Fig. 2C, also see So and Danielian, 1999). By E9.5, expression of the β-galactosidase fusion protein in the caudal intermediate mesoderm is expanded to the somitic mesoderm (Fig. 2D, E). In addition, strong β-galactosidase activity is detected in the fore- and hindgut endoderm, in the lung bud mesenchyme as well as in the myocardial cells in the dorsal wall of the common atrial chamber at this stage (Fig. 2E, F). These expression patterns of the β-galactosidase fusion protein in the Odd1+/− embryos exactly match that of the Odd1 mRNA detected by in situ hybridization of embryo sections (Fig. 6 and data not shown). By E10.5, expression of both Odd1 mRNA and β-galactosidase fusion protein is activated in the developing branchial arches and limb buds in the heterozygous embryos (Fig. 2G, H). These data indicate that expression of the β-galactosidase fusion protein from the Odd1tm1RJ allele recapitulates the wild-type Odd1 gene expression pattern and provides a good reporter of spatiotemporal Odd1 gene activity during mouse embryogenesis.
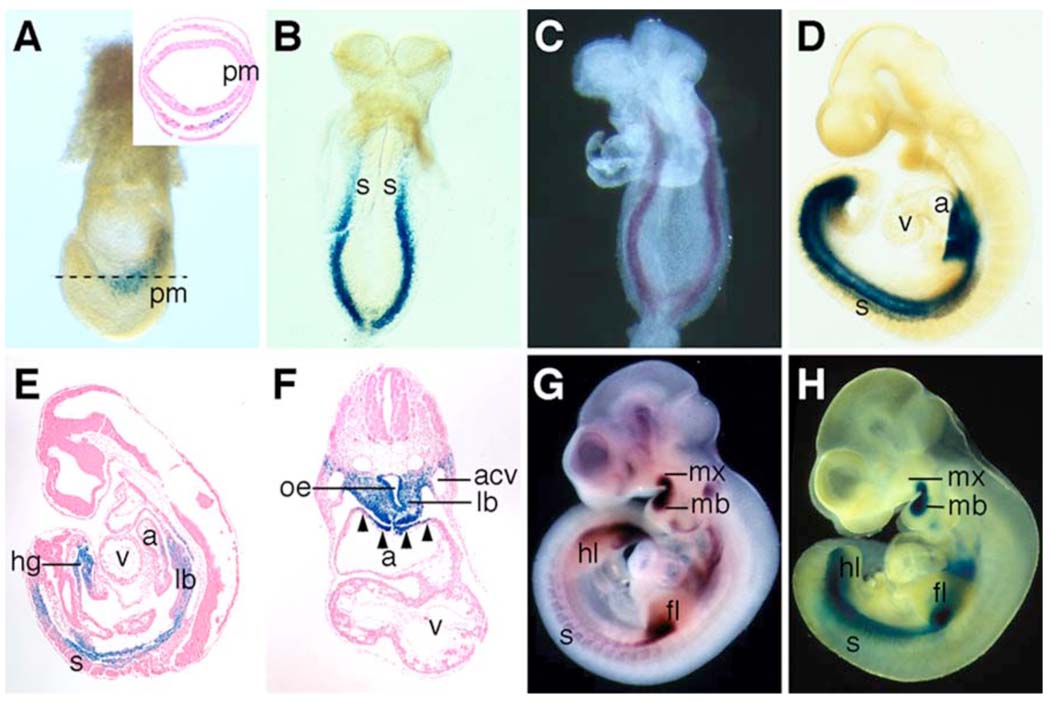
Fig. 2.
β-galactosidase fusion protein expression from the Odd1tm1Jian allele recapitulates Odd1 mRNA expression pattern during mouse embryogenesis. (A) Whole mount X-gal staining shows strong Odd1-LacZ expression (blue color) in a 7.5 dpc mouse embryo. The inset is a transverse section of a X-gal stained E7.5 embryo, which shows Odd1-LacZ expression in a few cells mixed with non-expressing cells in the intermediate region of the mesoderm layer. No staining is detected in the primitive streak cells. (B) At E8.5, Odd1-LacZ is expressed throughout the intermediate mesoderm caudal to the cardiac cresent. The expression pattern is identical to Odd1 mRNA expression pattern at this stage (C). (D) By E9.5, Odd1-LacZ expression is detected, in addition to the intermediate mesoderm, at the dorsal region of the atrium and in the caudal somites. (E,F) Sagittal and transverse sections, respectively of E9.5 heterozygous embryos stained with X-gal. Strong Odd1-LacZ expression is detected in the lung bud mesenchyme, the fore- and hindgut endoderm, and in the dorsal atrial wall. (G,H) By E10.5, both Odd1-LacZ (G) and Odd1 mRNA are expressed in identical patterns in the first branchial arches, limb buds, and somites. a, atrium; acv, anterior cardinal vein; fl, forelimb bud; hg, hindgut; hl, hindlimb bud; lb, lung bud; mb, mandibular process; mx, maxillary process; oe, oesophagous; pm, primitive streak; s, somite; v, ventricle.
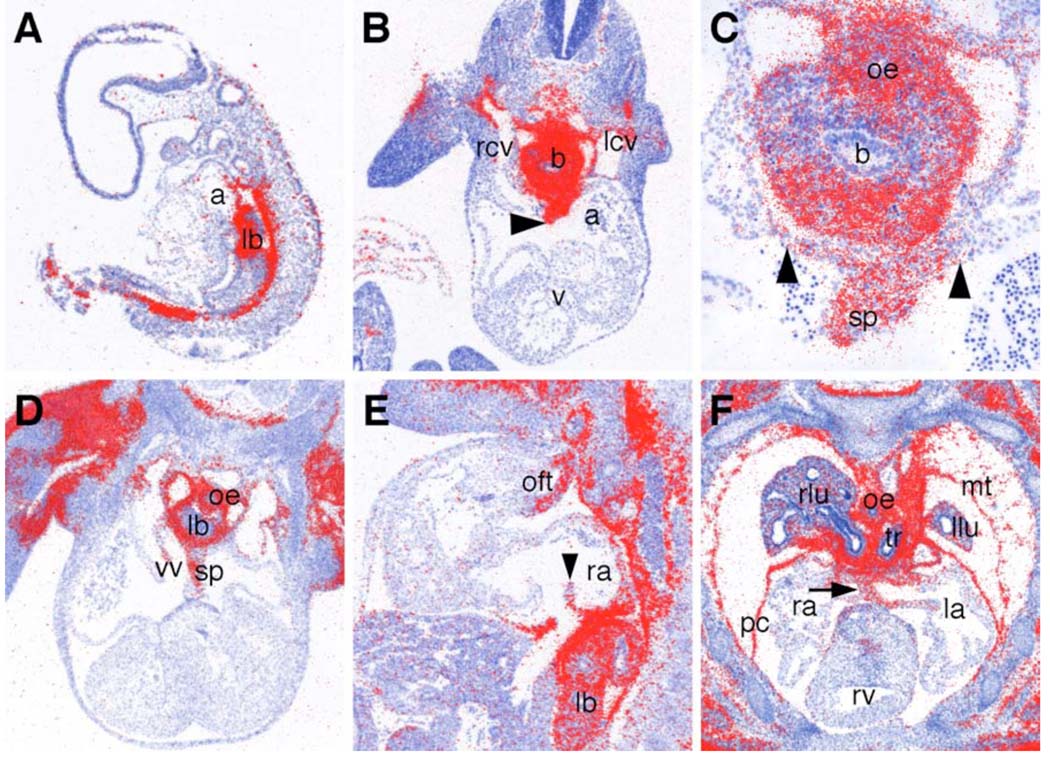
Fig. 6.
Odd1 mRNA expression during heart development. (A) Sagittal section of an E9.5 embryo showing Odd1 mRNA expression (red color) in the dorsal atrial wall. (B) Transverse section of an E10.5 embryo showing abundant Odd1 mRNA expression in the developing septum primum (arrowhead) and the dorsal atrial wall. Odd1 mRNA is also highly expressed in the cardinal vein. (C) Higher magnification of the lung bud region of the section in B. Odd1 mRNA is abundantly expressed in the oesophagus endoderm, the lung bud mesenchyme, the septum primum, and the dorsal atrial wall. Arrowheads mark the left and right boundaries of the dorsal atrial expression domain. (D, E) Transverse (D) and sagittal (E) sections of E11.5 embryos showing Odd1 mRNA expression in the left, but not right leaflet of the newly formed venous valves. (F) Frontal section through the dorsal thoracic region an E13.5 embryo showing strong expression of Odd1 mRNA in the dorsal atrial wall flanking the atrial septum (arrow) and in the mesothelium including the parietal pericardium. Low levels of Odd1 mRNA is detected in the lung mesenchyme at this stage. a, atrium; b, main bronchus; la, left atrium; lb, lung bud; lcv, left cardinal vein; llu, left lung; mt, mesothelium; oe, oesophagus; oft, outflow tract; pc, parietal pericardium; ra, right atrium; rcv, right cardinal vein; rv, right ventricle; v, ventricle; vv, venous valve; sp, septum primum; tr, trachea.
Odd1−/− mutants die prenatally with severe heart defects
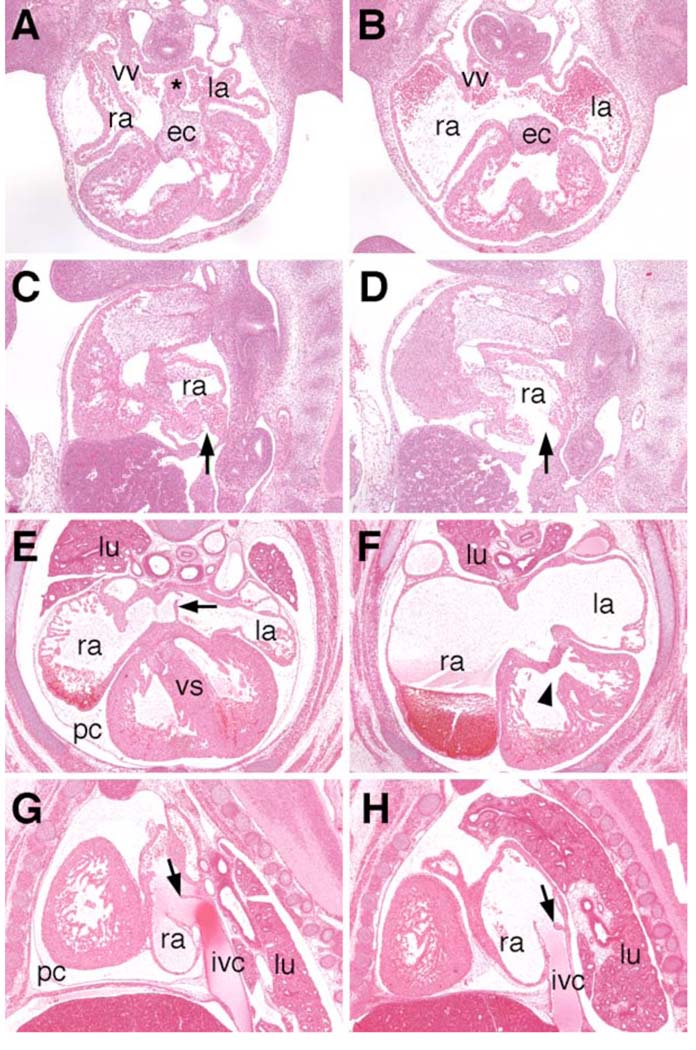
Mice heterozygous for the Odd1tm1RJ allele are normal and fertile. Heterozygous animals were intercrossed and genotypes of their progeny were determined two weeks after birth. No mice homozygous for the mutation were found. Careful examination of staged embryos and newborn mice from heterozygous intercrosses revealed that most of the Odd1−/− homozygous mutants died between E11.5 and E12.5, judging from their tail somite numbers and limb bud morphology (Kaufman, 1992). Analyses of embryos at E12.0 showed that homozygous mutant embryos have blood pooled in the liver and major veins while their yolk sac lacked blood circulation (Fig. 3B, D). A few Odd1−/− mutant embryos (approximately 5% of all homozygotes) survived to late embryonic stages and all of these exhibited generalized edema (Fig. 3F), indicating that these embryos were suffering from circulatory distress. All Odd1−/− mutant embryos examined at E11.5 by using histological analyses had severely dilated atria, lack of septum primum formation, and hypoplastic venous valves (Fig. 4B, D). All Odd1−/− mutant embryos that survived to late term also lacked the primary atrial septum and had dilated atria (Fig. 4F). These mutant embryos formed venous valves but the left leaflet of these valves appeared stumped and significantly reduced in size compared to that of heterozygous or wildtype littermates (Fig. 4H). In addition, these Odd1−/− embryos had malformed atrioventricular junction and ventricular septum (Fig. 4F), as well as clumped lung tissues and failure of mesothelium to form a complete parietal pericardium (Fig. 4F, H).
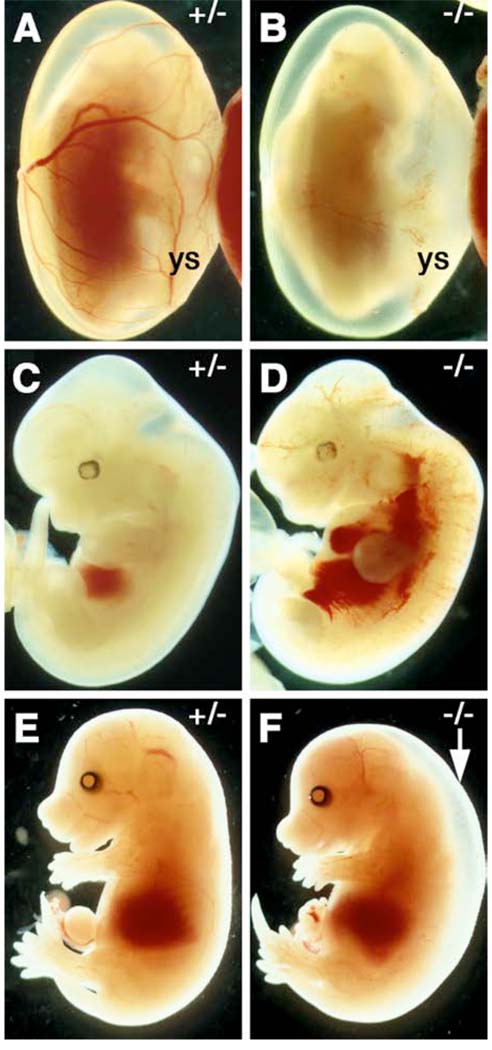
Fig. 3.
Odd1 homozyogus mutant embryos die prenatally. (A, C, E) newly dissected Odd+/− and (B, D, F) Odd−/− mutant embryos. Most Odd1−/− mutant embryos die at E12.0 (B) with blood pooled in the heart and major veins (D). (F) An E15.5 Odd−/− mutant embryo showing generalized edema (arrow). ys, yolk sac.
Fig. 4.
Histological analyses of Odd1 mutant heart defects. (A, B) Transverse sections of the wild-type and Odd−/− mutant embryos at E11.5 showing absence of septum primum (marked by the asterisk in A) and dilated atria in the Odd−/− mutant. (C, D) Sagittal sections of Odd+/− (C) and Odd−/− mutant (D) embryos at E11.5 show absence of venous valve in the homozygous mutant. The entrance of the inferior vena cava is wide open in the homozygous mutant (arrow in D). (E, F) Transverse sections of Odd+/− (E) and Odd−/− mutant (F) embryos at E15.5. Arrow in E points to the primary atrial septum, which is absent in the Odd−/− mutant heart (F). The Odd−/− mutant (F) also had severely dilated atria, defective atrioventricular junction and ventricular septum (arrowhead), as well as lack of parietal pericardium surrounding the ventricles. (G,H) Sagittal sections of wildtype (G) and Odd−/− mutant (H) embryos show malformed venous valve at the entrance of the inferior vena cava. The left leaflet of the venous valve in the mutant heart is stumped and less than half the length of that in the control embryos (arrows in G and H). The lung tissues are clumped together in the homozygous mutants (F,H) compared to the well-extended left and right lungs in the wildtype (E) and heterozygous (G) embryos. ec, endocardial cushion; ivc, inferior vena cava; la, left atrium; lu, lung; pc, pericardium; ra, right atrium; vv, venous valve.
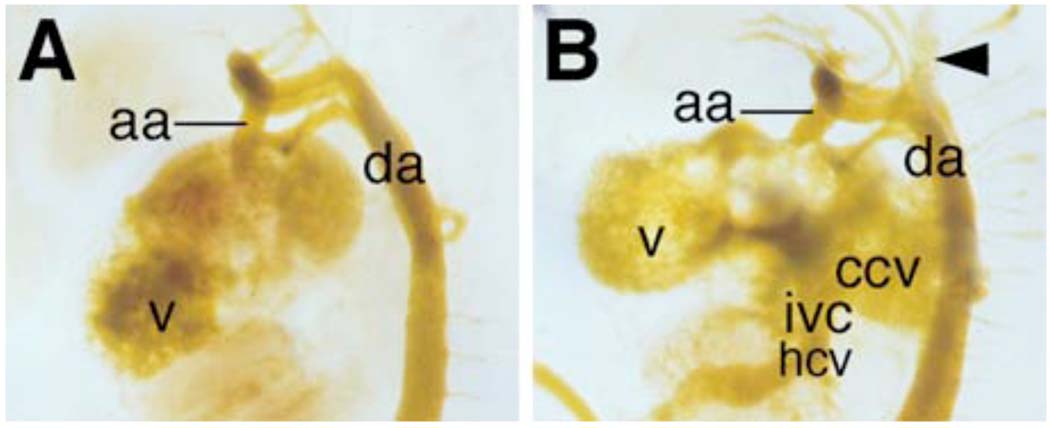
To investigate the cause of circulation defects and embryonic lethality, we injected yellow casting dye into the left ventricles of newly dissected E11.5 embryos to trace blood flow and found that the Odd1−/− mutant embryos exhibited blood backflow from the ventricle into the atria and the inflow tract (Fig. 5). We also injected diluted Indian ink into E11.5 embryonic ventricles to trace blood flow and found that all homozygous mutant embryos had the blood backflow problem whereas control wildtype and heterozygous embryos rarely exhibited backflow of either the yellow dye or the Indian ink into the inflow tract (data not shown). These data indicate that the defective atrioventricular junction and venous valves in the Odd1−/− mutants are the most likely cause of circulation distress and embryonic lethality.
Fig. 5.
Odd1−/− mutant embryos suffer blood backflow from the heart into the inflow tract. (A) An E11.5 wildtype embryo showing normal blood flow from the heart into the outflow tract as traced by yellow casting dye. (B) An Odd−/− littermate of the embryo shown in A was similarly injected with yellow casting dye into the left ventricle. Although most of casting dye followed blood flow into the aortas, a significant amount of the dye backflowed into the common cardinal vein, the anterior cardinal vein (arrowhead), and hepato-cardiac vein. aa, asending aorta; ccv, common cardinal vein; da, dorsal aorta; hcv, hepato-cardiac vein; ivc, inferior vena cava; v, ventricle.
Defects in heart development in Odd1−/− mutant embryos correlate with the normal developmental pattern of Odd1 mRNA expression
It has not previously been reported whether Odd1 mRNA is expressed during heart development. To investigate whether the heart defects in the homozygous mutant embryos are due to primary roles of Odd1 in heart development, we analyzed Odd1 mRNA expression at different stages of heart morphogenesis by using in situ hybridization analyses of serial sections. As shown in Fig. 6, Odd1 mRNA is highly expressed in the dorsal atrial myocardium as early as E9.5 during normal mouse embryogenesis (Fig. 6A). By E10.5, the septum primum grows into the common atrial chamber from the dorsal midline. Odd1 mRNA is strongly expressed in the developing septum primum and the flanking dorsal atrial wall, with the lateral boundary of expression coinciding with the entrance of the cardinal vein (Fig. 6B, C). By E11.5, the venous valve leaflets are clearly visible on transverse and sagittal sections through the atrial chamber in normal embryos. Odd1 mRNA is strongly expressed in the septum primum and the flanking dorsal wall, including the left leaflet of the venous valves (Fig 6D, E). Strong Odd1 mRNA expression is also detected in the outflow tract mesenchyme at this stage (Fig. 6E). At E13.5, in addition to expression in the dorsal atrial wall and the outflow tract, strong Odd1 mRNA expression is observed throughout the mesothelium lining the thoracic cavity, including the newly formed parietal pericardium (Fig. 6F). Odd1 mRNA expression also persists in the developing lung mesenchyme but at reduced levels compared with earlier stages (Fig. 6F). Thus, the defects in atrial septum and venous valve formation in the Odd1−/− mutants correlate well with the spatiotemporal pattern of Odd1 mRNA expression during normal heart development.
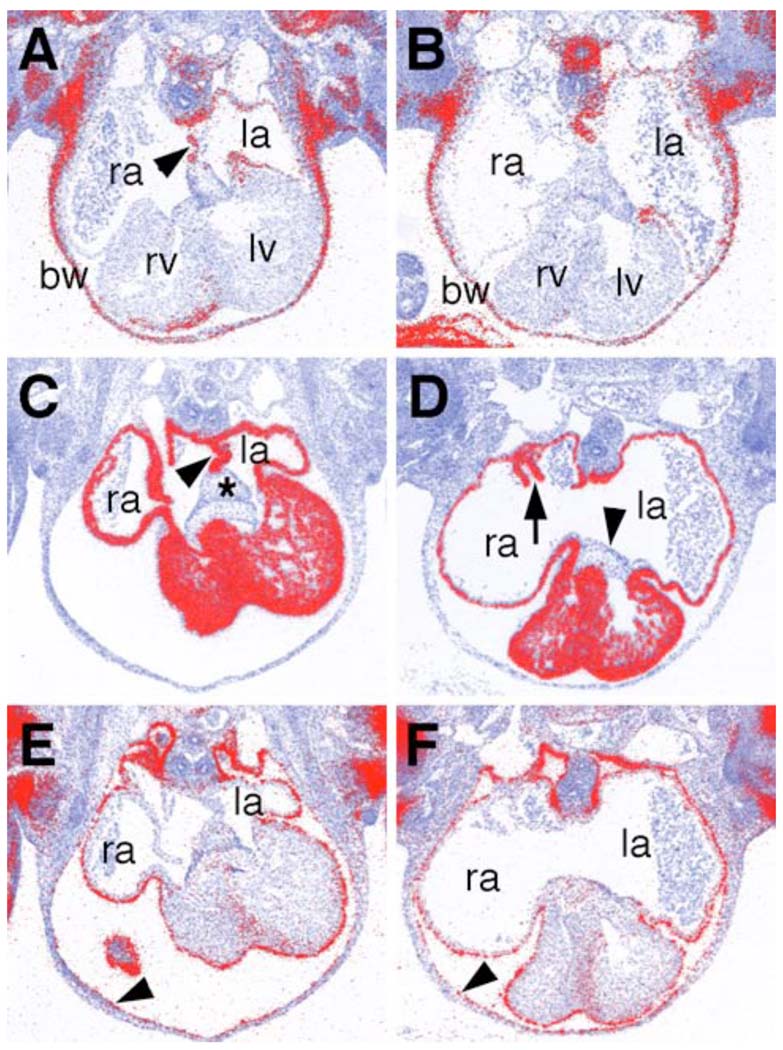
Defects in atrial septum development have been reported in association with disruptions of left-right asymmetry of the heart (Icardo and Sanchez de Vega, 1991; Gage et al., 1999). In particular, Pitx2 mRNA is normally expressed only in the left but not in the right atrial myocardium and mouse embryos homozygous for a null mutation in Pitx2 lack atrial septum formation and exhibit venous valve defects (Gage et al., 1999). The atrial septum myocardiam also expresses Pitx2 mRNA, indicating that it is a left atrial structure (Fig. 7A). Whereas the Odd1−/− mutant heart lacks atrial septum, Pitx2 mRNA is correctly expressed in the left but not in the right atrial myocardium (Fig. 7B). Several other transcription factors, including Nkx2.5 and Tbx5, have been previously shown to play essential roles in human and or mouse atrial septum development (Basson et al., 1997; Li et al., 1997; Schott et al., 1998; Biben et al., 2000). Nkx2.5 mRNA is expressed throughout the myocardial cells, including in the atrial septum and venous valves (Fig. 7C). Nkx2 mRNA expression pattern is unchanged in the Odd1−/− mutant embryos except for the lack of the atrial septum structure (Fig. 7D). Similarly, expression patterns of Gata4 and Tbx5 mRNAs are unaltered in Odd1−/− mutant hearts compared with that of the wildtype littermates (data not shown). These data indicate that Odd1 acts in a novel molecular pathway to regulate atrial septum formation. We also examined the expression pattern of Tbx18, an early epicardial marker, and found that its expression is unaltered either during heart development in the Odd1−/− mutants compared with that of the wildtype littermates (Fig. 7E, F), indicating that the parietal pericardial defect seen at later embryonic stages is not a result of early epicardial defect.
Fig. 7.
Comparison of molecular marker expression during heart development in the Odd1−/− mutant (B, D, F) and wildtype control (A, C, E) embryos at E11.5. (A, B) Pitx2 mRNA expression is restricted to the left atria in both wildtype (A) and Odd1−/− mutant embryos. Arrowhead in A points to the primary atrial septum, which is absent in the Odd1−/− mutant embryo. (C, D) Nkx2.5 mRNA is expressed throughout the myocardium in both the wildtype (C) and the mutant (D) embryos. Arrowhead in C points to the primary atrial septum, which is absent in the mutant embryos. The asterisk in C marks the mesodermal cap of the atrial septum, which is also absent in the mutant embryos. Arrow in D points to the short stomped venous valve in the mutant atrium whereas arrowhead in D points to the endocardial cushion, which is present at the atrioventicular junction in both wildtype and mutant embryos. (E, F) Tbx18 mRNA is expressed in the epicardium in both wildtype (E) and mutant (F) embryos. Arrowheads in E and F point to the mesothelial inner lining of the body wall, which expresses Tbx18 mRNA similarly in wildtype and mutant embryos. bw, body wall; la, left atrium, lv, left ventricle; ra, right atrium; rv, right ventricle.
Odd1 is required for intermediate mesoderm and kidney development
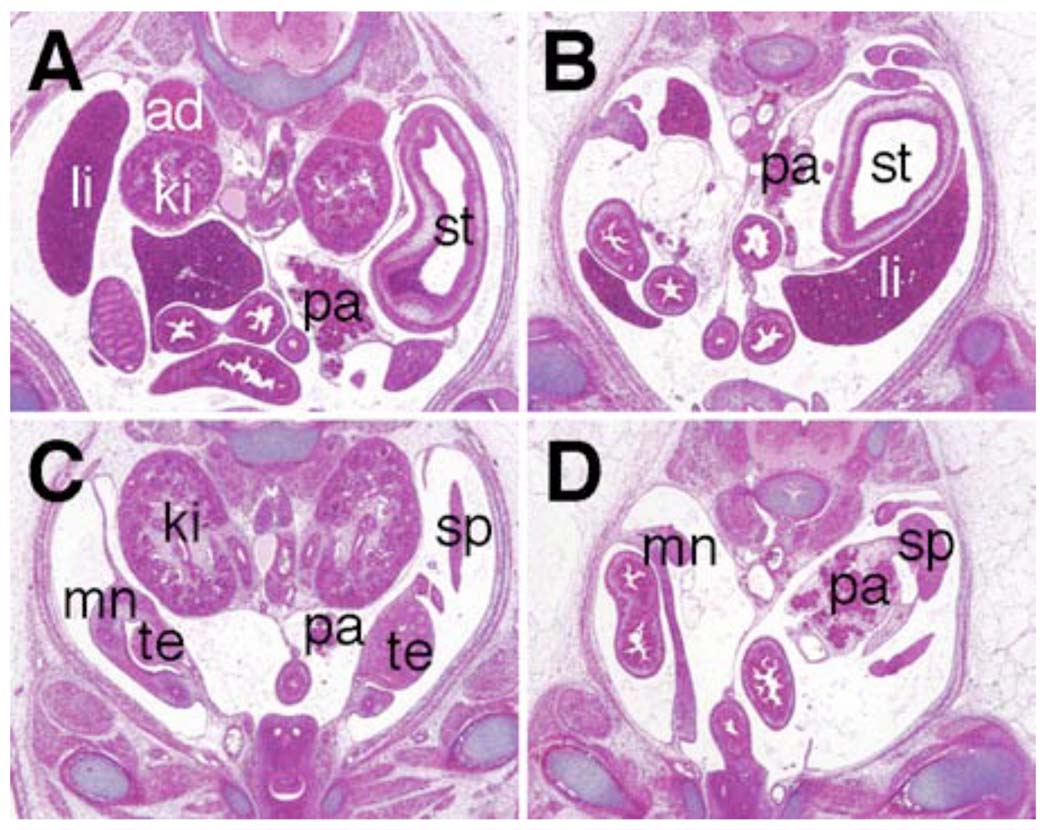
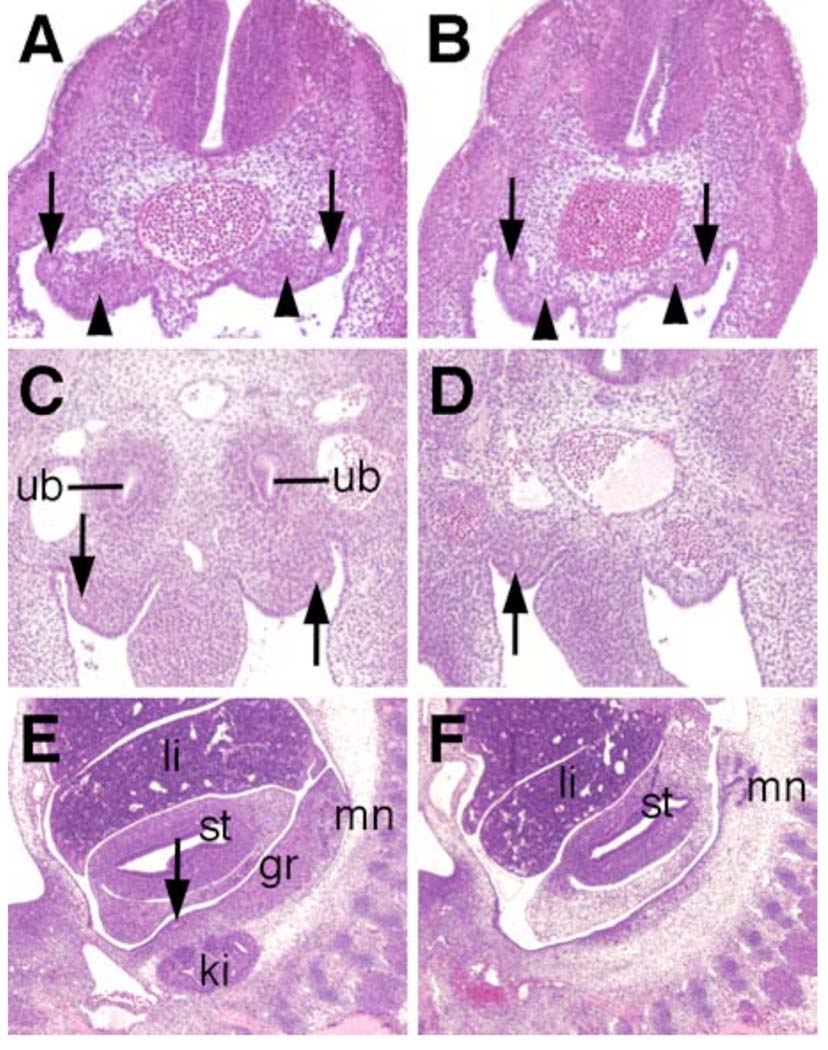
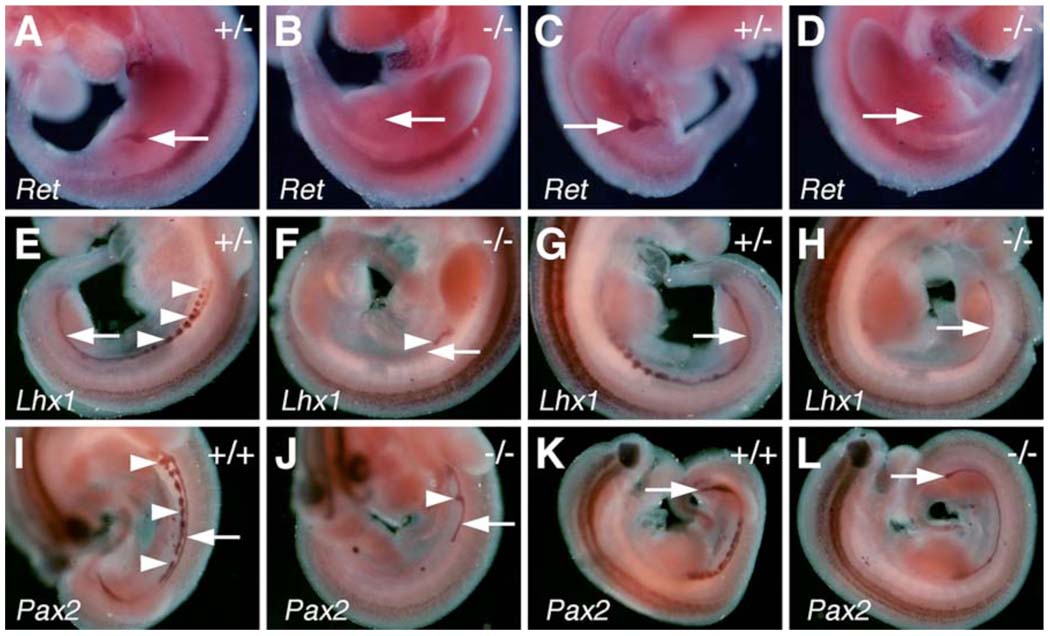
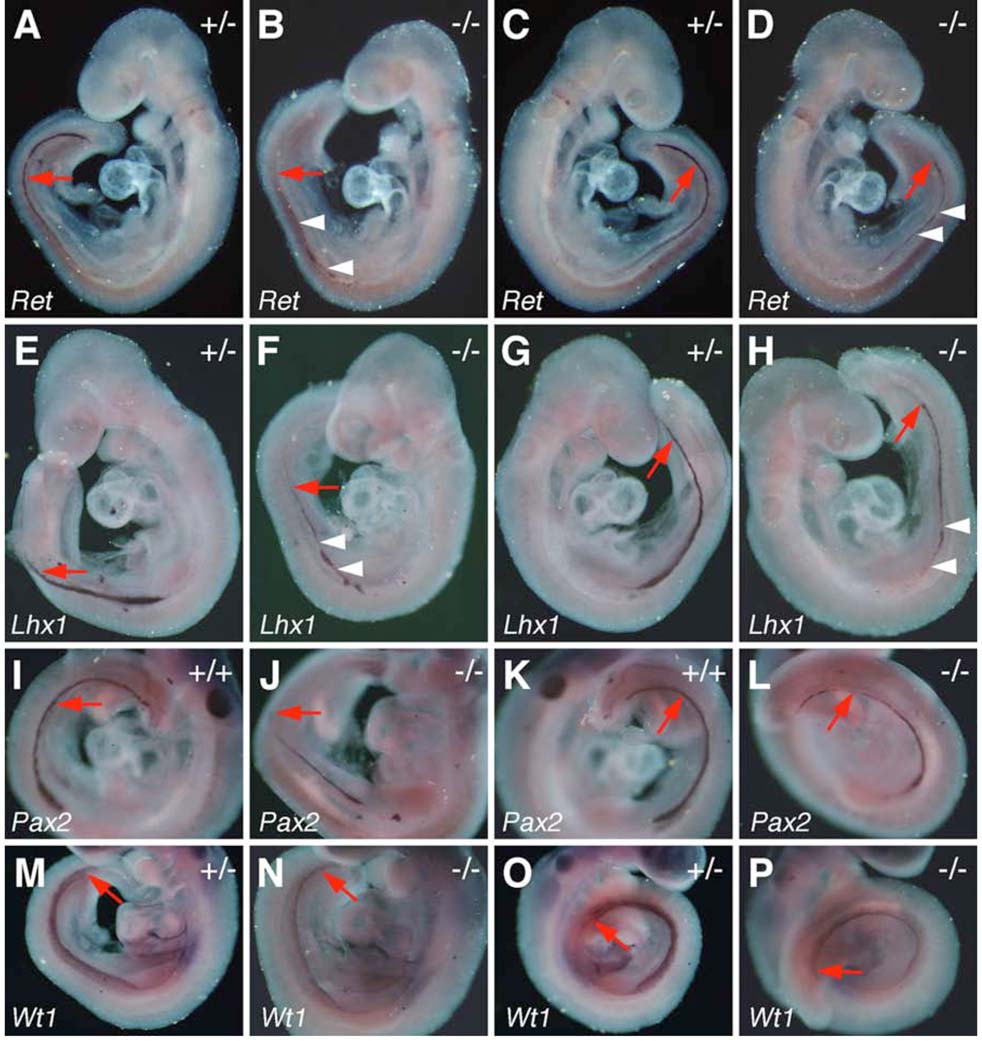
Odd1 mRNA expression is the earliest molecular marker of the intermediate mesoderm compartment in the mouse embryo. The intermediate mesoderm gives rise to the kidneys and gonads. From anterior to posterior, the intermediate mesoderm differentiates into three types of nephric tissues: the pronephros, mesonephros, and metanephros. We carried out detailed histolgical analyses of five Odd1−/− mutant embryos that survived to late gestation (15.5 – 17.5 dpc). All five Odd1−/− mutants completely lacked metanephric kidneys and gonads (Fig. 8B, D). Detailed histological analyses of embryos from E10.5 to E11.5 showed that the Odd1−/− mutants never initiated metanephric kidney formation: no signs of metanephric mesenchyme condensation or ureteric bud formation were observed in Odd1−/− mutants (Fig. 9B, D). Compared with wildtype and heterozygous littermates, there are also fewer mesonephric tubules and the gonadal ridge is retarded in the mutant embryos at E11.5 (Fig. 9F). In addition, the Wolffian duct is often malformed or absent in the posterior trunk region of the mutant embryos (Fig. 9D, F). To investigate further the etiology of kidney developmental defects in the Odd1−/− mutants, we carried out detailed molecular marker expression analyses of embryos at E9.5 and E10.5, before the Odd1−/− mutant embryos exhibited any signs of circulatory distress. At E10.5, the ureteric buds, which are specifically marked by Ret mRNA expression, branch off from the caudal ends of the Wolffian ducts and grow into the metanephrogenic mesenchyme on both sides of the wild-type embryos (Fig. 10A, C). In contrast, no comparable Ret-expressing ureteric bud structures form in the Odd1−/− mutant embryos (Fig. 10B, D), consistent with the histology data (Fig. 9D). Both Lhx1 (also known as Lim1) and Pax2 are expressed in the Wolffian duct and mesonephric vesicles in wildtype and heterozygous embryos at E10.5 (Fig. 10E, G, I, K). In contrast, the Odd1−/− mutant embryos at this stage show much fewer Lhx1- and Pax2-expressing mesonephric vesicles and disrupted or posteriorly truncated Wolffian ducts (Fig. 10F, H, J, L). Examination of expression of Ret, Lhx1, and Pax2, which are all expressed in the Wolffian duct epithelial cells at E9.5, showed that Wolffian duct development is already disrupted in the mutant embryos at this early stage compared with wildtype and heterozygous littermates (Fig. 11). Interestingly, the wolffian duct defect is always more severe on the left side than on the right side of the Odd1−/− mutant embryo such that the left Wolffian duct is often truncated posteriorly (Fig. 11B, F, J). On the other hand, expression of Wt1, which is normally expressed throughout the nephrogenic mesenchyme as early as E9.0 and its loss of function also causes agenesis of adrenal glands, kidneys and gonads as well as defective parietal pericardium (Armstrong et al., 1993; Kreidberg et al., 1993; Moore et al., 1999), is significantly down-regulated throughout the anterioposterior axis of the Odd1−/− mutant embryos but its expression domain is not truncated posteriorly and does not show left-right asymmetry (Fig. 11N, P). The overlaps in developmental expression patterns and in mutant phenotypes indicate that Odd1 and Wt1 normally function in a common molecular pathway to regulate urogenital development.
Fig. 8.
Odd1−/− mutant embryos lack metanephric kidneys. Representative transverse sections of E15.5 wildtype (A, C) and Odd1−/− mutant (B, D) littermates show the absence of kidneys, gonads, and adrenal glands in the mutant embryo. Due to the absence of kidneys, the internal organs such as pancreas and spleens were displaced in the mutant embryos but they were morphologically normal compared to the wildtype littermates. ad, adrenal gland; ki, metanephric kidney; li, liver; mn, mesonephros; pa, pancreas; sp, spleen; st; stomach; te; testis.
Fig. 9.
Defects in kidney development in Odd1−/− mutants. (A, B) Transverse sections of E10.0 wildtype (A) and Odd1−/− mutant embryos at the levels just rostral to the hindlimb buds. The wildtype embryo (A) shows condensation of the mestanephric mesenchyme (arrowhead) next to the nephric duct (arrow) whereas the nephrogenic mesenchyme does not condense in the mutant embryo (B). (C) At E10.5, ureteric bud invades the condensed metanephric mesenchyme in the wildtype embryo. (D) A Odd1−/− mutant littermate of the embryo in C do not have ureteric bud structure and lack nephric duct (arrow) on the left side of the caudal trunk. (E) Sagittal section of an E11.5 wildtype embryo showing the mesonephros, gonadal ridge, and metanephric kidney. (F) Sagittal section of an Odd1−/− mutant littermate showing lack of metanephric kidney and hypoplastic gonadal ridge. gr, gonadal ridge; ki, metanephric kidney; li, liver; mn, mesonephros; st, stomach; ub, ureteric bud.
Fig. 10.
In situ hybridization analyses of molecular marker gene expression during kidney development in E10.5 control and Odd1−/− mutant embryos. (A, C) Left and right sides, respectively of an E10.5 Odd1+/− embryo showing normal patterns of Ret mRNA expression in the newly initiated ureteric bud structures (arrows). (B, D) Odd1−/− mutant embryos at E10.5 lack Ret-expressing ureteric bud structures (red arrow). (E, G) Odd1+/− embryos show normal patterns of Lhx1 mRNA expression in the nephric duct (arrow) and mesonephric vesicles (arrowheads). (F, H) Odd1−/− mutant littermates of the embryos shown in E and G have significantly reduced Lhx1 mRNA expression in regions corresponding to mesonephros and nephric ducts. (I – L) Similarly, Pax2 mRNA is expressed in the nephric ducts (arrow) and mesonephric vesicles (arrowheads) in wildtype embryos (I, K) and is significantly reduced in the Odd1−/− mutant embryos (J, L). The Lhx1 and Pax2 expression patterns indicate that the left nephric duct is absent in the posterior trunk of mutant embryos (F, J), consistent with histology data (Fig. 8D). +/+, wildtype; +/−, heterozygous; −/−, homozygous mutant.
Fig. 11.
In situ hybridization of expression of kidney development marker genes in E9.5 control and Odd1−/− mutant embryos. Both left and right sides of the embryos are shown. Expression of Ret (A-D), Lhx1 (E-H), and Pax2 (I-L) mRNAs is significantly reduced in the Odd1−/− mutant embryo nephric ducts compared to their wildtype and heterozygous littermates. Expression domains of all three mRNAs are posteriorly truncated on the left side of the homozygous mutant embryos (B, F, J). Arrowhead point to additional areas of patchy disruption of the gene expression patterns in the mutant embryos. (M-P) Wt1 mRNA expression in the nephrogenic mesenchyme is significantly reduced throughout the anteroposterior axis in the homozygous mutants (N, P) compared to the heterozygous control littermates (M, O). +/+, wildtype; +/−, heterozygous; −/−, homozygous mutant.
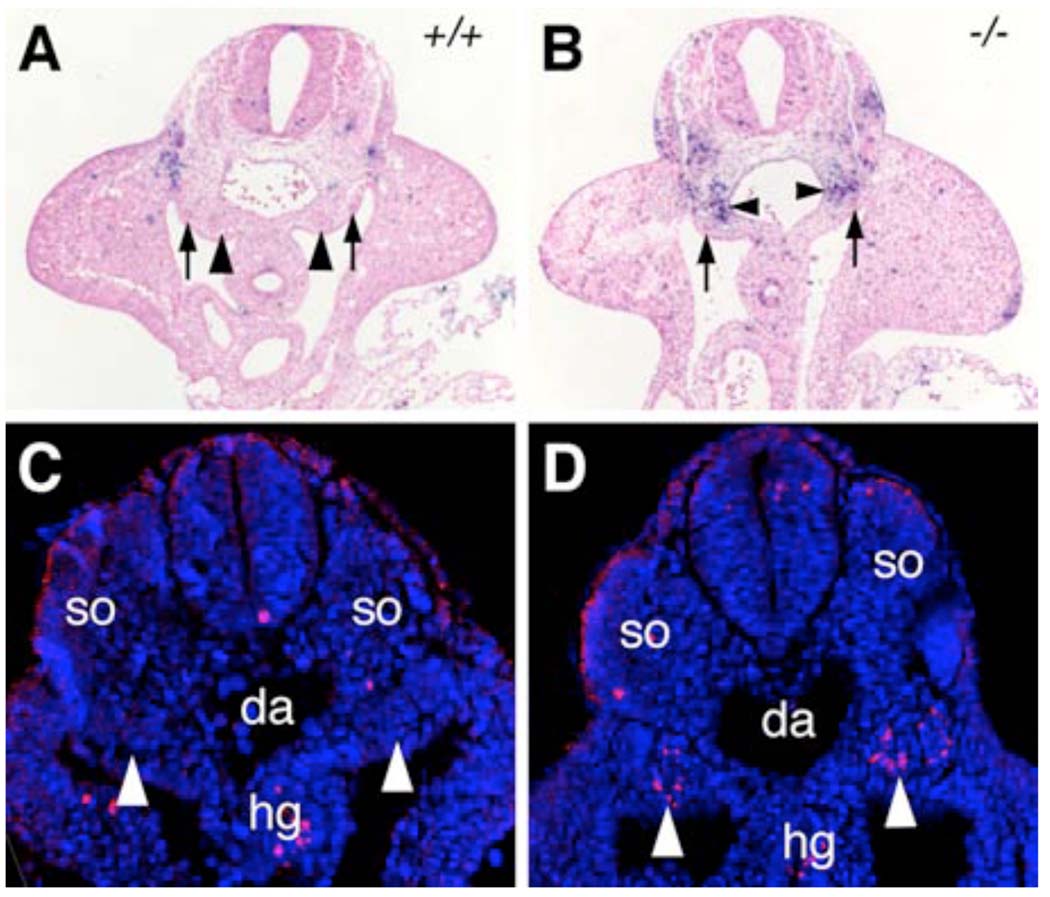
Disruptions of early kidney development often cause apoptosis of the nephrogenic mesenchyme (e.g., Fernandez-Teran et al., 1997; Bouchard et al., 2000; 2002; Ostrom et al., 2000). Thus, we analyzed patterns of apoptosis in E9.5 and E10.5 embryos. Compared with wildtype littermates, the Odd1−/− mutant embryos showed massive cell death in the nephrogenic mesenchyme by E10.5 as detected by TUNEL assay (Fig. 12A, B). Moreover, we detected significant numbers of cells expressing activated Caspase-3, a marker for early stages of apoptosis, specifically in the nephrogenic mesenchyme in Odd1−/− mutant embryos at E9.5 (Fig. 12D). Together with the data showing down-regulation of early kidney developmental genes in the homozygous mutants (Fig. 10, Fig. 11), these data indicate that Odd1 deficiency prevents the intermediate mesoderm from following the normal nephrogenic differentiation program and leads to apoptosis specifically in this tissue.
Fig. 12.
Increased nephrogenic mesenchyme cell death in the Odd1−/− mutant embryos. (A, B) Transverse sections at hindlimb bud levels of E10.5 wildtype (A) and Odd1−/− mutant (B) embryos were stained for apoptotic cells using TUNEL assay. Arrows point to the nephric ducts and arrowheads point to metanephric mesenchyme. (C, D) Transverse sections of lower trunk regions of E9.5 wildtype (C) and Odd1−/− mutant (D) embryos were stained for Caspase-3 expression (red). The sections were counterstained with DAPI (blue) to show cell nuclei. Arrowheads point to the nephrogenic mesenchyme, which contains significant numbers of Caspase-3 positive cells in the Odd1−/− mutant embryo (D). da, dorsal aorta; hg, hindgut; so, somite; +/+, wildtype; −/− homozygous mutant.
Discussion
Defects in heart development account for the most common birth defects and are the leading cause of birth defect-related deaths. Whereas several transcription factors essential for heart development have been identified recently through genetic studies, the molecular mechanisms controlling atrial septum and venous valve development are not well understood (Vaughan and Basson, 2001). During mammalian heart development, the systemic venous tributaries initially join with the primary atrium through the sinus venosus from both sides of the embryo. During human embryogenesis, the left horn of the sinus venosus diminishes in size and becomes incorporated into the left atrioventricular junction as the coronary sinus in subsequent developmental stages while left anterior cardinal vein becomes connected to the right horn of sinus venosus via the newly formed brachiocephalic vein to drain into the right side of the primary atrium (Anderson et al., 2002; Kaufman and Richardson, 2005). In mice, however, the brachiocephalic vein does not form and the left horn of the sinus venosus persists and drains via the coronary sinus into the post-hepatic part of the inferior vena cava (Kaufman and Richardson, 2005). Despite the species differences, the venous system rearrangement in both species results in the entire systemic venous compartment draining into the right side of the atrium. As the venous system rearrangements occur, venous valves form at the junction between the venous compartment and the primary atrium to prevent blood backflow (Anderson et al., 2002). As heart growth and development continue, the primary atrial septum, also called septum primum, grows into the atrial chamber from the dorsal midline. In this paper, we show that mouse embryos homozygous for a targeted null mutation in the Odd1 gene lack the primary atrial septum and have hypoplastic venous valves. These heart defects correlate with specific Odd1 mRNA expression in the dorsal atrial wall, including the developing primary atrial septum and the venous valve, indicating that Odd1 plays critical roles during normal atrial septum and venous valve morphogenesis. However, examination of the expression patterns of several transcription factor genes known to be essential for atrial septum development, including Nkx2.5, Pitx2, and Tbx5, showed that atrial myocardial differentiation and left-right patterning are unaltered, indicating that Odd1 does not regulate the expression of these known atrial septum regulators. Odd1 is a mouse homolog of the Drosophila Odd-skipped family transcription factors, which include Odd, Bowl, Sob, and Drm (Coulter et al., 1990; Hart et al., 1996; Wang and Coulter, 1996; So and Danielian, 1999; Lan et al, 2001; Green et al., 2002). Interestingly, both Bowl and Drm are required for the development of the proventriculus, a valve-like structure at the foregut-midgut junction that regulates food passage in the Drosophila embryo (Pankratz and Hoch, 1995; Wang and Coulter, 1996; Campos-Ortega and Hartenstein, 1997; Liu et al., 1999; Green et al., 2002, Johansen et al., 2003, reviewed by Myat, 2005). Proventriculus development begins when cells at the posterior boundary of the foregut tube evaginate to form a keyhole structure. In subsequent developmental stages, the keyhole epithelium folds back over the foregut to form the valve-like structure (Pankratz and Hoch, 1995; Campos-Ortega and Hartenstein, 1997; Johansen et al., 2003; Myat, 2005). All four members of the Drosophila odd-skipped family genes are expressed in the keyhole epithelium and mutations in either bowl or drm disrupt keyhole formation (Wang and coulter, 1996; Johansen et al., 2003), suggesting that Bowl and Drm normally regulates the cellular processes of foregut evagination during proventriculus development. Further evidence that the Odd-skipped family transcription factors regulate morphogenetic processes came from studies of Drosophila leg joint formation. Hao et al. (2003) showed that bowl mutant clones disrupted epithelial invagination processes during leg joint formation and that ectopic expression of any one of the four odd-skipped family genes induced invaginations in the leg disc epithelium characteristic of endogenous invaginating leg joint cells. Remarkably, formation of both the atrial septum and venous valves in mammals involve morphogenetic tissue folding: the atrial septum is initiated by protrusion of the dorsal mesocardium, the extracardiac mesoderm at the dorsal midline, into the primary atrial chamber whereas formation of the venous valves involves protrusion of the sinuatrial foramen into the lumen of the atrium during heart development in both mice and human (Webb et al., 1998; Anderson et al., 1999; Wessels et al., 2000; Lamers and Moorman, 2002). The expression pattern of Odd1 mRNA in the dorsal atrial wall and extracardiac mesoderm as well as in the developing venous valve, together with the defects in the development of these structures in the Odd1−/− mutants suggest that the Odd-skipped family transcription factors act in an evolutionarily conserved molecular pathway in valve formation, including the morphogenesis of proventriculus in insects and the atrial septum and venous valves in vertebrates.
The identification of Odd1 as an essential regulator of atrial septum development provides a new foundation for characterization of the molecular pathways regulating atrial morphogenesis. One particularly disputed area in studies of atrial development is with regard to the role of extracardiac mesoderm in atrial septum formation. van Gils (1981) described the leading edge of the atrial septum is formed from extracardiac source as “an ingrowth of tissue from the dorsal mesocardium”. However, Arrechedera et al. (1987) and Gerety and Watanabe (1997), through structural and histochemical analyses, suggested that the septum primum mesenchymal tissue originated from epitheliomesenchymal transformation from endocardial cells, similar to that seen for the atrioventricular cushions. Wessels et al. (2000) carried out detailed spatiotemporal immunohistochemical analyses of human atrial development and showed that the dorsal mesocardium protrudes into the dorsal wall of the primary atrium and is continuous with the mesenchymal cap on the leading edge of the developing primary atrial septum. Although it was shown that neither the mesenchymal tissues of the dorsal protrusion nor the mesenchymal cap of the primary atrial septum expressed a specific endocardial tissue antigen at all developmental stages investigated, Wessels et al (2000) suggested that the cells in the dorsal protrusion could originate from either endocardial or extracardiac tissues. Since Odd1 is expressed in both the dorsal atrial myocardium and the extracardiac somatic mesoderm before atrial septum formation, failure of atrial septum formation in the Odd1−/− mutant embryos may result from defects in either the extracardiac dorsal mesocardium or the dorsal atrial myocardium. Future studies using tissue-specific gene inactivation approaches will enable characterization of the roles of the Odd1 molecular pathway in cardiac and extracardiac mesenchyme, respectively during atrial septum development.
We show that Odd1 gene expression is first activated in the nascent intermediate mesoderm shortly after their migration from the primitive streak at the midgastrulation stage during mouse embryonic development. The intermediate mesoderm lies between the paraxial and lateral mesoderms and undergoes extensive epithelial transformation to generate the ducts and tubules of the urogenital tracts and kidneys (Saxen, 1987). Previously, it was demonstrated that Odd1 mRNA expression marks the intermediate mesoderm fate in normal and mutant mice (Wilm et al., 2004). Our mutation analysis indicates that Odd1 is not required for intermediate mesoderm specification. Rather, it is involved in the control of intermediate mesoderm differentiation, as derivatives of the intermediate mesoderm including the nephric ducts, mesonephros, metanephros and gonads are either defective or absent in the Odd1−/− mutants. Gene targeting studies in mice have shown that many transcription factors, including Lhx1, Pax2, Wt1, Emx2, Sall1 and the Hox11 paralogs (Kreidberg et al., 1993; Shawlot and Behringer, 1995; Torres et al., 1995; Miyamoto et al., 1997; Tsang et al., 2000; Nishinakamura et al., 2001; Bouchard et al., 2002; Wellik et al., 2002), as well as several signaling molecules, such as the GDNF-Ret ligand-receptor pair, Wnt4, and Wnt11 (Stark et al., 1994; Schuchardt et al., 1994; 1996; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Majumdar et al., 2003), are essential for normal kidney development. We show that the expression of Lhx1, Pax2, and Wt1 is each significantly reduced in the Odd1−/− mutant embryos as early as E9.5, indicating that Odd1 acts upstream of these known transcription factors in regulating early urogenital development. At E10.5, while wildtype and heterozygous littermates have initiated ureteric bud outgrowth into the metanephric mesenchyme, the Odd1−/− homozygous mutant embryos lacked metanephric mesenchyme condensation and ureteric bud induction. Instead, the Odd1−/− mutant nephrogenic mesenchyme initiates Caspase-3 activation at E9.5 and undergoes massive apoptosis by E10.5. It has been reported that disruption of nephric duct elongation results in nephrogenic mesenchyme cell death due to lack of induction (Fernandez-Teran et al., 1997). Furthermore, a two-fold reduced expression of Pax2 in Pax2+/− embryos caused significant nephrogenic mesenchyme death whereas complete absence of Pax2 and Pax8 resulted in massive death of the intermediate mesoderm as early as E9.5 (Bouchard et al., 2000; 2002; Ostrom et al., 2000). Thus, the cell death of the nephrogenic mesenchyme in the Odd1−/− mutants is most likely an indirect effect of reduced Pax2 gene expression and/or disrupted nephric duct development. Our data, demonstrating that Odd1 is expressed from the earliest stage of intermediate development and required for nephrogenic mesenchyme differentiation, suggest that Odd1 acts as a competence factor in the control of normal intermediate mesoderm development.
The Odd1−/− mutant phenotype is strikingly similar to that of the Wt1−/− mutant embryos. In addition to lack of ureteric bud induction and gonad development, both Odd1−/− and Wt1−/− mutant embryos have fewer mesonephric tubules at E11.5 as well as generalized edema, clumped lungs, failure of mesothelium to form a complete parietal pericardium, and lack of adrenal glands at later stages of embryonic development (Kreidberg et al., 1993; Moore et al., 1999; Hammer et al., 2005; and this study). Wt1, originally identified as a Wilms’ tumor suppressor gene, encodes a transcription factor with four zinc finger domains (Call et al., 1990; Gessler et al., 1990). The earliest expression of Wt1 mRNA during mouse embryogenesis was detected in the intermediate mesoderm at E9.0 and it is subsequently expressed in many tissues, including nephrogenic condensations, spinal cord, brain, and the differentiating mesothelium (Armstrong et al., 1993; Moore et al., 1999). We showed that Wt1 mRNA expression is significantly down-regulated throughout the intermediate mesoderm in Odd1−/− mutant embryos at E9.5. Since Odd1 expression is activated prior to the earliest stage of Wt1 in the intermediate mesoderm (Armstrong et al., 1993, and this study), the down-regulation of Wt1 mRNA expression in the Odd1−/− mutant embryos and the phenotypic similarities in urogenital, pericardial and adrenal gland development between the Odd1−/− and Wt1−/− mutants suggest that Odd1 acts upstream of Wt1 in the transcriptional network controlling normal intermediate mesoderm differentiation. The fact that nephrogenesis is blocked earlier in the Odd1−/− mutants than that reported for the Wt1−/− mutants, for instance metanephric mesenchyme condensation occurred in the Wt1−/− mutants but not in the Odd1−/− mutants, is consistent with this hypothesis. Alternatively, it is possible that Odd1 and Wt1 normally interact with each other to regulate intermediate mesoderm differentiation such that lack of either factor results in similar defects in urogenital and pericardial development. The fact that two Drosophila homologs, Drm and Bowl interact with other transcription factors through their first zinc finger domain that is conserved in all invertebrate and vertebrate Odd-skipped family members suggest that the vertebrate Odd-skipped class transcription factors may also function through protein-protein interactions with other transcription factors (Green et al., 2002; Buckley et al., 2004; Hatini et al., 2005). Since mutations in WT1 have been associated with multiple urogenital malformations and Wilms’ tumors in humans (reviewed in Pritchard-Jones, 1999; Chesney, 2002, and references therein), further characterization of the role of Odd1 and its interaction with Wt1 will provide new insights into the molecular mechanisms underlying normal urogenital development as well as tumor formation.
Acknowledgements
We thank Tom Schultheiss for communication of data prior to publication and comments on the manuscript. We thank Tom Gridley for the CJ7 ES cells and in situ hybridization probes, Liam Casey for assistance with immunofluorescent staining, and the University of Rochester Transgenic Mouse Facility for generation of chimeric mice from targeted ES cells. This work was supported by the NIH/NIDCR grant R01DE013681 to RJ.
References
- Anderson RH, Webb S, Brown NA. Clinical anatomy of the atrial septum with reference to its developmental components. Clin. Anat. 1999;12:362–374. doi: 10.1002/(SICI)1098-2353(1999)12:5<362::AID-CA6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Brown NA, Webb S. Development and structure of the atrial septum. Heart. 2002;88:104–110. doi: 10.1136/heart.88.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB. The expression of the wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 1993;40:85–97. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- Arrechedera H, Alvarez M, Strauss M, Ayesta C. Origin of mesenchymal tissue in the septum primum: a structural and ultrastructural study. J. Mol. Cell. Cardiol. 1987;19:641–651. doi: 10.1016/s0022-2828(87)80372-3. [DOI] [PubMed] [Google Scholar]
- Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nature Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- Biben C, Weber R, Kesteven S, Stanley E, McDonald L, Elliott DA, Barnett L, Koentgen F, Robb L, Feneley M, M, Harvey RP. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circ. Res. 2000;87:888–895. doi: 10.1161/01.res.87.10.888. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Pfeffer P, Busslinger M. Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development. 2000;127:3703–3713. doi: 10.1242/dev.127.17.3703. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MS, Chau J, Hoppe PE, Coulter DE. Odd-skipped homologs function during gut development in C. elegans. Dev. Genes Evol. 2004;214:10–18. doi: 10.1007/s00427-003-0369-x. [DOI] [PubMed] [Google Scholar]
- Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, Jones C, Housman DE. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer; 1997. [Google Scholar]
- Chesney RS. Why is the oncogene WT1 in the developing kidney and what is it doing there? Pediatr. Nephrol. 2002;17:990–992. doi: 10.1007/s00467-002-0927-x. [DOI] [PubMed] [Google Scholar]
- Coulter DE, Swaykus EA, Beran-Koehn MA, Goldberg D, Wieschaus E, Schedl P. Molecular analysis of odd-skipped, a zinc finger encoding segmentation gene with a novel pair-rule expression pattern. EMBO J. 1990;9:3795–3804. doi: 10.1002/j.1460-2075.1990.tb07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DE, Wieschaus E. Gene activities and segmental patterning in Drosophila: analysis of odd-skipped and pair-rule double mutants. Genes Dev. 1988;2:1812–1823. doi: 10.1101/gad.2.12b.1812. [DOI] [PubMed] [Google Scholar]
- DiNardo S, O’Farrell PH. Establishment and refinement of segmental pattern in the Drosophila embryos: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1987;1:1212–1225. doi: 10.1101/gad.1.10.1212. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Piedra ME, Simandl BK, Fallon JF, Ros MA. Limb initiation and development is normal in the absence of the mesonephros. Dev. Biol. 1997;189:246–255. doi: 10.1006/dbio.1997.8680. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gerety M, Watanabe M. Polysialyted NCAM expression on endocardial cells of the chick primary atrial septum. Anat. Rec. 1997;247:71–84. doi: 10.1002/(SICI)1097-0185(199701)247:1<71::AID-AR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gessler M, Poustka A, Cavenee W, Neve RL, Orkin S, Bruns GAP. Homozygous deletion in Wilms’ tumors of zinc-finger gene identified by chromosome jumping. Nature. 1990;343:774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Green RB, Hatini V, Johansen KA, Liu X-J, Lengyel JA. Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development. 2002;129:3645–3656. doi: 10.1242/dev.129.15.3645. [DOI] [PubMed] [Google Scholar]
- Hammer GD, Parker KL, Schimmer BP. Transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–1024. doi: 10.1210/en.2004-1385. [DOI] [PubMed] [Google Scholar]
- Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odds-kipped family of zinc finger genes promotes Drosophila leg segmentation. Dev. Biol. 2003;263:282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Hart MC, Wang L, Coulter DE. Comparison of the structure and expression of odd-skipped and two related genes that encode a new family of zinc finger proteins in Drosophila. Genetics. 1996;144:171–182. doi: 10.1093/genetics/144.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Green RB, Lengyel JA, Bray SJ, DiNardo S. The Drumstick/Lines/Bowl regulatory pathway linkes antagonistic Hedgehog and Wingless signaling inputs to epidermal cell differentiation. Genes Dev. 2005;19:709–718. doi: 10.1101/gad.1268005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Icardo JM, Sanchez de Vega MJ. Spectrum of heart malformations in mice with situs solitus, situs inversus, and associated visceral heterotaxy. Circulation. 1991;84:2547–2558. doi: 10.1161/01.cir.84.6.2547. [DOI] [PubMed] [Google Scholar]
- Johansen KA, Gree RB, Iwaki DD, Hernandez JB, Lengyel JA. The Drm-Bowl-Lin relief-of-repression hierarchy controls fore- and hindgut patterning and morphogenesis. Mech. Dev. 2003;120:1139–1151. doi: 10.1016/j.mod.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. London: Academic Press; 1992. [Google Scholar]
- Kaufman MH, Richardson L. 3D reconstruction of the vessels that enter the right atrium of the mouse heart at Theiler stage 20. Clin. Anat. 2005;18:27–38. doi: 10.1002/ca.10242. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Lamers WH, Moorman AFM. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ. Res. 2002;91:93–103. doi: 10.1161/01.res.0000027135.63141.89. [DOI] [PubMed] [Google Scholar]
- Lan Y, Kingsley PD, Cho E-S, Jiang R. Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech. Dev. 2001;107:175–179. doi: 10.1016/s0925-4773(01)00457-9. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ovitt EE, Cho E-S, Maltby KM, Wang Q, Jiang R. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 2004;131:3207–3216. doi: 10.1242/dev.01175. [DOI] [PubMed] [Google Scholar]
- Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis ARJ, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nature Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- Liu X, Kiss I, Lengyel JA. Identification of genes controlling malpighian tubule and other epithelial morphogenesis in Drosophila melanogaster. Genetics. 1999;151:685–695. doi: 10.1093/genetics/151.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Philips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev. Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145. [DOI] [PubMed] [Google Scholar]
- Myat MM. Making tubes in the Drosophila embryo. Dev. Dyn. 2005;232:617–632. doi: 10.1002/dvdy.20293. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev. Biol. 2000;219:250–258. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- Pankraz MJ, Hoch M. Control of epithelial morphogenesis by cell signaling and integrin molecules in the Drosophila foregut. Development. 1995;121:1885–1898. doi: 10.1242/dev.121.6.1885. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K. The Wilms tumor gene, WT1, in normal and abnormal nephrogenesis. Pediatr. Nephrol. 1999;13:620–625. doi: 10.1007/s004670050757. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Saulier-Le Drean B, Nasiadka A, Dong J, Krause HM. Dynamic changes in the functions of Odd-skipped during early Drosophila embryogenesis. Development. 1998;125:4851–4861. doi: 10.1242/dev.125.23.4851. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the Kidney. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- Schott J-J, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret−/− mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- So PL, Danielian PS. Cloning and expression analysis of a mouse gene related to Drosphila odd-skipped. Mech. Dev. 1999;84:157–160. doi: 10.1016/s0925-4773(99)00058-1. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Swiatek P, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev. Biol. 2000;223:77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- van Gils FA. The fibrous skeleton in the human heart: embryological and pathological considerations. Virchows Arch. 1981;393:61–73. doi: 10.1007/BF00430871. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Basson CT. Molecular determinants of atrial and ventricular septal defects and patent arteriosus. Am. J. Med. Genet. 2001;97:304–309. doi: 10.1002/1096-8628(200024)97:4<304::aid-ajmg1281>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wang L, Coulter DE. bowel, an odd-skipped homolog, functions in the terminal pathway during Drosophila embryogenesis. EMBO J. 1996;15:3182–3196. [PMC free article] [PubMed] [Google Scholar]
- Webb S, Brown NA, Wessels A, Anderson RH. Development of the murine pulmonary vein and its relationship to the embryonic venous sinus. Anat. Rec. 1998;250:325–334. doi: 10.1002/(SICI)1097-0185(199803)250:3<325::AID-AR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16:1423–1432. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, Anderson RH, Markwald RR, Webb S, Brown NA, Viragh SZ, Moorman AFM, Lamers WH. Atrial development in the human heart: an immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat. Rec. 2000;259:288–300. doi: 10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wilm B, James RG, Schultheiss TM, Hogan BLM. The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev. Biol. 2004;271:176–189. doi: 10.1016/j.ydbio.2004.03.034. [DOI] [PubMed] [Google Scholar]