Abstract
Stress-induced activation of hypothalamic paraventricular nucleus (PVN) corticotropin releasing hormone (CRH) neurons triggers CRH release and synthesis. Recent findings suggest that this process depends on the intracellular activation (phosphorylation) of extracellular-regulated kinase 1 and 2 (ERK1/2) within CRH neurons. We have recently shown that the presence of glucocorticoids constrains stress-stimulated phosphorylation of PVN ERK1/2. In some peripheral cell types, dephosphorylation of ERK has been shown to be promoted by direct glucocorticoid upregulation of the MAP-kinase phosphatase-1 (Mkp-1) gene. In this study we tested the prospect that glucocorticoids regulate Mkp-1 mRNA expression in neural forebrain (medial-prefrontal cortex,mPFC, and PVN) and endocrine tissue (anterior pituitary) by subjecting young adult male Sprague-Dawley rats to various glucocorticoid manipulations ± acute psychological stress (restraint). Restraint led to a rapid increase in Mkp-1 mRNA within the mPFC, PVN and anterior pituitary, and this increase did not require glucocorticoid activity. In contrast to glucocorticoid upregulation of Mkp-1 gene expression in peripheral tissues, we found that the absence of glucocorticoids (via adrenalectomy) augmented basal mPFC and stress-induced PVN and anterior pituitary Mkp-1 gene expression. Taken together, this study indicates that the presence of glucocorticoids may constrain Mkp-1 gene expression in neural forebrain and endocrine tissues. This possible constraint may be an indirect consequence of the inhibitory influence of glucocorticoids on stress-induced activation of ERK1/2, a known upstream positive regulator of Mkp-1 gene transcription.
Keywords: MKP-1, ACTH, paraventricular nucleus of hypothalamus, medial prefrontal cortex, corticosterone, ERK1/2
Introduction
Corticotropin releasing hormone (CRH) neurons of the hypothalamic-pituitary-adrenal (HPA) axis integrate stress-dependent change in neural input and direct negative feedback effects of glucocorticoids (Dallman et al. 1987; Sawchenko et al. 1996; Bali et al. 2008). Excitation of CRH neurons is often coupled to not only CRH neurohormone secretion, but also altered gene expression and neurohormone synthesis (Kovács & Sawchenko 1996; Watts 2005; Pace et al. 2009). Recent evidence indicates that a molecular element of this coupling process within CRH neurons is phosphorylation/activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Khan & Watts 2004; Khan et al. 2007; 2011). ERK1/2 are members of the mitogen-activated protein kinase (MAP-kinase) family. MAP-kinases are essential intracellular signaling proteins for virtually all cell types, including neurons and endocrine cells (Grewal et al. 1999). We recently reported that acute exposure to psychological stress (restraint) increased the activated (phosphorylated) form of ERK1/2 in hypothalamic CRH neurons, and this ERK1/2 activation was constrained by tonic glucocorticoid activity (Osterlund et al. 2011).
The active phosphorylation state of ERK1/2 is regulated by both the kinase MEK and by phosphatases, including MAP kinase phosphatase-1 (MKP-1, also known as DUSP1) (Keyse 2000; Patterson et al. 2009). MKP-1 is a dual specificity phosphatase that inactivates MAP-kinases by dephosphorylating tyrosine and threonine residues essential for catalytic activity (Camps et al. 2000; Theodosiou & Ashworth 2002). A key feature of MKP-1 is that its encoding gene (Mkp-1) is rapidly induced in a wide range of cell types by a large number of excitatory stimuli (Sun et al. 1993; Caunt & Keyse 2013). Interestingly, in vitro studies find that glucocorticoids increase Mkp-1 mRNA levels in a variety of peripheral cell types and cell lines (Clark 2003; Clark et al. 2008). Glucocorticoid treatment of mice has also been shown to rapidly increase Mkp-1 mRNA in lung, spleen and liver (Wang et al. 2008; Vandevyver et al. 2012). In vitro studies have also shown that glucocorticoid suppression of some MAP-kinase dependent cellular processes depends on glucocorticoid mediated upregulation of Mkp-1 gene expression (Kassel et al. 2001; Issa et al. 2007; Zhou et al. 2007; Komatsu et al. 2008; Nicoletti-Carvalho et al. 2010; Burke et al. 2012).
Glucocorticoids provide a protein synthesis dependent negative feedback inhibition of the HPA axis that is evident within 1-3 hr after glucocorticoid treatment (Dallman et al. 1987; Shipston 1995; Osterlund & Spencer 2011). This short-term glucocorticoid negative feedback action is likely due to glucocorticoid rapid induction of one or more genes that encode proteins important for the coupling of cellular excitation with (neuro)hormone secretion (Shipston 1995; Osterlund & Spencer 2011). We considered the possibility that glucocorticoid suppression of stress-induced ERK1/2 activation in hypothalamic PVN CRH neurons depends at least in part on glucocorticoid rapid upregulation of Mkp-1 gene expression. To determine whether induction of the Mkp-1 gene could be a mechanism of short-term glucocorticoid regulation of the HPA axis, we examined Mkp-1 mRNA levels (in situ hybridization) in the hypothalamic paraventricular nucleus (PVN), medial prefrontal cortex (mPFC) and anterior pituitary of male rats subjected to acute stress and CORT manipulations. The PVN contains the HPA axis-related CRH neuron cell bodies. For comparison purposes, we also examined Mkp-1 mRNA levels in another brain region important for modulation of HPA axis activity, the mPFC (Radley et al. 2006; Weinberg et al. 2010), as well as in an additional anatomical element of the HPA axis, the anterior pituitary. For these studies we challenged rats with the acute stressor restraint, which is considered a moderate intensity psychological stressor (Herman & Cullinan 1997; Dayas et al. 2001; Pace et al. 2005). We manipulated CORT levels by either removing the presence of endogenous CORT (adrenalectomy) or by treating rats with an acute injection of CORT. Although to date there is very limited study of Mkp-1 gene expression in mammalian brain, there is evidence for it to be regulated in an activity dependent manner, to contribute to neuronal axonal plasticity, and to be associated with major depressive disorder (Sgambato et al. 1998; Kodama et al. 2005; Doi et al. 2007; Jeanneteau et al. 2012).
Materials and Methods
Animals
Young adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IA) weighed between 280 and 305 g at time of experimentation, and were housed two per-cage. The colony room lights were maintained on a 12 hour light/dark cycle and rats were given rat chow (Purina Rat Chow, Ralston Purina, St. Louis, MO, USA) and tap water ad libitum. Rats were given at least a 2 week acclimation period to the colony room before initiation of experimental procedures. All experiments were performed during the first half of the rats' inactive period, when basal CORT secretion is at its circadian trough. Handling and testing of all rats were approved by the University of Colorado Institutional Animal Care and Use Committee.
Surgery
Rats were adrenalectomized bilaterally (ADX) or were sham-ADX under halothane anesthesia. Adrenal glands were excised and removed through bilateral incisions that were made through the dorsal-lateral skin and peritoneal wall in close proximity to each kidney. Sham-ADX rats experienced the same surgical procedure as ADX rats, except that adrenal glands were left in place after their localization. All ADX and sham-ADX rats were given 4 days to recover from surgery before the experimental test day. ADX rats were given 0.9% saline drinking water ad libitum.
Restraint Stress
Acute stress challenge consisted of placing rats in clear plexiglas tubes (23.5 cm in length and 7 cm in diameter; with multiple air holes). The size of the tube restricted lateral, forward and backward movement but did not interfere with breathing. Restraint is widely accepted as a psychological stressor within the stress neurobiology field, which has not only a conceptual basis, but is also supported by neurocircuit activity studies (Dayas et al. 2001).
Experimental Procedures
Experiment 1
Effect of acute stress on PVN Mkp-1 mRNA expression. Rats were exposed to 15 or 30 min of stress or no-stress (n = 3-4). Immediately after restraint, or at the same time of day (no stress group) rats were killed by guillotine decapitation. Brains were flash frozen in isopentane (chilled between -30 and -40 °C), and then stored at -80 °C until subsequent analysis.
Experiment 2
effect of long-term absence of endogenous glucocorticoid (ADX) and acute stress challenge (restraint) on ACTH secretion and Mkp-1 mRNA expression in the PVN, anterior pituitary and mPFC. On the test day, ADX and sham-ADX rats were either subjected to 30 min of restraint or left in their respective home cage (2 × 2 between-subjects factorial design; n=6, N=24). Immediately after decapitation, brains were removed, flash frozen in isopentane (chilled between -30 and -40 °C) and then stored at -80 °C until subsequent analysis. In addition, trunk blood was collected for subsequent plasma ACTH hormone measurement.
Experiment 3
effect of a phasic 1 or 3 hour corticosterone (CORT) pretreatment on mPFC and PVN Mkp-1 mRNA expression of non-stressed adrenal intact rats. On the test day, rats were given an injection of CORT (2.5 mg/kg, i.p.) or vehicle (1 ml/kg, i.p.) 1 or 3 hours before decapitation (2 × 2 factorial between-subjects factorial design; n=6; N=24). This exogenous CORT treatment procedure produces plasma CORT levels in rats that closely match the endogenous CORT levels and time course associated with a moderate intensity stressor, such as restraint (Pace et al. 2001; Barnum et al. 2008). One to three hours post CORT treatment is within the time range that another study observed a significant increase in mast cell Mkp-1 gene expression after glucocorticoid treatment (Kassel et al. 2001). After each injection, rats were returned to their home cage and home room. In order to minimize stress induced CORT increase in the vehicle treated rats, we habituated rats to the injection procedure by poking rats with the blunt end of a 1 ml syringe (no needle attached) for 2 min over a 3-day period before testing,. CORT was purchased from Steraloids Inc. (Newport, RI, USA) and dissolved in vehicle (10% ethanol, 30% propylene glycol and 60% sterile saline).
ACTH Radioimmunoassay
Trunk blood was collected into ethylenediaminetetraacetic acid-containing vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ, USA), placed on wet ice and then centrifuged for 15 min (4 °C). Plasma was then aliquoted into microfuge tubes and snap-frozen on dry ice. The blood processing procedure was completed within 45 min after decapitation. ACTH (pg/ml) was determined in duplicate (100 μl of plasma) by a competitive radioimmunoassay protocol as described previously (Osterlund & Spencer 2011). Radiolabeled 125-I ACTH-Tracer was obtained from DiaSorin (DiaSorin, Stillwater, MN, USA) and primary ACTH anti-serum Rb 7 (diluted to a final concentration of 1:30,000) was provided courtesy of Dr W. Engeland (University of Minnesota, twin cities campus, department of Neuroscience). The detection limit for this assay was 15 pg/ml; the intra-assay coefficient of variability was 4%.
CORT enzyme-linked immunosorbent assay
Plasma CORT samples were measured using 20 μl of plasma with an enzyme immunoassay kit (Assay Design, Ann Arbor, MI, USA). All samples were diluted 1:50 in assay buffer and incubated in 70 °C water bath for 1 hour to denature corticosteroid binding globulin. Heat inactivated samples were then processed per instructions from the assay kit. Sensitivity for the CORT assay, as reported by the manufacturer is 27 pg/ml. The intra-assay coefficient of variability was 6.3%.
in situ Hydridization Assay
Coronal brain sections and horizontal pituitary sections (12 μm thick) were sectioned with a cryostat (model 1850; Leica Microsystems, Nussloch, Germany) and thaw mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Generation of 35S-UTP labeled cRNA probes for Mkp-1 mRNA used a cDNA template of a portion of the Rattus norvegicus gene Dusp1 (accession number AF357203) corresponding to a 206 nucleotide mRNA sequence (nt 817-1022 from origin) that was cloned within the Spencer lab into a transcription vector (pSCA, Agilent Technologies). The Mkp-1 cDNA containing plasmids were subsequently linearized with the restriction endonuclease HindIII and transcribed using T7 RNA polymerase. The identity of Mkp-1 cloned DNA was verified by DNA sequencing (University of Colorado Molecular, Cellular and Developmental Biology sequencing facility). After the hybridization assay procedure (Girotti et al. 2006), slides were exposed to x-ray film (Kodak Biomax MR film) for 14 days. Semiquantitative analyses were performed on digitized images from x-ray films using the linear range of the gray values obtained from an acquisition system (Northern Lights lightbox, model B 95, Ontario, Canada; CCD camera, model XC-77, Sony, Tokyo, Japan; image capture with National Institutes of Health scion Image v1.59 software) as previously described (Campeau et al. 2002). Brain regions of interests (PVN, and prelimbic and infralimbic subregions of mPFC) were determined by matching digitized rat hypothalamic structures to rat brain atlas diagrams (Paxinos & Watson 2007). Quantification and analysis of images were performed by individuals that were blind to treatment condition assignments. For the PVN, bilateral measurements were taken from 4 tissue sections (approximately 1.8 mm posterior to bregma) for each rat (4-8 measurements for each rat brain). Measurements were also taken from 4-6 sections of the anterior pituitary. For the prelimbic (PrL) and infralimbic (IL) subregions of the mPFC, bilateral measurements were taken from 2-4 tissue sections (approximately 3.2 mm anterior to bregma) for each rat (4-8 measurements for each rat brain). Average uncalibrated optical densities for each region of interest were measured using the program NIH ImageJ (version 1.42q).
Statistical Analysis
One-way (Experiment 1) or two-way (Experiment 2 and 3) between groups analysis of variance was used to analyze the dependent measures (Statistical Package for the Social Sciences, SPSS, Macintosh computer version, Chicago, IL, USA). Significant F-test results were followed with a Fishers Least Significant Difference (FLSD) post hoc test in order to assess the statistical significance between pairs of groups. Additionally, a Pearson's r correlational analysis was performed (SPSS) for experiment 2. An alpha-level of P≤0.05 was used to determine statistical significance. All graphical presentation of data represent group means ± SEM.
Results
Experiment 1
PVN Mkp-1 mRNA increased after 15 and 30 minute restraint challenge.
In this first experiment we examined whether acute psychological stress (restraint) would lead to a rapid increase in Mkp-1 mRNA levels within the PVN. Under no-stress conditions there was very low Mkp-1 mRNA detected within the PVN. Restraint substantially increased PVN Mkp-1 mRNA; F(3,11) = 4.2, P < 0.05. Post-hoc tests indicate that there was a significant increase of PVN Mkp-1 mRNA within 15 min of restraint and a progressively greater increase after 30 min of restraint (Fig 1).
Figure 1.
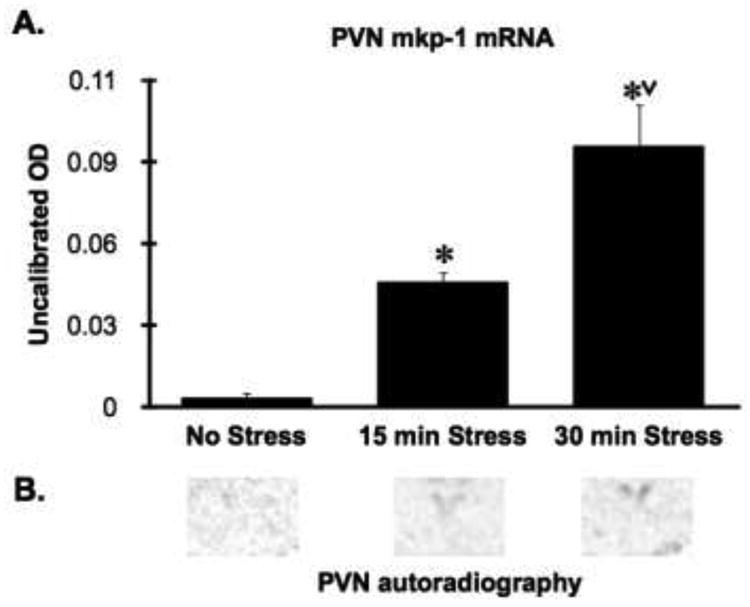
Stress rapidly increased Mkp-1 mRNA in the PVN. Panel A: Mkp-1 mRNA (optical density of autoradiographs) was significantly increased after 15 min of restraint, and was increased to a greater extent after 30 min of restraint. *, significant stress effect compared to the no stress group; V, significant stress effect compared to 15 minute stress group, p < 0.05, FLSD. Panel B: representative portions of autoradiographs surrounding the PVN taken from a rat within each treatment group as denoted in the aligned Panel A bar graph. Mkp-1 mRNA levels in the PVN were not visible on basal condition autoradiographs.
Experiment 2
Restraint increased Mkp-1 mRNA in the PVN, anterior pituitary and PFC, and this increase in the PVN and anterior pituitary was augmented by the long-term absence of endogenous glucocorticoids (ADX).
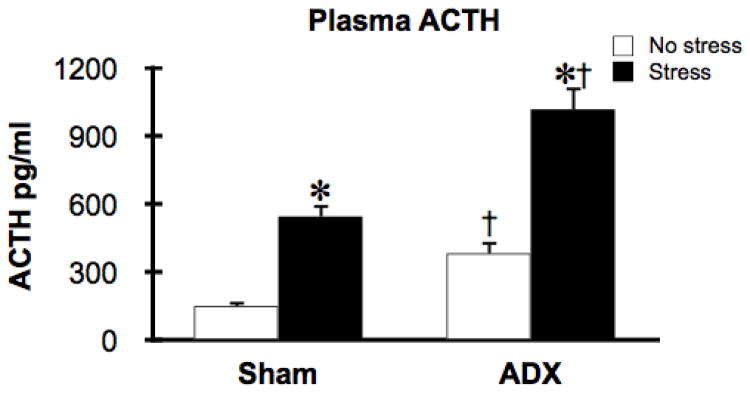
In this second experiment we examined whether acute stress (30 min restraint) would also lead to a rapid increase in Mkp-1 mRNA in an additional anatomical element of the HPA axis (the anterior pituitary) as well as in a separate brain region (mPFC). We also examined whether a stress-induced increase in Mkp-1 mRNA depended on stress-induced increases in endogenous CORT. As expected, restraint challenged rats displayed an increase in ACTH secretion (stress effect: F(1,19) = 78.3, p<0.05), and the absence of glucocorticoids resulted in a substantial increase in basal and stress-stimulated ACTH secretion (ADX effect: F(1,19) = 33.8, p<0.05; Fig 2). Plasma CORT analysis indicated that sham-ADX non-stressed rats displayed normal low basal levels of CORT (M = 48.8, ± SEM 16.0 ng / ml) and stressed sham-ADX rats had a significant increase in plasma CORT levels (M = 464.4, ± SEM 21.8 ng / ml). All ADX rats had CORT levels near or below the detection threshold of the assay.
Figure 2.
Both basal and stress-stimulated ACTH plasma levels were augmented in ADX rats. Four days after ADX or sham-ADX surgery rats were challenged with 30 min restraint. *, significant stress effect compared to the no stress group within the same surgical condition;, significant tonic CORT condition (ADX) effect compared to the sham group within same stress condition; p < 0.05, FLSD.
Under non-stress conditions, Mkp-1 mRNA levels were very low in the PVN and anterior pituitary (Fig. 3). Within the PrL and IL subregions of the mPFC, Mkp-1 mRNA levels were also low but clearly visible on autoradiograms (Fig 4). Sham rats challenged with 30 minutes of restraint displayed a significant increase in Mkp-1 mRNA expression within PVN (stress effect: F(1,19) = 37.4, p<0.05), anterior pituitary (stress effect: F(1,19)=29.8, p<0.05), PrL cortex (stress effect: F(1,19) = 14.7, p<0.05) and IL cortex (stress effect: F(1,19)=42.8, p<0.05). Stress-stimulated CORT levels of sham rats was not significantly correlated with stress-induced increase in Mkp-1 mRNA levels in the PVN (r=0.27; p>0.05), PrL cortex (r=0.34; p>0.05), or IL cortex (r=-0.063; p>0.05). Interestingly, there was a trend for a negative correlation between CORT levels and stress-induced Mkp-1 mRNA levels in the anterior pituitary (r=-0.64, p=.244), suggesting that acute CORT may have had an inhibitory influence on stress-induced Mkp-1 mRNA levels. Rather than attenuating stress-induced Mkp-1 mRNA levels, as predicted, ADX augmented stress-stimulated Mkp-1 mRNA levels within the PVN (stress x ADX interaction F(1,19)=11.0, p<0.05) and anterior pituitary (stress x ADX F(1,19)=5.1, p<0.05). There was also a trend for greater stress-induced Mkp-1 mRNA levels in the PrL and IL cortex of ADX rats, however, there was not a significant stress x ADX interaction in either brain region. On the other hand, within the IL cortex there was a significant increase in no-stress Mkp-1 mRNA levels of ADX rats compared to sham rats (FLSD, p < 0.05).
Figure 3.

Stress-stimulated Mkp-1 mRNA levels were augmented in PVN and anterior pituitary of ADX rats. Panel A: relative Mkp-1 mRNA levels for sham or ADX rats ◈ 30 min restraint challenge 4 days after surgery. *, significant stress effect compared to the no stress group within the same surgical condition; #, significant tonic CORT condition (ADX) effect compared to the sham group, within same stress condition; p < 0.05, FLSD. Panel B: representative autoradiographs for Mkp-1 mRNA expression in PVN (coronal brain section) and anterior pituitary (horizontal section); regions of interest are denoted on a representative autoradiograph taken from a rat within the ADX-Stress condition.
Figure 4.
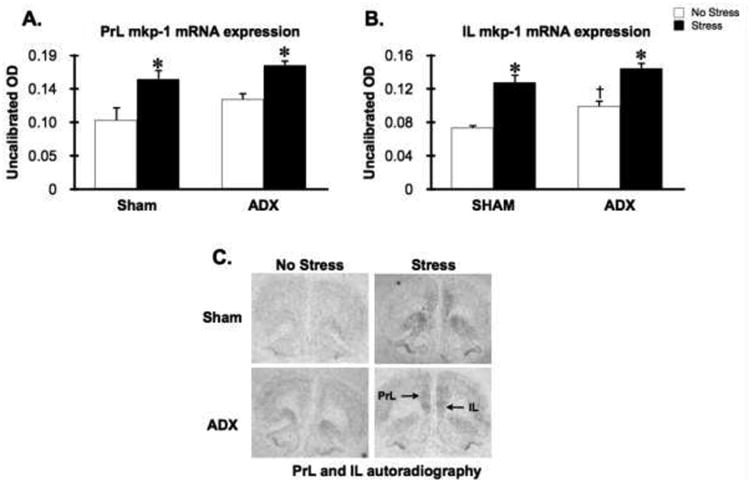
Stress increased Mkp-1 mRNA levels to a comparable degree in the mPFC of sham and ADX rats. Four days after ADX or sham-ADX surgery rats were challenged with 30 min restraint. Restraint increased Mkp-1 mRNA levels in the prelimbic (PrL, panel A) and infralimbic (IL, panel B) subregions of the mPFC, and ADX increased basal Mkp-1 mRNA levels in the IL. *, significant stress effect compared to the no stress group within the same surgical condition;, significant tonic CORT condition (ADX) effect compared to the sham group within same stress condition; p < 0.05, FLSD. Panel C: representative autoradiographs for Mkp-1 mRNA expression in mPFC (coronal brain section); regions of interest are denoted on a representative autoradiograph taken from a rat within the ADX-Stress condition. Note that there was low but visible Mkp-1 mRNA levels in the mPFC on basal condition autoradiographs.
Experiment 3
Acute CORT treatment was not sufficient to upregulate PVN or mPFC Mkp-1 mRNA levels.
Although Experiment 2 demonstrated that an increase in endogenous CORT was not necessary for a stress-induced increase in Mkp-1 mRNA, there is still the possibility that an acute increase in CORT is sufficient to produce an increase in Mkp-1 mRNA in PVN and mPFC, which perhaps may be masked by the effect of restraint stress. Thus, this experiment examined the effect of vehicle or CORT injection in the absence of restraint stress on subsequent Mkp-1 mRNA. As expected, plasma CORT measures indicated that there was a greater level of plasma CORT present 1 hr after CORT injection (M = 149.1, ± SEM 51.3 ng/ml) compared to vehicle injection (M = 33.4, ± SEM 13.0 ng/ml). By 3 hr after CORT injection, the exogenous CORT had cleared such that plasma CORT levels were low in both CORT injected rats (M = 7.5, ± SEM 1.5 ng/ml) and vehicle injected rats (M = 22.0, ± SEM 11.2 ng/ml).
We observed no difference in Mkp-1 mRNA levels of CORT vs vehicle injected rats in either brain region (Fig 5). Similar to non-stressed conditions in experiment 1 and 2 we observed almost undetectable levels of Mkp-1 mRNA within the PVN. Within the PrL there was a moderate level of Mkp-1 mRNA expression present 1 hr after injection, but it did not differ between CORT or vehicle treatment. Interestingly, for both CORT and vehicle treatment groups there was a lower level of Mkp-1 mRNA expression in PrL 3 hr after injection compared to 1 hr after injection (post injection time: F1,10 = 2.4, P < 0.05), perhaps indicating that the stress of injection produced a transient increase in Mkp-1 mRNA levels in PrL that was evident 1 hr, but less so by 3 hr after injection. A similar pattern of Mkp-1 mRNA was observed in IL (data not shown).
Figure 5.
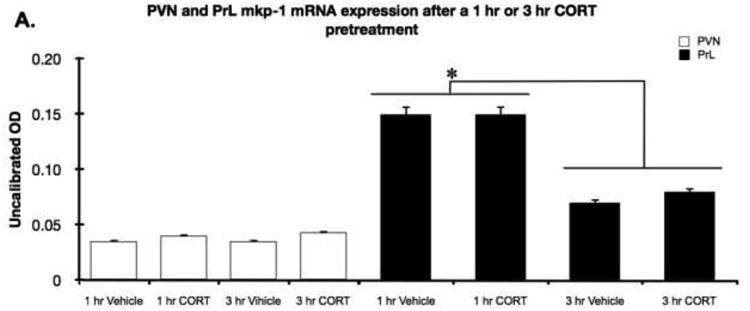
Acute CORT treatment did not increase PVN or prelimbic cortex Mkp-1 mRNA levels. Adrenal-intact rats were injected with CORT (2.5 mg/kg i.p.) or vehicle 1 or 3 hr prior to sacrifice. There was very low Mkp-1 mRNA expression in the PVN for the 4 treatment groups. There was a moderately high level of Mkp-1 mRNA expression in the prelimbic (PrL) subregion of the mPFC 1 hr after injection and lower levels 3 hr after injection (p < 0.05), but the levels did not differ between CORT or vehicle injected rats. *, significant pretreatment-time difference effect in the PrL.
Discussion
In this study we found that Mkp-1 mRNA was rapidly increased by acute psychological stress within anatomical elements of the HPA axis (PVN and anterior pituitary) and in a stress-responsive brain region that provides regulatory modulation over the HPA axis (mPFC) (Diorio et al. 1993; Radley et al. 2006; Weinberg et al. 2010). Contrary to predictions based on studies of glucocorticoid regulation of Mkp-1 gene expression in peripheral tissues and cell lines (Clark et al. 2008), we found that acute CORT treatment was not sufficient to increase Mkp-1 mRNA within the brain and endocrine tissues examined. Moreover, stress-induced CORT secretion was not necessary for the rapid increase in Mkp-1 mRNA observed after acute stress. Instead, we found that stress-induced Mkp-1 gene expression was augmented within the PVN and anterior pituitary of rats that lacked endogenous adrenal glucocorticoids. These results suggest that Mkp-1 expression is dynamically regulated in brain and neuroendocrine tissue, and that endogenous glucocorticoids may provide a tonic suppressive role in regulating Mkp-1 gene expression in these tissues, perhaps by indirectly constraining activity-dependent regulation of MAP-kinase (see discussion below).
A number of studies have found that the Mkp-1 gene behaves as an activity-dependent immediate early gene in response to a wide variety of stimuli within various peripheral cell types and transformed cell lines (Clark 2003; Patterson et al. 2009; Caunt & Keyse 2013). Initial indication that the Mkp-1 gene may be regulated in a similar fashion within mammalian neural tissue was provided by studies that observed a rapid increase in Mkp-1 mRNA in striatal and hippocampal subregions of rodent brain after direct electrical stimulation or electroconvulsive seizure (Sgambato et al. 1998; Davis et al. 2000; Kodama et al. 2005). Subsequently, activity-dependent Mkp-1 gene induction in the mammalian brain has also been observed in the suprachiasmatic nucleus in response to a light pulse during the subjective night (Doi et al. 2007). Our first experiment showed that PVN Mkp-1 gene expression was rapidly increased within 15 minutes of restraint, an acute stress challenge that is predominantly psychological in nature (Herman & Cullinan 1997; Dayas et al. 2001). Our second experiment showed that this same stressor produced a rapid increase in Mkp-1 mRNA within the anterior pituitary and mPFC (PrL and IL). Despite the contrasting influence that the PrL and IL cortex provide over the PVN (inhibitory and excitatory, respectively) (Radley et al. 2006), both subregions of the medial-PFC display stress-dependent rapid induction of other experience-dependent genes such as c-fos and Fra-2 (Weinberg et al. 2007). We observed nearly undetectable levels of Mkp-1 mRNA within the PVN and the anterior pituitary under no-stress conditions, whereas there was a greater degree of constitutive Mkp-1 mRNA expression within the mPFC. Moreover, our third experiment suggests that there may be a greater sensitivity of Mkp-1 mRNA induction to the mild stress of vehicle injection in the mPFC than the PVN. Overall, our study extends the extent of known activity-dependent operation of the Mkp-1 gene to neural and endocrine elements of the HPA axis and to the mPFC. In addition, our results indicate that a moderate intensity stressor is an effective experience for producing a rapid and substantial increase of Mkp-1 mRNA in the adult rat brain.
In this study we explored the prospect that CORT serves as both an intercellular and intracellular stress-dependent signal for Mkp-1 gene induction (Clark 2003; Clark et al. 2008). We found, however, that the presence of endogenous CORT was not necessary for the stress-induced increase in Mkp-1 mRNA within the tissue examined. In addition, acute CORT treatment was not sufficient to increase Mkp-1 mRNA levels. An in vitro study has also noted that glucocorticoid treatment alone does not increase Mkp-1 gene expression, but that study found that glucocorticoid treatment augmented Mkp-1 gene induction to other cellular stimuli (Zhou et al. 2007). In contrast to the finding of that study, we observed an opposite effect. In the anatomical elements of the HPA axis there was a greater stress-induced increase in Mkp-1 mRNA in rats lacking endogenous CORT. It should be noted that the mPFC, PVN, and anterior pituitary all express high levels of GR (Gustafsson et al. 1987; Herman 1993; Francis et al. 2006). Further, the levels of CORT typically secreted in response to restraint are sufficient to activate GR in those tissues (Reul & de Kloet 1985; Spencer 1993). Thus, we conclude that CORT-activated GR does not contribute to a rapid induction of Mkp-1 mRNA in the PVN, mPFC and anterior pituitary.
Several functional glucocorticoid response elements have recently been identified in the distal promoter region of the human Mkp-1 gene (Shipp et al. 2010; Tchen et al. 2010). Although some of those putative GREs exhibit high homologous nucleotide sequences at approximately the same upstream locations of the rat Mkp-1 gene, they fail to exhibit comparable functional responsiveness to glucocorticoids when assessed in gene transcription reporter assays (Tchen et al. 2010). Whether glucocorticoids influence transcriptional processes is often dependent on cellular function and location. For example, glucocorticoids provide suppressive transcriptional regulation of the crh gene in the PVN CRH neuroendocrine neurons (Swanson & Simmons 1989; Ginsberg et al. 2003; Bali et al. 2008; Sharma et al. 2013), but in other forebrain regions, such as the amygdala and bed nucleus of the stria terminalis, glucocorticoids either upregulate crh gene expression or have no effect (Makino et al. 1995; Schulkin et al. 1998). Glucocorticoid regulation of the Mkp-1 gene may also vary with cell phenotype and cell state, and these factors may differ across species (Tchen et al. 2010).
The profile of glucocorticoid regulation of Mkp-1 gene expression in the PVN that we observed in this study is similar to what we have seen in previous studies for the immediate early genes c-fos, zif268 and ngfi-b (Girotti et al. 2007; Pace et al. 2009). Whereas the absence of endogenous glucocorticoids produced a large increase in both basal and stimulated ACTH hormone levels, for each of these immediate early genes only stimulated levels were augmented in the PVN of ADX rats. This expression profile may indicate that glucocorticoids indirectly regulate the expression of Mkp-1 and other immediate early genes within the PVN. One possible mechanism for this indirect effect could be alteration of CRH neuron responses to acute stress by altering CRH receptor (CRH-R1) expression within PVN CRH neurons (Imaki et al. 2002, Luo et al. 1995). For example, ADX has been shown to increase CRH-R1 expression in the rat PVN (Luo et al. 1995). Thus, the long-term absence of CORT may lead to an augmented positive CRH feedback loop onto PVN CRH neurons, resulting in increased signaling activity (e.g. MAP-kinase activation), transcriptional processes (e.g. Mkp-1 gene expression) and neuropeptide release (e.g. CRH) within these neuroendocrine cells. Regardless of the mechanism, there appears to be some process by which the tonic presence of glucocorticoids constrains the general reactivity of these cells to excitation (Pace et al. 2009; Osterlund et al. 2011; Osterlund & Spencer 2011; Weiser et al. 2011).
Although the specific process remains undetermined, the augmented Mkp-1 gene expression in the absence of glucocorticoids may be secondary to an augmentation of ERK1/2 activation in CRH neurons. We have previously observed an increased number of stress-induced phospho-ERK1/2 positive cells in the PVN of ADX rats, which was normalized by giving ADX rats CORT in their drinking water (Osterlund et al. 2011). MAP-kinase pathway activation has been found to converge on Mkp-1 gene expression in a number of cell types (Brondello et al. 1997; Camps et al. 2000; Kassel et al. 2001). This Mkp-1 gene induction may serve as a form of intracellular negative feedback control over the pluripotent MAP-kinase intracellular signaling network. There is some evidence in neural tissue that activity-dependent Mkp-1 gene induction depends specifically on ERK1/2 (Sgambato et al. 1998; Jeanneteau et al. 2012). Interestingly, in cultured cortical neurons, ERK1/2 activation was necessary for activity-dependent Mkp-1 gene induction, with the subsequent upregulated MKP-1 protein responsible for negative regulation of the phosphorylation state and function of a different MAP-Kinase (c-jun N-terminal kinase) (Jeanneteau et al. 2012). Basal CORT levels may not only constrain Mkp-1 expression within the HPA axis, they may also have an important modulatory influence on Mkp-1 gene expression in a variety of extrinsic hypothalamic forebrain regions. In this study we saw a trend for greater stress-induced Mkp-1 mRNA in the mPFC of ADX rats. In addition, there was a significant increase in basal Mkp-1 mRNA in the IL subregion of the mPFC of ADX rats.
Our results, taken together with others, suggests that the Mkp-1 gene may be induced in a wide-range of brain regions by an extensive set of experiential conditions. Thus, the activity dependence of Mkp-1 gene expression may resemble that of some other activity-dependent immediate early genes, such as c-fos, Zif268, and Arc that are critical for experience-dependent neuroplasticity (Guzowski et al. 2001; Loebrich & Nedivi 2009; Okuno 2011). Given the importance of the MAP-kinase intracellular signaling network for neuroplasticity in the developing and adult brain, it would not be surprising that tight regulation of this molecular network is critical for optimal function (Sweatt 2001; Waltereit & Weller 2003; Davis & Laroche 2006; Ayroldi et al. 2012). Mice lacking the Mkp-1 gene exhibit a range of heightened inflammatory processes, presumably due to the overactivity of MAP-kinases (Chi et al. 2006; Maier et al. 2007; Wang et al. 2008; Patterson et al. 2009; Vandevyver et al. 2012). This antiinflammatory role of MKP-1 may extend to brain microglia (Zhou et al. 2007). Cortical neurons cultured from Mkp-1 gene knockout mice are deficient in their axonal branching response to brain derived neurotrophic factor (Jeanneteau et al. 2012). Support for the importance of Mkp-1 gene expression levels in human brain function is provided by a study that found increased Mkp-1 mRNA levels in the postmortem brains of individuals with major depressive disorder (Duric et al. 2010). The dynamic interaction between MAP-kinases and MKP-1 appears to be an important element of neural function, and may be an effective pharmacotherapeutic target. Further study of how that interaction may be modulated by tonic changes in glucocorticoids associated with chronic stress and various mental and physical disorders is warranted.
Acknowledgments
We thank Erin Jarvis for providing technical assistance of the cloning of rat MKP-1 cDNA.
Funding: This work was supported by National Institute of Mental Health (NIH) grants MH75968 and MH065977, and the University of Colorado Undergraduate Research Opportunity Program.
Footnotes
Declaration of Interest: The authors declared no conflict of interest that could be perceived as prejudice the impartiality of the research reported.
References
- Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2012;26:4805–4820. doi: 10.1096/fj.12-216382. [DOI] [PubMed] [Google Scholar]
- Bali B, Ferenczi S, Kovács KJ. Direct inhibitory effect of glucocorticoids on corticotrophin-releasing hormone gene expression in neurones of the paraventricular nucleus in rat hypothalamic organotypic cultures. Journal of Neuroendocrinology. 2008;20:1045–1051. doi: 10.1111/j.1365-2826.2008.01759.x. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Eskow KL, Dupre K, Blandino P, Deak T, Bishop C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta. Neuroscience. 2008;156:30–41. doi: 10.1016/j.neuroscience.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondello JM, Brunet A, Pouysségur J, McKenzie FR. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. The Journal of Biological Chemistry. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- Burke SJ, Goff MR, Updegraff BL, Lu D, Brown PL, Minkin SC, Biggerstaff JP, Zhao L, Karlstad MD, Collier JJ. Regulation of the CCL2 gene in pancreatic β-cells by IL-1β and glucocorticoids: role of MKP-1. PloS One. 2012;7:e46986. doi: 10.1371/journal.pone.0046986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress (Amsterdam, Netherlands) 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2000;14:6–16. [PubMed] [Google Scholar]
- Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. The FEBS Journal. 2013;280:489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? Journal of Endocrinology. 2003;178:5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- Clark AR, Martins JRS, Tchen CR. Role of Dual Specificity Phosphatases in Biological Responses to Glucocorticoids. The Journal of Biological Chemistry. 2008;283:25765–25769. doi: 10.1074/jbc.R700053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Progress in Hormone Research. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pagès C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. The Journal of Neuroscience. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes, Brain, and Behavior. 2006;5(2):61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European Journal of Neuroscience. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Cho S, Yujnovsky I, Hirayama J, Cermakian N. Cato ACB & Sassone-Corsi P 2007 Light-inducible and clock-controlled expression of MAP kinase phosphatase 1 in mouse central pacemaker neurons. Journal of Biological Rhythms. 22:127–139. doi: 10.1177/0748730406298332. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nature Medicine. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis AB, Pace TWW, Ginsberg AB, Rubin BA, Spencer RL. Limited brain diffusion of the glucocorticoid receptor agonist RU28362 following i.c.v. administration: implications for i.c.v. drug delivery and glucocorticoid negative feedback in the hypothalamic-pituitary-adrenal axis. Neuroscience. 2006;141:1503–1515. doi: 10.1016/j.neuroscience.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS, Spencer RL. Differential responses of hypothalamus-pituitary-adrenal axis immediate early genes to corticosterone and circadian drive. Endocrinology. 2007;148:2542–2552. doi: 10.1210/en.2006-1304. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Current Opinion in Neurobiology. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA, Carlstedt-Duke J, Poellinger L, Okret S, Wikström AC, Brönnegård M, Gillner M, Dong Y, Fuxe K, Cintra A. Biochemistry, molecular biology, and physiology of the glucocorticoid receptor. Endocrine Reviews. 1987;8:185–234. doi: 10.1210/edrv-8-2-185. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. The Journal of Neuroscience. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cellular and Molecular Neurobiology. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Issa R, Xie S, Khorasani N, Sukkar M, Adcock IM, Lee K-Y, Chung KF. Corticosteroid inhibition of growth-related oncogene protein-alpha via mitogen-activated kinase phosphatase-1 in airway smooth muscle cells. Journal of Immunology. 2007;178:7366–7375. doi: 10.4049/jimmunol.178.11.7366. [DOI] [PubMed] [Google Scholar]
- Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotropin-releasing hormone type 1 receptor in paraventricular nucleus after acute stress. Neuroendocrinology. 2001;73(5):293–301. doi: 10.1159/000054646. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. The EMBO Journal. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Current Opinion in Cell Biology. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Khan AM, Watts AG. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology. 2004;145:351–359. doi: 10.1210/en.2003-0539. [DOI] [PubMed] [Google Scholar]
- Khan AM, Kaminski KL, Sanchez-Watts G, Ponzio TA, Kuzmiski JB, Bains JS, Watts AG. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. Journal of Neuroscience. 2011;31:18479–18491. doi: 10.1523/JNEUROSCI.4785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. Journal of Neuroscience. 2007;27:7344–7360. doi: 10.1523/JNEUROSCI.0873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Russell DS, Duman RS. Electroconvulsive seizures increase the expression of MAP kinase phosphatases in limbic regions of rat brain. Neuropsychopharmacology. 2005;30:360–371. doi: 10.1038/sj.npp.1300588. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Jono H, Lim JH, Imasato A, Xu H, Kai H, Yan C, Li JD. Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phosphatase-1-dependent inhibition of p38 MAPK. Biochemical and Biophysical Research Communications. 2008;377:763–768. doi: 10.1016/j.bbrc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. The Journal of Neuroscience. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Nedivi E. The function of activity-regulated genes in the nervous system. Physiological Reviews. 2009;89:1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Kiss A, Rabadan-Diehl C, Aguilera G. Regulation of hypothalamic and pituitary corticotropin-releasing hormone receptor messenger ribonucleic acid by adrenalectomy and glucocorticoids. Endocrinology. 1995;136(9):3877–3883. doi: 10.1210/endo.136.9.7649095. [DOI] [PubMed] [Google Scholar]
- Maier JV, Brema S, Tuckermann J, Herzer U, Klein M, Stassen M, Moorthy A, Cato ACB. Dual specificity phosphatase 1 knockout mice show enhanced susceptibility to anaphylaxis but are sensitive to glucocorticoids. Molecular Endocrinology. 2007;21:2663–2671. doi: 10.1210/me.2007-0067. [DOI] [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith MA, Pacák K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- Nicoletti-Carvalho JE, Lellis-Santos C, Yamanaka TS, Nogueira TC, Caperuto LC, Leite AR, Anhê GF, Bordin S. MKP-1 mediates glucocorticoid-induced ERK1/2 dephosphorylation and reduction in pancreatic ß-cell proliferation in islets from early lactating mothers. American Journal of Physiology- Endocrinology and Metabolism. 2010;299:E1006–E1015. doi: 10.1152/ajpendo.00341.2010. [DOI] [PubMed] [Google Scholar]
- Okuno H. Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neuroscience Research. 2011;69:175–186. doi: 10.1016/j.neures.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Osterlund CD, Jarvis E, Chadayammuri A, Unnithan R, Weiser MJ, Spencer RL. Tonic, But Not Phasic Corticosterone, Constrains Stress ActivatedExtracellular-Regulated-Kinase 1/2 Immunoreactivity Within the Hypothalamic Paraventricular Nucleus. Journal of Neuroendocrinology. 2011;23:1241–1251. doi: 10.1111/j.1365-2826.2011.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund C, Spencer R. Corticosterone pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action and de novo protein synthesis. The Journal of Endocrinology. 2011 doi: 10.1530/JOE-10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T, Cole M, WARD G, Kalman B, Spencer R. Acute exposure to a novel stressor further reduces the habituated corticosterone response to restraint in rats. Stress. 2001;4:319–331. doi: 10.3109/10253890109014755. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. The European Journal of Neuroscience. 2005;22:1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. The Biochemical Journal. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. Journal of Neuroscience Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. The Journal of Neuroscience. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovács KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Progress in Brain Research. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pagès C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. The Journal of Neuroscience. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone Induces a Putative Repressor Complex and Chromatin Modifications in the CRH Promoter. Molecular Endocrinology. 2013;27:1142–1152. doi: 10.1210/me.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp LE, Lee JV, Yu CY, Pufall M, Zhang P, Scott DK, Wang JC. Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PloS One. 2010;5:e13754. doi: 10.1371/journal.pone.0013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipston MJ. Mechanism(s) of early glucocorticoid inhibition of adrenocorticotropin secretion from anterior pituitary corticotropes. Trends in Endocrinology and Metabolism: TEM. 1995;6:261–266. doi: 10.1016/1043-2760(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Spencer RL. Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology. 1993;133:1941–1950. doi: 10.1210/endo.133.5.8404640. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. The Journal of Comparative Neurology. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tchen CR, Martins JRS, Paktiawal N, Perelli R, Saklatvala J, Clark AR. Glucocorticoid regulation of mouse and human dual specificity phosphatase 1 (DUSP1) genes: unusual cis-acting elements and unexpected evolutionary divergence. The Journal of Biological Chemistry. 2010;285:2642–2652. doi: 10.1074/jbc.M109.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-reviews3009. REVIEWS3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, Van Bogaert T, Kleyman A, Liu Y, Tuckermann J, Libert C. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. The Journal of Clinical Investigation. 2012;122:2130–2140. doi: 10.1172/JCI60006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Molecular Neurobiology. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Wang X, Nelin LD, Kuhlman JR, Meng X, Welty SE, Liu Y. The role of MAP kinase phosphatase-1 in the protective mechanism of dexamethasone against endotoxemia. Life Sciences. 2008;83:671–680. doi: 10.1016/j.lfs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Frontiers in Neuroendocrinology. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168:744–756. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg M, Girotti M, Spencer R. Restraint-induced fra-2 and c-fos expression in the rat forebrain: Relationship to stress duration. Neuroscience. 2007;150:478–486. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Osterlund C, Spencer RL. Inhibitory Effects of Corticosterone in the Hypothalamic Paraventricular Nucleus (PVN) on Stress-Induced Adrenocorticotrophic Hormone Secretion and Gene Expression in the PVN and Anterior Pituitary. Journal of Neuroendocrinology. 2011;23:1231–1240. doi: 10.1111/j.1365-2826.2011.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ling EA, Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. Journal of Neurochemistry. 2007;102:667–678. doi: 10.1111/j.1471-4159.2007.04535.x. [DOI] [PubMed] [Google Scholar]