Summary
The adoptive transfer of T cells specific for native tumor antigens (TAs) is an increasingly popular cancer treatment option because of the ability of these cells to discriminate between normal and tumor tissues and corresponding lack of short or long-term toxicities. Infusions of antigen-specific CD4+ and CD8+ T cells targeting viral antigens derived from Epstein Barr virus (EBV) induce sustained complete tumor remissions in patients with highly immunogenic tumor’s such as post-transplant lymphoproliferative disease, although resistance occurred when the infused T-cell population had restricted antigen specificity. T cells specific for EBV antigens have also produced complete remissions of EBV-positive nasopharyngeal carcinomas and lymphomas developing in immunocompetent individuals, even though in these patients tumor survival is dependent on their ability to evade T-cell immunity. Adapting this strategy to non-viral tumors is more challenging, as the target antigens expressed are less immunogenic and the tumors lack the potent danger signals that are characteristic of viruses. The goals of current studies are to define conditions that promote expansion of antigen-specific T cells ex vivo and to ensure their in vivo persistence and survival by combining with maneuvers such as lymphodepletion, checkpoint inhibition, cytokine infusions, or genetic manipulations. More pragmatic goals are to streamline manufacturing to facilitate the transition of these therapies to late phase trials and to evaluate closely histocompatibility antigen (HLA)-matched banked antigen-specific T-cells so that T-cell therapies can be made more broadly available.
Introduction
The exquisite specificity, safety, and efficacy of therapeutic T cells with native receptor specificity has been demonstrated repeatedly in trials of donor-derived, virus-specific T cells (VSTs) for the prevention and treatment of virus-associated diseases and malignancies in the hematopoietic stem cell transplant (HSCT) setting (1–3). The lymphopenic environment that results from a T-cell-depleted HSCT promotes the proliferation of transferred T cells and antigenic stimulation provided by poorly controlled viruses ensures rapid T-cell expansion and repopulation of the memory compartment.
VSTs have also produced impressive clinical responses outside of the transplant setting in patients with Epstein-Barr virus (EBV)-associated lymphoma and nasopharyngeal carcinoma (4–6). However, in these diseases, T cells must contend with an evolving array of immune evasion strategies that impede both afferent and efferent arms of the immune response: most tumors produce inhibitory cytokines and ligands, recruit cohorts of inhibitory cell types and subvert the function of proinflammatory cell types (7,8). To advance T-cell therapies for cancer, strategies to counteract these inhibitory mechanisms must be developed.
T cells specific for non-viral tumor antigens (TAs) must contend not only with immune evasion mechanisms but with the weakness of the TAs they recognize. Non-viral TAs are generally ‘self’ antigens, and since high affinity T cells with self-specificity are deleted by central and peripheral tolerance mechanisms, only T cells with relatively weak affinities remain. Further, tumor cells are generally poor antigen-presenting cells (APCs), they lack the potent danger signals provided by pathogens, and they can inactivate professional APCs so that TAs may never be presented adequately to T cells. Nevertheless, an increasing number of self or modified-self TAs has been described, and reactive T-cells can be detected in healthy donors and cancer patients. Further, ex vivo-expanded antigen-specific tumor-infiltrating lymphocytes (TILs) have produced impressive results in patients with melanoma, if they receive intensive prior conditioning (9). However, in most cases it is a major challenge to reactivate and expand sufficient TA-specific T-cells for clinical use in vitro.
Even when protocols for the in vitro generation of TA-specific T cells for clinical use have been developed, strategies are required to ensure that the infused T cells access the immunosuppressive tumor environment and then continue to proliferate and function. Lymphodepletion is commonly used to reduce the number of inhibitory cells within tumor tissues and to provide space and homeostatic cytokines to enhance T-cell proliferation, and this has dramatically enhanced response rates in melanoma (10). There is also increasing interest in combining T cells with biological response modifiers such as antibodies to inhibitory ligands like programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA4) or epigenetic modifiers like histone deacetylase (HDAC) inhibitors or demethylating agents (11, 12). T cells are amenable to genetic modification and can be rendered resistant to immune inhibition or can be used as delivery vehicles for immunostimulatory or oncolytic agents. Finally, more pragmatic hurdles remain. Manufacturing strategies must meet standards that are increasingly restrictive as promising cell therapy product progresses to late phase trials. This is particularly onerous in Europe, where excessive regulatory impediments have frustrated the implementation of T-cell therapies, even at phase I.
Viral antigens and immunogenicity
VSTs used in the stem cell transplant setting have provided a paradigm for adoptive T-cell immunotherapy. Small numbers of VSTs proliferate exponentially after infusion, persist for up to 10 years, remain capable of re-expanding in vivo in response to virus reactivation and both prevent and cure virus-associated diseases. This has been demonstrated clearly for EBV, cytomegalovirus (CMV), and adenoviruses, and clinical studies targeting other common community viruses that produce morbidity and mortality in immunocompromised patients are in early clinical trials. The reasons for the success of T cells in this lymphopenic setting are that an excess of homeostatic cytokines are available to expand infused T-cells and viruses are poorly controlled providing antigens for T-cell stimulation. Outside of the transplant setting, most clinical interest in the use of VSTs has been to target virus-associated tumors and human immunodeficiency virus (HIV).
Oncogenic viruses account for about 15% of the world’s cancer, and the number of identified tumor-associated viruses continues to increase. These include five directly oncogenic virus groups; human papillomaviruses (HPV), EBV, human herpesvirus 8 (Kaposi sarcoma virus), human T-cell leukemia virus (HTLV 1), and the Merkel cell polyoma virus (MCPyV). Indirectly oncogenic viruses, such as the hepatitis B and C, produce chronic inflammation that has been shown to lead to hepatocellular carcinoma, while non-oncogenic viruses, such as CMV or measles, opportunistically infect pre-existing tumors thereby providing target antigens for T cells. Even when opportunistic viruses do not infect all tumor cells they carry danger signals that can enhance anti-tumor immunity and induce the activation and expansion of T-cells specific for non-viral TAs (epitope spreading) and may also produce direct tumor cell lysis. This phenomenon has been illustrated by tumor regression following naturally acquired infections with non-oncogenic viruses such as measles or varicella zoster (13). One challenge for the use of VSTs to treat tumors is to identify target antigens that elicit therapeutic T cells. The antigens that induce T-cells that best protect against viral infection, usually virion proteins and immediate early proteins, may be optimal for the treatment of tumors carrying opportunistic passenger viruses but may not be suited to the treatment of tumors carrying non-replicating oncogenic viruses.
Human papilloma viruses
About 18 oncogenic HPVs have so far been identified, the highest risk strains being HPVs 16, 18, 31, and 45 that are associated with genital and oropharyngeal carcinomas and account for about 5.2% of new cancer cases worldwide. As tumors progress from pre-neoplastic lesions to carcinoma, their episomal viral genomes are lost, leaving integrated fragments encoding the E6 and E7 oncogenes that provide targets for T cells. While virus-like particle vaccines have had outstanding success in preventing primary infection with HPV (14), no clinical trials of either vaccines or T cells have produced a major impact on established HPV+ carcinoma, although long peptides overlapping the E6 and E7 sequences of HPV 16 produced CD4+ and CD8+ HPV-specific T cells in patients with resected cervical cancer.(15)
Epstein-Barr virus
EBV has a complex life cycle involving infection of oropharyngeal epithelial cells and B cells at different stages of differentiation and maturation. All EBV-associated tumors involve viral latency and generally three different patterns of viral latent gene expression are found in tumors. Nine latency proteins, including nuclear (EBNAs), membrane proteins (LMPs), and the secreted BARF1 gene product, are expressed in the B-cell lymphomas that arise in immunocompromised patients (16). These antigens are immunogenic and immunostimulatory and a result the lymphomas are highly immunogenic and never found in immunocompetent hosts. The reactivation and expansion of T cells specific for EBV latency antigens from healthy seropositive donors is simple and reproducible and effectively prevents and treats EBV+ lymphomas after allogeneic HSCT. By contrast, tumors that arise in immunocompetent persons express a more limited array of antigens, LMP1, LMP2, EBNA1, and BARF1. These are less immunogenic but can still be targeted by T cells. LMP1 and LMP2-specific T cells have been effective in the context of lymphoma and NPC, producing complete tumor responses in over 50% of patients with relapsed lymphoma and 20–30% of patients with NPC (5,17). Trials of T cells for the treatment of Burkitt’s lymphoma that express only EBNA1 have not been reported, although EBNA1-specific T cells have been used successfully to treat patients with PTLD following HSCT (18).
Human herpesvirus 8 and Merkel cell polyomaviruse
The use of T cells to treat Kaposi’s sarcoma (HHV8), adult T-cell leukemia (HTLV1), and Merkel cell carcinoma [Merkel cell polyomavirus (MCPyV)] has not yet been reported, although T cells specific for these viruses have been identified (19). The best therapy for patients infected with viruses, such as hepatitis C virus (HCV), that impart a high risk of malignancy may be T cells that prevent virus replication, but tumor cells carrying oncogenic viruses must be targeted by T cells specific for antigens expressed within the tumors. For example, T cells specific for the Tax and HBZ proteins of HTLV-1 are associated with a low proviral load and a reduced risk of developing leukemia, while for MCPyV the large T antigen is an essential oncogene and an obvious target (20). Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers (21).
In patients with hepatitis B and C, boosting the immune system with broad spectrum VSTs may facilitate viral clearance and reduce the risk of developing HCC (22). However, in patients with HCC, while viral antigens are detected in tumor cells, they are also detected in normal infected hepatocytes, and so treatment with potent VSTs should be considered with caution.
Cytomegalovirus
CMV DNA and antigens are detected in glioblastoma, and several clinical studies are using CMV-specific T cells as therapy (23). So far the immunodominant CMV matrix protein pp65 has been targeted, but preclinical studies have shown that T-cells specific for both pp65 and the immediate early protein (IE) can readily be generated from patients with CMV+ glioma and kill CMV-infected glioma cells (23). More recently, CMV has been detected in colon cancer, EBV-negative Hodgkin’s lymphoma, cervical cancer, prostate cancer, breast cancer, neuroblastoma, and medulloblastoma (24–26), and there is increasing speculation that the virus might have oncomodulatory effects or even have direct oncogenicity (27). CMV-specific T cells therefore may play a role in the treatment of these tumors in the future.
Oncolytic viruses
Anecdotal observations that tumor regression can follow viral infection has led to the burgeoning field of oncolytic virotherapy, in which wildtype or recombinant viruses that preferentially infect and replicate in tumors, often relying on the active tumor metabolism for gene expression, have been used to treat tumors. A wide range of viruses, including vaccinia, adenovirus, reovirus, measles, herpes simplex, and Newcastle disease virus have been used in this fashion, usually modified genetically to enhance their tumor specificity and their ability to activate the immune response. T-cell immune responses to the viruses may play an important role in tumor cell killing and epitope spreading, so that VSTs could potentially be used to enhance tumor cell lysis but also may limit virus spread throughout the tumor.
Manufacture of virus-specific T cells
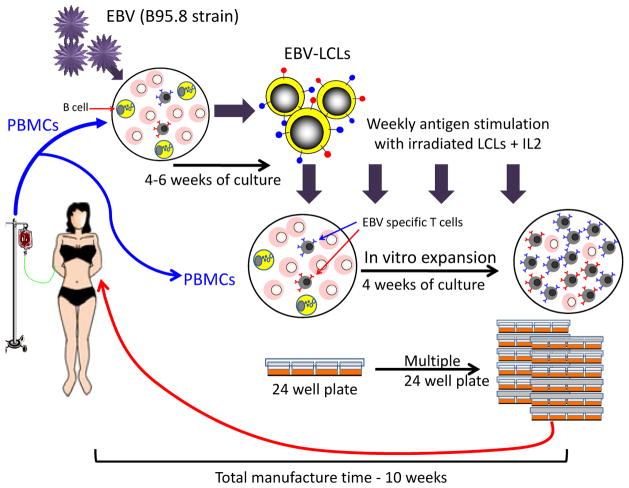
The selective expansion of virus-reactive T-cell lines in vitro requires repetitive antigenic stimulation in the presence of cytokines. Although there is a wealth of evidence supporting the safety and activity of such cells when used clinically (Tables 1 to 4), extension of this approach beyond specialized centers has been restricted by the cost and complexity associated with manufacture and the requirement for individualized products. For example, we have traditionally propagated EBV-directed T-cell lines by weekly stimulation of peripheral blood mononuclear cells (PBMCs) with autologous EBV-transformed B-lymphoblastoid cell lines (EBV-LCLs) in the presence of IL-2 using conventional tissue-culture treated 24-well plates (28) (Fig. 1). The laboratory strain of EBV (B95-8) required to produce the EBV-LCL that is used as a source both of viral antigens and APCs for VST generation is expensive to make and test, while the 8 to 10 weeks of culture (4–6 weeks for EBV-LCL and ~4 weeks for VST) required to produce sufficient cells for infusion and testing has the unfortunate consequence that cells must be manufactured in advance for high-risk patients. To overcome these obstacles, we and others have explored more rapid strategies for generating virus-specific T cells (Fig. 2).
Table 1.
Donor cytotoxic T lymphocytes post hematopoietic stem cell transplant.
| Patient number | Type of CTL | Antitumor l effects | Reference |
|---|---|---|---|
| 113 | LCL-activated EBV-specific T-cells |
Prophylaxis: None of 101 developed PTLD Treatment: Induced CR in 11/13 patients |
Rooney et al(33) Heslop et al(32) Rooney et al(31) Heslop et al(3) |
| 14 | LCL-activated EBV-specific T-cells | CRs in 10 patients 4 with progressive disease |
Doubrovina et al 2012(2) |
| 6 | LCL-activated EBV-specific T-cells | Decreased EBV DNA levels in 5 patients; 1 patient died of PTLD | Gustafsson et al, 2000(91) |
| 1 | LCL-activated EBV-specific T-cells | Patient attained CR | Lucas et al(37) |
| 1 | LCL-activated EBV-specific T-cells | Patient failed to respond | Imashsuki(92) |
| 4 | LCL-activated EBV-specific T-cells | CRs in 3 patients with recurrent PTLD post Rituximab; decrease in EBV DNA in patient without overt PTLD | Comoli et al(93) |
| 6 | HLA A2-restricted multimer-selected | CRs in 3 patients; no response in 3 others | Moosmann et al(43) |
| 10 | IFN-γ capture of cells responding to EBNA1 protein or peptides | CR in 7 of 10 patients | Icheva et al 2012 (44) |
| 40 | Multivirus activated by monocytes and LCLs transduced with adenoviral vector encoding CMVpp65 | No EBV reactivation as prophylaxis 6/6 with EBV cleared infection including 1 with PTLD |
Leen et al 2006(94) Bollard et al(95) Leen et al 2009(96) |
| 3 | Induced by dendritic cells pulsed with EBV-LMP2, CMV-pp65 and CMV-IE peptides | Treatment: Cleared in 2/2 Prophylaxis: No infections in 1 |
Dong et al 2010(97) |
Abbreviations: CTL, cytotoxic T lymphocyte, LCL, lymphoblastoid cell line; EBV, Epstein Barr virus; CR, complete remission; PR, partial remission; HLA, human leukocyte antigen
Table 4.
Clinical trials with third party epstein barr virus cytotoxic T lymphocytes
| Study | Patient number | Type of transplant or cancer targeted | (CTL line | GVHD | Results |
|---|---|---|---|---|---|
| Haque et al. (102) | 8 | SOT | Closely matched allogeneic EBV specific T cells | None | 3 attained CR; 2 did not respond, 3 did not complete treatment |
| Haque et al. (86) | 33 | SOT and HSCT | Closely matched allogeneic EBV specific T cells | None | 14 attained CR, 3 had a PR and 16 had no response at 6 months |
| Gandhi et al. (103) | 3 | SOT | Closely matched allogeneic EBV specific T cells | None | 2 attained CRs, |
| Sun et al. (90) | 2 | SOT | Closely matched allogeneic EBV specific T cells | None | 2 attained CRs (one also received radiotherapy) |
| Barker et al. (104) Doubrivina et al. (2) |
5 | HSCT including cord | Closely matched allogeneic EBV specific T cells | None | 4 attained CR: 1 had progressive disease |
| Uhlin et al. (42) | 1 | Cord | Haploidentical GLC-peptide separated T cells | None | 1 attained CR Recurrence 9 months later responded to 2nd infusion |
| Leen et al. (87) | 9 | HSCT | Closely matched allogeneic trivirus specific T cells | 1 | 6 attained CR or PR: 2 had no response |
| Comoli et al. (88) | 1 | Nasopharyngeal cancer | Matched sibling EBV-specific T cells | None | Disease stabilization |
| Sun et al. (90) | 4 | EBV lymphoma | Matched sibling (2) or closely matched (2) EBV-specific T cells | None | 1 CR, 1CR but with radiation as well, 1PR |
| Lucas et al. | 6 | EBV Positive Hodgkin lymphoma | Closely matched allogeneic EBV specific T cells | None | 5 patients with measurable responses |
Abbreviations: SOT, solid organ transplant; PTLD, post transplant lymphoproliferative disease; CR, complete remission; PR, partial remission
Fig. 1. Schematic of conventional Epstein-Barr virus cytotoxic T lymphocyte (EBV-CTL) manufacturing.
Traditionally EBV-CTLs have been generated by weekly stimulation of donor PBMCs with autologous EBV-LCLs in the presence of IL-2. This is a prolonged process, requiring 4–6 weeks for EBV-LCL establishment with a further 4 weeks to generate sufficient CTLs for both testing and infusion.
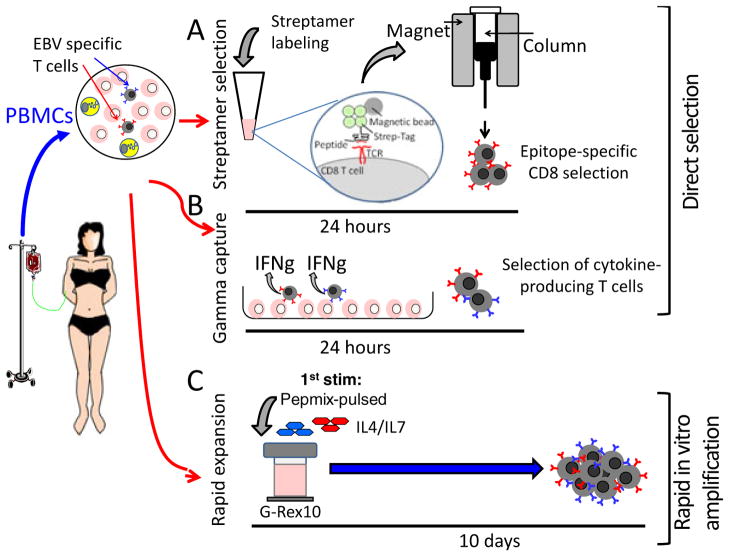
Fig. 2. Schematic of direct selection or rapid expansion protocols.
Virus-specific T cells can be directly isolated from peripheral blood using either streptamer selection (A) or γ-capture (B) for immediate infusion. Alternatively, in a 10-day procedure, virus-specific T cells can be rapidly and selectively enriched by stimulation with overlapping peptides and culture of cells in a specialized G-Rex in the presence of growth-promoting cytokines (C).
Direct selection from PBMCs
A number of groups have investigated strategies to directly isolate antigen-specific T cells from donor peripheral blood for immediate infusion. Antigen-specific T cells may be selected directly using HLA-peptide-streptamers or by capture of T cells that secrete IFNγ or express activation markers such as CD154 after antigenic stimulation. Clinical studies using these strategies have demonstrated that small numbers of T cells can expand rapidly after infusion into an HSCT recipient and control viral infections. However, these approaches require a large starting blood volume, which is not always available (e.g. in the unrelated donor setting), and cannot be applied to viruses with low circulating T-cell precursor frequencies.
Rapid in vitro expansion
As an alternative, our group and others have investigated strategies to simplify and accelerate T-cell expansion with minimal cell handling, while ensuring that specificity and function is maintained. The first step was to replace EBV-LCLs, which provide an unlimited source of APCs that present EBV latent and early lytic cycle antigens for T-cell stimulation but also can be genetically modified to present heterologous antigens. To eliminate the six weeks required for EBV-LCL manufacture, we investigated two alternate sources of biohazard-free antigen sources (plasmids and overlapping peptide libraries) using either dendritic cells (DCs) or APCs present in peripheral blood to present antigen. Plasmids are non-infectious, do not replicate in mammalian cells, and can be rapidly and cost-effectively produced at clinical grade with excellent long term stability. We have recently completed a phase I/II treatment study using DCs nucleofected with DNA plasmids encoding EBV, CMV, and adenovirus antigens to generate trivirus-directed T-cell lines, in a manufacturing process requiring just 15 days. These T cells were effective in treating active infections associated with all three viruses without adverse effects (29). More recently we have explored the clinical activity of VST lines generated in 10 days by directly stimulating PBMCs with a peptide mixture spanning 12 immunogenic antigens from 5 viruses (EBV, CMV, adenovirus, HHV6, and BK virus) that are frequent causes of post-HSCT morbidity and mortality (29). By eliminating the requirement for DC manufacture, we shaved 7 days off the manufacturing time.
In both of these studies, the manufacturing time was further reduced by the addition of IL-4 and IL-7 to the cultures. These cytokines reduce activation induced T-cell death and promote proliferation of antigen-specific T cells, which correspondingly helps increase the frequency, repertoire and size of virus-specific populations that are high avidity, Th1-polarized, produce multiple effector cytokines upon stimulation, and are able to kill virus-infected targets without demonstrating alloreactivity in vitro or in vivo. Using our new strategy, large numbers of VSTs can be produced from small amounts of blood within 10 days.(29)
Transition to G-Rex cultureware to increase safety and enhance T-cell expansion
Traditionally, VSTs have been generated in standard tissue-culture treated plates, flasks or bags, with weekly restimulation. These systems are labor intensive and difficult to scale, since the cells require frequent media changes and manipulation to optimize nutrient levels and remove waste products as well as to achieve sufficient T-cell proliferation. To overcome these limitations, we have evaluated a novel gas-permeable rapid expansion cultureware (G-Rex) system in which O2 and CO2 are exchanged across a silicone membrane at the base. This allows for an increased depth of medium above, providing cells with more nutrients, while waste products are diluted. These culture conditions have allowed us to decrease the frequency of culture manipulations by 80% while increasing cell output by 3–20-fold, allowing a shortened culture time. In the case of our virus-specific T cells, the process has been shortened from at least 23 days to about 10 days. The increased fold expansion results largely from decreased cell death, so that there is no increase in late T-cell differentiation markers compared to standard cultures (30).
This G-Rex platform is linearly scalable, and T cells will expand from about 1.5 × 105 PBMCs per cm2 to around 2×107 per cm2 whether grown on surface area of 10 cm2 or 600 cm2, producing ~2 × 108 T cells per G-Rex10 or ~12 × 1010 per G-Rex600, allowing for the production of both individualized (patient-specific) products as well as T-cell banks for third party use. These advantages together with small footprint of the G-Rex chambers, compared to rocking and stirring bioreactors, facilitate the extension of T-cell therapies to larger numbers of patients.
Clinical results with T cells specific for viral antigens
As previously discussed, viral tumor antigens are more immunogenic than other TAs and have proved robust in numerous clinical trials. EBV lymphomas arising in immunosuppressed recipients of hemopoietic or solid organ transplants are highly immunogenic, and T-cell therapies targeting these lymphomas have been in use for almost 20 years (3, 31–33). Similar approaches have been taken to target the less immunodominant EBV antigens expressed on tumors such as nasopharyngeal cancer, Hodgkin’s lymphoma, and some types of non-Hodgkin’s lymphoma occurring in individuals with normal immune systems, but are more challenging but have undergone significant process development.
EBV-associated post-transplant lymphoproliferative disease
Lymphoproliferative diseases after hemopoietic stem cell transplant
EBV-associated post-transplant lymphoproliferative disease (EBV-PTLD) is a relatively rare complication after HSCT and usually develops in the first 6–12 months after transplant. Risk factors include the degree of HLA-mismatch between donor and recipient, the use of a stem cell product selectively depleted of T cells, the intensity of immunosuppression, and the use of antibody and serotherapy regimens that selectively deplete T cells (34). In most cases the outgrowing EBV-infected B-cells are of donor origin and express even the most immunostimulatory EBV latent cycle antigens as well as T-cell costimulatory molecules and hence develop only the severely immunocompromised. Since the balance between EBV-infected B cells and EBV-specific T cells seems to be a crucial factor in pathogenesis, there has been a longstanding interest in using T-cell therapies to reconstitute EBV-specific T-cell function (3, 31–33). Initial studies used unmanipulated donor lymphocyte infusions (DLI), and since most EBV seropositive individuals have a high frequency of EBV-specific T-cell precursors, this strategy resulted in an expansion of this specific precursor population and resolution of EBV-PTLD in over 70% of cases (2, 35–37). However, unmanipulated T cells have an even higher frequency of alloreactive T cells, so this approach is limited by the risk of GVHD (2, 36, 37).
To reduce the risk of alloreactivity, our group has used EBV-specific T cells generated using repeated stimulations with EBV-transformed LCLs (28). When these cells were administered as prophylaxis to 101 recipients considered high risk for developing PTLD because either they were receiving T-cell-depleted transplants from mismatched or unrelated donors, or because they had a previous history of EBV lymphoma, or had a diagnosis that conferred a high risk of developing this complication, none developed PTLD compared with an 11.5% incidence in controls (38). The first 26 patients in this study received VSTs that had been genetically marked by transduction with a retroviral vector encoding the neomycin resistance gene as a marker that allowed us to track the infused cells for up to 10 years, showing that small numbers, up to 5 × 107 antigen-specific T-cells, can expand and persist when administered early post-transplant in a lymphodepleted milieu. Additional factors that may have promoted long term persistence include the polyclonality of the infused cell lines that contained both CD4+ and CD8+ cells and the fact that infused cells would receive stimulation by EBV-infected B cells.
EBV-VSTs were also effective when administered to treat active disease, with 11 of 13 patients achieving sustained complete remission. Of note, EBV VSTs did not induce alloreactivity, with no development of de novo GVHD after infusion. The main adverse effect was reversible inflammatory reactions at disease sites in four patients with PTLD (3). Similar results were seen in a study by O’Reilly and colleagues (2), where 10 of 14 patients with active PTLD attained complete remissions after infusion of donor-derived EBV-specific T-cells.
Investigation of causes of failure in the patients who failed to respond to EBV-VSTs revealed important tumor evasion mechanisms. In three patients in the Sloan Kettering series, the VST recognized the EBV-LCL transformed with the B-95 laboratory strain of EBV used for T-cell generation but did not recognize the tumor cells or spontaneous LCLs outgrowing from the patients’ blood, suggesting that antigenic differences between EBV strains causing PTLD may be a limitation when LCL are used as a source of EBV antigens (2). Analysis of one of our treatment failures revealed an EBV deletion mutant that co-existed with a wildtype strain prior to VST infusion. Only the deleted strain persisted in the tumor after VSTs(39). This patient and her donor expressed HLA-A11, which is a dominant restricting allele in the immune response to EBV; the activity in the line was biased to two HLA-A11-restricted epitopes in the viral EBNA-3B antigen that were removed by the deletion. Subsequently, it has been shown that EBNA3B, while dispensable for transformation, is a tumor suppressor whose inactivation promotes immune evasion and virus-driven lymphomagenesis (40). Thus, the failure in this patient likely can be ascribed not only to the limited T-cell repertoire but also to the loss of a viral tumor suppressor gene. A similar phenomenon was reported in a Sloan Kettering patient, who also received a line from an HLA-A11 donor which was biased towards EBV antigens recognized through this allele and failed to respond since the recipient lacked HLA11 (2).
The overall results from these studies along with those from smaller series performed at several centers worldwide (Table 1) show the potential of donor-derived EBV-VSTs to prevent and treat PTLD after HSCT. Recent studies have tested whether EBV VSTs generated using the more rapid manufacturing strategies or selected by direct isolation have equivalent activity. Our group evaluated short ex vivo expansion protocols to generate ‘rapid VSTs’ that target multiple viruses including EBV. In the study evaluating VSTs generated using DCs nucleofected with plasmids encoding viral antigens a response rate of 80% was seen, which included one patient with a biopsy-proven EBV-lymphoma (41). We are currently using overlapping peptides as a source of antigen with optimized cytokines in an even shorter ex-vivo culture(29), and these VSTs have induced complete responses in two patients with EBV lymphoma (Papadopoulou et al., manuscript in preparation).
In a study by Uhlin and colleagues (42), HLA-peptide multimers were used to isolate HLA A2-restricted T cells specific for epitopes in two EBV antigens from a haploidentical parent to treat EBV PTLD in a cord blood transplant recipient. A small number of these directly selected cells expanded after infusion, inducing a complete clinical response (42). However, the T cells may not have persisted long term, since EBV-PTLD recurred at 12 months after transplant, perhaps because CD4+ T cells were not co-infused. However, a second selection procedure and infusion induced a second response. The multimer strategy is limited by the HLA restriction of antigen recognition, the availability of clinical grade multimers and lack of HLA class II multimers. An alternative rapid approach uses IFN-γ capture technology to select T cells that secrete IFN-γ after stimulation with viral antigens. This technique is not restricted by HLA-phenotype and both CD4+ and CD8+ T cells are selected. In one study, donor mononuclear cells were incubated with 23 class I and II peptides derived from 11 EBV antigens, and T-cells that secreted IFN-γ in response to these peptides were selected for infusion into 6 patients with EBV-PTLD (43). While three patients responded, three with more advanced disease progressed (43). Whether these patients would have responded had they received higher numbers of effector T cells remains an important question for future development. Icheva and colleagues (44) targeted the EBNA-1 antigen and pulsed APCs with either whole EBNA-1 protein or EBNA-1 overlapping peptide pools and then selected responding T-cells by IFN-γ capture. Ten patients with PTLD were treated, and seven had clinical responses. No significant toxicities have been seen in any of these studies (Table 1). Results from these rapid isolation approaches are therefore encouraging, although the numbers are small, so it is not yet clear whether there will be a lower overall response rate when only a limited number of EBV antigens or epitopes are targeted. A second limitation to this strategy may be the requirement for large numbers of starting T cells from unrelated donors in a short time frame.
Lymphoproliferative diseases after solid organ transplant
As with HSCT recipients, solid organ graft recipients most commonly develop EBV-PTLD in the first year post-transplant. However, with solid organ transplant recipients, PTLD most commonly arises from recipient lymphoid cells. The incidence can vary from 1 to 30% depending on the type of organ transplanted, with lowest rates seen in renal transplant recipients and the highest in small bowel recipients. Other risk factors include recipient EBV-seronegative status prior to transplantation and higher-intensity immunosuppressive regimens. Since children are more often EBV-seronegative at the time of transplantation, they have a higher incidence of PTLD.
In solid organ transplant recipients, reduction of immunosuppression is usually the first line therapy for EBV-PTLD, followed by the use of the CD20 monoclonal antibody rituximab, either alone or in combination with chemotherapy. As these therapies can be toxic and can fail, there is interest in the use of EBV VSTs. An initial challenge was to decide on the source of VSTs as patients usually remain on long-term immunosuppression. This proved a non-issue, since several groups showed that VSTs could be generated, even from recipients with active PTLD (Table 2). We infused autologous EBV-VSTs to two solid organ graft recipients with active disease and eight with high EBV DNA levels (45). In the two patients with disease, we observed one complete and one partial response while none of the eight prophylaxis patients developed PTLD, although the effects on EBV viral load were not consistent or impressive (45). Other groups have reported similar results in treating active disease or elevated EBV viral load (46–48) (Table 2). The time taken to generate VSTs in this setting has led to interest in using partially HLA-matched third party cells, which instantly available.(49)
Table 2.
Clinical trials: autologous Epstein Barr virus cytotoxic T lymphocytes in solid organ transplant
| Study | Patient number | Type of transplant | Prophylaxis or therapy | Results |
|---|---|---|---|---|
| Comoli et al. (46) | 7 | SOT | Prophylaxis | No PTLD |
| Haque et al. (98) | 3 | SOT | Prophylaxis | No PTLD |
| Khanna et al. (47) | 1 | SOT | Therapy | Significant regression |
| Sherrit et al. (48) | 1 | SOT | Therapy | CR |
| Comoli et al. (99) | 5 | SOT | Therapy | CRs (used as adjuvant after chemotherapy and Rituxan) |
| Savoldo et al. (45) | 12 | SOT | Prophylaxis and Therapy | No PTLD 1 of 2 patients treated with PTLD attained CR and the other a PR |
Abbreviations: SOT, solid organ transplant; CR, complete remission; PR, partial remission, PTLD, post transplant lymphoproliferative disorder
EBV CTLs for Type 2 latency tumors
The success of EBV VST therapy in treating the immunogenic Type 3 latency tumors that develop in patients after transplant led to interest in treating other EBV-associated malignancies, such as Hodgkin’s lymphoma, some types of non-Hodgkin’s lymphoma (NHL). and nasopharyngeal carcinoma (NPC). These tumors, which develop in immunocompetent individuals, express a more restricted array of EBV-encoded antigens (Type 2 latency) with only the weakly immunogenic EBV antigens (BARF, EBNA1, LMP1, and LMP2) being expressed.
Studies in lymphomas arising in immunocompetent persons
In our first study outside of the HSCT setting, we used EBV-VSTs for the treatment of 14 patients with relapsed EBV+ Hodgkin’s lymphoma (50). Using immunoassays, we showed that T cells reactive with the subdominant viral TA, LMP2, did indeed expand after infusion as detected in peripheral blood. The infused VSTs also had clinical activity, inducing two complete responses, one partial response, and five patients had stable disease (Table 3). To increase the frequency of clones recognizing the appropriate viral tumor antigens, we next evaluated the efficacy of VSTs activated with APCs transduced with adenoviral vectors expressing either LMP2 alone (n=17) or both LMP2 and ΔLMP1 (n=33)(17, 51). Twenty eight of 29 patients with high-risk or multiply relapsed disease who received LMP-CTL as adjuvant therapy remained in remission at a median of 3.1 years after VST infusion. Twenty-one patients with active disease received LMP VSTs, and 13 had clinical responses, including 11 complete responses. Of note, T cells specific for LMP antigens could be detected in the blood after VST infusion and in some responding patients T cells reactive with nonviral TAs (epitope spreading) also became detectable.
Table 3.
Clinical trials of Epstein Barr virus cytotoxic T lymphocytes in type II latency tumors
| Patient number | Type of CTL | Antitumor l effects | Reference |
|---|---|---|---|
| Non-Hodgkin’s and Hodgkin’s Lymphoma | |||
| 10 | LCL-induced | 2 CRs, 1 PR, 5 stable disease | Bollard et al. 2004 (50) |
| 50 | LMP-specific T cells using autologous dendritic cells and LCL transduced with an adenoviral vector expressing LMP2 or LMP1/2 | Prophylaxis: 28 of 29 remain disease-free with median of 3 years follow up Treatment. 11 of 21 CRs, 2 PRs |
Bollard et al. 2007(51) Bollard et al. 2013 (17) |
| 3 | LCL-induced from HLA-identical sibling (2) or autologous (1) | Stable disease for > 3 years in 2 | Cho et al. 2006 (100) |
| Nasopharyngeal cancer | |||
| 4 | LCL-induced | Reduction in viral load but no clinical responses | Chua et al. 2001(101) |
| 10 | LCL-induced | 2 PR and 4 with stable disease | Comoli et al. 2005 (6) |
| 23 | LCL-induced | Prophylaxis: 5 of 8 patients treated adjuvantly remained disease-free for 25 to 82 months and 3 relapsed. Treatment. 5 with CR, 2 with PR, 3 with Stable Disease, 5 with progression |
Straathof et al. 2005 (53) Louis et al. 2010 (5) |
| 8 | LCL-induced after lymphodepletion with CD45 | 1 CR and 2 stable diseases | Louis et al. 2009 (54) |
| 11 | LCL-induced after cyclophosphamide and fludarabine | 2 PRs, 3 with stable disease, 1 minor response | Secondino et al. (55) |
| 35 | LCL-induced after standard chemotherapy for metastatic disease | 2-year overall survival OS was 67.2% | Teo et al. (30) |
Abbreviations: LCL, lymphoblastoid cell line; LMP, latent membrane protein; CR, complete remission; PR, partial remission,
Studies in nasopharyngeal cancer
Over 95% of cases of NPC express EBV antigens, and advanced stage disease continues to have a poor prognosis with a 5-year survival rate of less than 50% (52). Moreover, current therapies are associated with significant morbidity making this cancer an attractive target for EBV-based immunotherapies (Table 3). Comoli et al. (6) treated 10 patients with progressive NPC after conventional therapy with autologous EBV-specific CTLs and observed partial responses in two and stable disease in four. Responses were associated with an increase in LMP2-specific responses in the peripheral blood. Our group has also infused LCL-activated EBV CTLs to patients with NPC and observed 10 responses in 15 patients treated with active disease (5 CRs, 2 PRs, and 3 stable disease) (5, 53). An additional eight patients were treated in their second or subsequent remission, and five remain disease free with follow up of 17 to 75 months. To try and improve these response rates both groups next evaluated the effect of infusing CTLs after lymphodepletion. Our group used CD45 depleting antibodies (54) and the Italian group using cyclophosphamide and fludarabine (55). In both studies, the addition of lymphodepletion did not alter the response rate (54, 55) (Table 3).
In NPC studies from both groups, a consistent finding has been that EBV-CTLs produce measurable benefit only when the product contains LMP2 reactive clones that expand after infusion. This was confirmed in a larger study from Singapore where patients with metastatic NPC received four cycles of chemotherapy followed by up to six infusions of EBV-CTLs. In 35 patients, the 2-year overall survival OS was 67.2%, a rate much higher than that seen in historic controls receiving chemotherapy alone, again with an association of benefit with specificity for EBV-LMP2 in the infused line (56). Current studies are therefore testing strategies to enrich the infused line for clones reactive with LMP antigens.
Other Virus CTLs
Although as discussed earlier, many other tumors express viral antigens that provide targets for T-cell-based immunotherapies, and there have been reports of preclinical studies of VSTs to target other tumors (23, 57). There are, as yet, no reported clinical trial results.
T-cell therapy for non-viral tumor-associated antigens
Given the success of adoptive transfer approaches for the treatment of virus-associated diseases and malignancies, this approach has also been considered as a therapeutic modality for the treatment of non-viral cancers. In this context, identification of the optimal tumor-expressed target is more complex. To limit collateral damage, the model TA should be universally and selectively expressed on tumor cells and ideally should be essential for the maintenance of the oncogenic phenotype of the tumor. However, the majority of non-viral TAs does not meet these criteria and are often expressed in normal cells against which peripheral blood T cells are tolerized by central and peripheral mechanisms. Initially it was thought that T cells specific for self-antigens should not exist at all, but over the last 20 years, increasing numbers of antigens that may serve as tumor-specific targets have been described. Tumor associated antigens (TAAs) can be classified into four main groups based on their expression and tissue distribution.
Tumor antigens and T-cell immunogenicity
Mutated antigens are common to certain tumors (e.g. Bcr-Abl) or unique to each tumor and are expressed exclusively in neoplastic cells and hence are considered ideal for immunotherapy. However, single point mutations are immunogenic only in the context of particular HLA alleles or not at all. Recently, tumor transcriptome analysis has demonstrated that many tumors express a plethora of mutated proteins (58, 59). In principal, therefore, peptide libraries encompassing all mutated sequences could be generated and used to stimulate tumor-specific T cells for each patient, increasing the likelihood of generating T cells specific for multiple TAs. In practice, such a highly customized product would be expensive, and it would be difficult to confirm that each peptide was truly tumor-specific. However, as tumor proteomics become more standardized and if many mutations are shared between patients, such an approach may eventually be reduced to practice by selecting a peptide cocktail from a library of clinical grade peptides.
Lineage-restricted tumor antigens are expressed on tumor cells as well as on their normal tissue of origin, such as the melanoma associated antigens MART, gp100, or Melan-A, that were first discovered as targets of melanoma-infiltrating lymphocytes. Subsequently, these antigens have proved immunostimulatory to a degree that is almost equivalent to that of weak viral antigens, enabling the efficient and relatively simple generation and expansion of tumor-specific T cells from both patients and healthy donors with minimal in vitro manipulation. These antigens can be targeted if they are overexpressed on tumors relative to normal tissues or if they are differentially processed (60, 61). Nevertheless, infusion of melanoma-specific T-cells can result in destruction of normal melanocytes, resulting in vitiligo as well as ocular and systemic autoimmunity (62). Indeed elevated numbers of GP100-specific T cells have been detected in patients with vitiligo (63). The decision to use T cells specific for antigens expressed on normal tissues must be considered with caution because of the potential longevity of infused T cells. The availability of effective suicide genes (64, 65) may mitigate the risks, thus facilitating the use of T cells with autoimmune potential.
Cancer/testis antigens (CTA) (e.g. shared tumor-specific TAAs MAGE, BAGE, GAGE, NY-ESO-1, SSX, PRAME) are found in a variety of malignant tumors. In normal tissues, their expression is limited to germ line tissues that are immune privileged and hence less susceptible to tolerance mechanisms. CTA-specific T cells can be produced on a large scale to provide broad-spectrum protection against a variety of tumors. CTAs have been targeted in both vaccine and T-cell therapy protocols, with evidence of clinical efficacy (66). Recent efforts to enhance the natural specificity of CTA-specific TCRs to generate a more potent T-cell product have resulted in off-target effects including lethal cardiac and cerebral toxicities (67, 68).
The last group are TAs that are overexpressed in many different tumors but are absent from or expressed at low levels in healthy tissue (e.g. hTERT, CEA, and survivin). T cells targeted to these antigens carry the risk of inducing collateral damage to normal tissues co-expressing the antigen (e.g. CEA and normal biliary epithelium), and there are limited clinical data available regarding the safety of targeting these antigens in vivo (69). However, survivin- and CEA-specific T cells have been isolated from the peripheral blood of patients who have cleared their tumors, and increases in survivin-specific T cells in patients receiving oncolytic viruses have been reported, suggesting that they can have efficacy without toxicity in patients. Survivin is expressed in proliferating T cells, and the fact that survivin-specific T cells can be grown in vitro illustrates the ability of T cells to distinguish between high and low levels of antigen expression or differential antigen processing and presentation.(70)
Optimizing cell culture protocols for tumor-specific T-cell generation
In addition to choosing the optimal TAs for T-cell stimulation, successful adoptive immunotherapy relies on effective protocols for activating and expanding tumor-specific T cells in vitro. In general, reactivation requires professional APCs, which not only present antigen but also provide costimulation and cytokines (e.g. IL-12) that drive T-cell differentiation down the appropriate effector pathway (Tc1/Th1).
Perhaps the most widely used APCs for tumor-specific T-cell activation are monocyte-derived DCs, although clinical utility is limited by the achievable numbers of these non-dividing cells, which in turn impedes large-scale T-cell production. Alternative autologous APC sources include activated T-APCs and CD40L-activated B-cell blasts, whose baseline immunostimulatory capacity has been genetically enhanced via the incorporation of costimulatory molecules (CD40, OX40, CD70, B7-1, ICAM-1, and LFA-3), or cytokines such as IL-12, IL-7, or IL-15. To further simplify manufacture, the use of ‘off the shelf’ artificial APCs (aAPCs) is also an area of active investigation as the incorporation of such an APC source would make tumor-specific T-cell generation significantly more cost effective and practical. Cellular aAPC platforms include human leukemia cell lines, insect cells, or mouse fibroblasts, which have been engineered to effectively present antigen. For example, because it is HLA-negative and unlikely to induce alloreactivity, the leukemia cell line K562 has been modified to express costimulatory molecules (e.g. CD137, CD80), to secrete a range of cytokines (IL-2), and to express HLA genes (71). Cell-free aAPC systems including micron-size latex, polyglycolide, magnetic beads, or lipid-based vesicles are also being evaluated (72). Ultimately, the optimal aAPC must be GMP-compliant, potent, and able to reproducibly support the efficient expansion of antigen-specific T cells in vitro. As cell therapy becomes more widely used, this area of development is likely to be of intense interest.
Tumor-specific T cells isolated from whole blood or tumor biopsy samples are often anergized/tolerized, and have poor proliferative capabilities. Thus, the effective induction of cellular anti-tumor immunity relies on culture supplementation with immune-modulating and growth-promoting cytokines. A variety of cytokines, including IL-2, IL-15, IL-12, IL-7, IL-21, and IL-6, or cytokine combinations have been tested for their ability to selectively expand tumor-specific effector T cells, enhance their effector function, promote their survival, and selectively inhibit Tregs. Indeed, in our preclinical studies, we demonstrated that a combination of IL-7, IL-12, IL-15, and IL-6 was optimal for the in vitro activation of T-cell lines with specificity for multiple lymphoma-expressed antigens (70). It should be noted, however, that combining individually effective cytokines may simply produce antagonistic or even paradoxical effects, and the combinations used and the sequence of their introduction needs careful analysis for each tumor-specific T-cell product.
Melanoma-targeted immunotherapy using tumor-infiltrating lymphocytes
By far the largest body of work using tumor-directed T cells clinically has been reported by the Rosenberg group from the NCI, who have pioneered the ex vivo expansion and adoptively transfer of tumor-infiltrating lymphocytes (TILs) to patients with metastatic melanoma, starting in the late 1980s (73). TILs were isolated from resected melanoma lesions, expanded in individual 24-well culture wells, tested for tumor-specificity by co-culture with autologous or HLA-matched tumor material, followed by expansion of subcultures with confirmed specificity using feeder cells, anti-CD3, and high dose (6000U/ml) IL2 (10) – a process requiring 4–8 weeks. In the initial clinical trial, 20 patients received a lymphodepleting dose of cyclophosphamide (25mg/kilogram), followed by TIL infusions ranging from 3 – 75 × 1010 TILs with IL-2 administered every eight hours until dose-limiting toxicity was reached. In this patient cohort, transient tumor reductions were seen but limited in vivo persistence produced sub-optimal anti-tumor effects (74). To address this issue, the same group modified their approach to incorporate a more intensive preparative regimen with or without additional radiation. This step was designed specifically to alter the host tumor microenvironment by eliminating inhibitory regulatory T cells/myeloid suppressor cells and reduce competition for endogenous IL-7 and IL-15 to maximize therapeutic benefit of the adoptively transferred TILs. Indeed, the long term outcomes of 93 patients with metastatic disease treated on three sequential trials with the initial and modified approach have been recently updated, with objective responses ranging from 49–72% reported, complete and durable response rates in up to 40% of patients (9, 10), and a trend towards superior clinical outcomes in those receiving increased lymphodepletion and TBI.
However, despite these impressive results, collection of autologous TILs for individual therapeutic use is restricted to those tumors that are easily accessible and not all tumors contain TILs. Furthermore, it is not possible to expand a tumor-specific T-cell population from every patient, and even when successful, the extended culture period (4–8 weeks) required for preparation of the large cell numbers (>1010 T-cells) is complex and expensive and produces a product of variable composition (phenotype and specificity), making it challenging to define the characteristics associated with clinical activity.
Melanoma-targeted immunotherapy using antigen/epitope-specific T cells
To extend applicability and generate a more defined tumor-specific product with respect to specificity and function, a number of groups have investigated the clinical use of tumor-specific T cells isolated from peripheral blood and selectively activated and expanded ex vivo. Mitchell and colleagues (75) used insect cells modified to express HLA-A2, CD80, and CD54 and loaded with an HLA-A2-restricted epitope peptide derived from the melanoma-expressed antigen tyrosinase to repetitively stimulate and selectively expand tyrosinase peptide-specific T cells. Infusion of 5×108 cells (of which between 10–30% were tyrosinase-specific) to 10 patients with tyrosinase-positive melanoma tumors was associated with clinical responses in two. Other groups have used autologous DCs to activate a tumor-directed product. For example, Yee and colleagues (76) generated CD8+ T-cell clones specific for HLA-A2 peptides derived from MART1 and gp100 using peptide-loaded DCs as stimulators. Four infusions (3.3×109/m2/infusion) of the tumor-directed clones without and subsequently with low dose IL-2 produced clinical benefit in 8 of 10 patients with metastatic melanoma, and IL-2 proved to be crucial for in vivo persistence (76). A follow-up study from this group incorporating a pre-conditioning step with high dose cyclophosphamide +/− low or high dose IL-2 was shown to further increase proliferation and persistence (77). However, the toxicities with high dose IL-2 were substantial, and this arm of the study was halted. Finally, MART1 was targeted by Mackensen et al. (78), who prepared tumor-directed T-cell lines using HLA-A2 peptide-loaded DCs to stimulate selected CD8+ T-cells. Eleven patients received at least 3 and up to 10 infusions of specific T cells (median 2.11 × 108 cells/infusion) with low dose IL-2. T cells homed to tumor sites detected and favorable clinical responses were reported in 3 patients (1 complete, 1 partial, and 1 mixed response) (78).
While the generation and adoptive transfer of CD8+ melanoma peptide-specific T-cells from peripheral blood is feasible, as in the TIL setting clinical benefit appears to be tightly linked with in vivo persistence, and T-cells could not be manufactured from all patients. One potential strategy to overcome this obstacle may be to infuse CD8+ peptide-directed T cells with antigen-specific CD4+ T-cell populations, which not only mediate tumor killing directly against HLA class II positive targets but also can support the survival and maintain the effector function of the transferred CD8+ T cells via the production of immunostimulatory cytokines and other signals upon antigen encounter. Perhaps a more general shortcoming of this peptide stimulation approach, however, is its limitation to individuals with a restricted HLA genotype due to the small number of class I-restricted epitopes used for T-cell activation. Finally, one emerging concern is the potential for such a targeted therapy to evade the immune system as was reported by Mackensen et al., who reported the emergence of MART1-negative tumors in two patients infused with MART1-targeted CD8+ T cells. Hence, many groups are now focusing on the use of peptide libraries to activate both CD4+ and CD8+ T cells
T-cell therapy to prevent leukemic relapse
Adoptive immunotherapy has also proved to be an effective strategy in the prevention of leukemic relapse after allogeneic HSCT. The first adoptive T-cell transfer protocols were based on the premise that donor peripheral blood contained T-cells that were able to mediate antitumor in vivo. Accordingly, unmanipulated DLI has been extensively used to provide anti-tumor immunity (79). However, the efficacy of this approach is limited by the low frequency of tumor-specific T cells and the relatively high frequency of alloreactive T cells, resulting in frequent and severe GVHD. One strategy to enhance the ‘graft versus leukemia’ effect without promoting GVHD is to selectively target tumor-expressed antigens using selectively expanded T-cell populations. The antigens targeted clinically fall into two categories: (i) minor histocompatibility antigens (mHAgs) expressed by leukemia progenitors, and (ii) TAs overexpressed by the leukemic cells.
mHAgs can differ between donor and recipient even when matched at major histocompatibility loci. Donor-derived mHAg-specific T-cells can have both GVL and GVHD effects in vivo (80). Much of the current research in this area is focused on identifying and selectively targeting mHAgs expressed exclusively on hematopoietic cells, thereby separating GVL from GVHD. Warren and colleagues (81) evaluated the safety of adoptively transferring donor-derived CD8+ T-cell clones recognizing such mHAgs to seven patients with relapse of acute leukemia after myeloablative allogeneic HSCT. The highest doses administered to each patient ranged from 2.25 – 6.6 × 109 cells. Pulmonary toxicity was seen in three patients and was severe in one, correlating with the level of expression of the mHAg-encoding genes in lung tissue. However, the administration of steroids coincided with a rapid reversal in pulmonary symptoms. Thus, the associated toxicity could be rapidly and effectively controlled (81).
In both the autologous and allogeneic-donor/HSCT setting, adoptive transfer approaches are being developed against a number of leukemia-associated antigens including Wilms Tumor gene 1 (WT1), proteinase 3 (Pr3), human neutrophil elastase (NE), melanoma-associated antigen A3 (MAGE-A3), and preferentially expressed antigen in melanoma (PRAME) (82,83). Of these, WT1 is perhaps the most extensively characterized with several groups evaluating T cells specific for defined CD4+ and CD8+ epitope peptides. Vaccines incorporating WT1 have produced clinical responses (84). This effect was associated with an increased frequency and long-term persistence of WT1-specific T cells in peripheral blood, showing the clinical relevance of immune responses directed to this antigen. Based on these and other studies, O’Reilly and colleagues (83) initiated a clinical trial of donor-derived WT1-specific T cells, activated using DCs loaded with an overlapping peptide library (15mers overlapping by 11 amino acids) spanning the entire sequence of the antigen, and infused as treatment of persistent minimal residual disease or recurrence of WT1+ AML, ALL, or MDS following allogeneic HSCT. In preliminary reports, T-cell infusions at the lowest dose levels were safe and well tolerated (83). Peptide libraries can present all possible HLA-class I-restricted epitopes as well as a proportion of HLA class II-restricted epitopes allowing the generation of T cells specific for multiple epitopes, regardless of HLA phenotype.(85)
Broadening applicability with third party VSTs
Although the adoptive transfer of autologous/donor-derived virus- and tumor-directed T cells has been associated with clinical activity, the delay and complexity of producing individual cell products for infusion remains a barrier, rendering this therapeutic modality impractical for widespread or urgent use. This limitation could be overcome by a bank of well-characterized, HLA-typed antigen-specific T-cell lines for administration as off-the-shelf reagents. This strategy was first shown to be safe and effective against EBV-PTLD in solid organ and bone marrow transplant recipients (Table 4). In the first and largest multicenter study, Haque et al. (86) used banked polyclonal EBV-specific T-cell lines to treat EBV-PTLD after HSCT or solid organ transplantation and reported an overall response rate of 52% at 6 months. Similar results have been reported from Memorial Sloan Kettering with four of five PTLD patients achieving CR in response to third party EBV-specific T cells (2). More recently, our group applied this approach to treat patients with refractory CMV, adenovirus, and EBV infections post allogeneic HSCT, achieving an overall response rate of 74%, including responses in 6 of 9 patients with refractory EBV-PTLD (87). Of note none of these studies report an increased risk of GVHD.
In the future, the third party strategy may be extended beyond the transplant setting to the treatment of both viral and non-viral malignancies. At present, there are only isolated reports outside the transplant setting, but some responses have been reported: Comoli et al. (88) reported temporary stabilization of disease in a patient with refractory nasopharyngeal cancer, while Lucas and colleagues (89, 90) reported responses in 2 patients with EBV lymphoma and 5 with EBV-positive Hodgkin lymphoma. One issue in this setting may be that in less immunocompromised patients, repeated cell infusions will be required to overcome their likely short in vivo persistence.
The development of banks of tumor-directed T cells is feasible, particularly in light of recent manufacturing improvements, including the optimization of protocols for non-viral tumor-specific T-cell generation. However, a comprehensive epitope/HLA restriction analysis must be performed to enable the identification of the ‘best’ T-cell line for each patient. In further development of this approach, it will be necessary to define criteria for HLA matching to provide coverage for a high percentage of potential patients, for example with the use of homozygous donors.
Conclusions
Cellular therapies have often been criticized for their ‘boutique’ application. However, with improved, simplified, and more rapid manufacturing, costs can be low. T cells with native receptor specificities can potentially offer long term protection with a single infusion and are not associated with hospitalization costs or with short or long-term iatrogenic morbidities that require additional therapeutic intervention. Therefore, when overall cost and quality of life are taken into consideration, T-cell therapies may in fact provide a low cost and more palatable alternative to current standard chemoradiotherapies.
T cells targeting native receptors have already produced encouraging results in the clinic, most notably when viral antigens are targeted in immunosuppressed patients. However, there remain significant obstacles to their more widespread use in immunocompetent patients, including their limited in vivo expansion and persistence in the presence of immunosuppressive tumors. Tumor cells employ a range of passive and active immune evasion strategies to avoid the consequences of immune activation, including the expression of inhibitory cytokines and ligands, recruitment of inhibitory cell types and failure to present TAs appropriately to the immune system. Tumors may respond to immunotherapies by increasing their expression of inhibitory molecules and producing variants that have lost the antigens targeted, a phenomenon referred to as ‘cancer immunoediting’(7). Future directions therefore include engineering of T cells to incorporate countermeasures, targeting multiple TAs simultaneously to minimize the potential for tumor immune escape, and combining immunotherapy approaches with targeted small molecule therapies or with checkpoint inhibitors to increase antitumor activity.
Acknowledgments
This work was supported in parts by NIH grants P50CA126752 and PO1 CA94237, a Specialized Center of Research Award from the Leukemia Lymphoma Society, CPRIT grant RP110553 and the Production Assistance for Cellular Therapies (PACT) program [NHLBI contract #HHSN268201000007C]. We also appreciate the support of shared resources by the Dan L Duncan Cancer Center support grant P30CA125123. CMR and HEH have a licensing agreement with Cell Medica for EBV VSTs in lymphoma and nasopharyngeal cancer. CMR has received research support from Cell Medica. JFV is a consultant for and has received research support from Wilson Wolf Manufacturing. The authors’ Center has a collaborative research agreement with Celgene for genetically modified T-cells.
Reference List
- 1.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9:510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doubrovina E, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2456. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollard CM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis CU, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comoli P, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 7.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everson TC, Cole WH. Spontaneous regression of malignant disease. J Am Med Assoc. 1959;169:1758–1759. doi: 10.1001/jama.1959.03000320060014. [DOI] [PubMed] [Google Scholar]
- 14.van der Burg SH, Arens R, Melief CJ. Immunotherapy for persistent viral infections and associated disease. Trends Immunol. 2011;32:97–103. doi: 10.1016/j.it.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Welters MJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 16.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 17.Bollard CM, et al. Sustained complete responses in lymphoma patients receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane protein. 2013 doi: 10.1200/JCO.2013.51.5304. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Icheva V, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31:39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 19.Gomez BP, et al. Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen. Cell Biosci. 2012;2:36. doi: 10.1186/2045-3701-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowan AG, Bangham CR. Is there a role for HTLV-1-specific CTL in adult T-cell leukemia/lymphoma? Leuk Res Treatment. 2012;2012:391953. doi: 10.1155/2012/391953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afanasiev OK, et al. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19:5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruner NH, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 23.Ghazi A, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012;35:159–168. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melnick M, Sedghizadeh PP, Deluca KA, Jaskoll T. Cytomegalovirus-induced salivary gland pathology: resistance to kinase inhibitors of the upregulated host cell EGFR/ERK pathway is associated with CMV-dependent stromal overexpression of IL-6 and fibronectin. Herpesviridae. 2013;4:1. doi: 10.1186/2042-4280-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marinho-Dias J, et al. Characterization of cytomegalovirus and epstein-barr virus infection in cervical lesions in Portugal. J Med Virol. 2013;85:1409–1413. doi: 10.1002/jmv.23596. [DOI] [PubMed] [Google Scholar]
- 26.Harkins LE, et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae. 2010;1:8. doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res. 2011;157:193–203. doi: 10.1016/j.virusres.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CA, et al. Production of genetically modified EBV-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995;4:73–79. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 29.Gerdemann U, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vera JF, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rooney CM, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 32.Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 33.Rooney CM, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 34.Landgren O, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos EB, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 36.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 37.Lucas KG, Burton RL, Zimmerman SE, Wang J, Cornetta KG, Robertson KA, et al. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 1998;91:3654–3661. [PubMed] [Google Scholar]
- 38.Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottschalk S, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 40.White RE, et al. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122:1487–1502. doi: 10.1172/JCI58092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerdemann U, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013 doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother. 2010;59:473–477. doi: 10.1007/s00262-009-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moosmann A, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 44.Icheva V, et al. Adoptive Transfer of Epstein-Barr Virus (EBV) Nuclear Antigen 1-Specific T Cells As Treatment for EBV Reactivation and Lymphoproliferative Disorders After Allogeneic Stem-Cell Transplantation. J Clin Oncol. 2013;31:39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 45.Savoldo B, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Comoli P, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. 2002;99:2592–2598. doi: 10.1182/blood.v99.7.2592. [DOI] [PubMed] [Google Scholar]
- 47.Khanna R, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherritt MA, et al. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation. 2003;75:1556–1560. doi: 10.1097/01.TP.0000058745.02123.6F. [DOI] [PubMed] [Google Scholar]
- 49.Haque T, McAulay KA, Kelly D, Crawford DH. Allogeneic T-cell therapy for Epstein-Barr virus-positive posttransplant lymphoproliferative disease: long-term follow-up. Transplantation. 2010;90:93–94. doi: 10.1097/TP.0b013e3181d7c424. [DOI] [PubMed] [Google Scholar]
- 50.Bollard CM, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bollard CM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan AT. Nasopharyngeal carcinoma. Ann Oncol. 2010;21(Suppl):vii308–vii312. doi: 10.1093/annonc/mdq277. [DOI] [PubMed] [Google Scholar]
- 53.Straathof KC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 54.Louis CU, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Secondino S, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol. 2012 Feb;23(2):435–41. doi: 10.1093/annonc/mdr134. [DOI] [PubMed] [Google Scholar]
- 56.Teo M, et al. Chemotherapy in combination with T-cell therapy results in significant antitumor activity and improved clinical outcomes for EBV-associated nasopharyngeal carcinoma. Mol Ther. 2011;19 (Suppl):S85–S86. [Google Scholar]
- 57.Ramos CA, et al. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes for adoptive immunotherapy of HPV-associated malignancies. J Immunother. 2013;36:66–76. doi: 10.1097/CJI.0b013e318279652e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt MW, Prindle MJ, Loeb LA. Implications of genetic heterogeneity in cancer. Ann N Y Acad Sci. 2012;1267:110–116. doi: 10.1111/j.1749-6632.2012.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong KM, Hudson TJ, McPherson JD. Unraveling the genetics of cancer: genome sequencing and beyond. Annu Rev Genomics Hum Genet. 2011;12:407–430. doi: 10.1146/annurev-genom-082509-141532. [DOI] [PubMed] [Google Scholar]
- 60.Sahin U, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James E, Bailey I, Sugiyarto G, Elliott T. Induction of protective antitumor immunity through attenuation of ERAAP function. J Immunol. 2013;190:5839–5846. doi: 10.4049/jimmunol.1300220. [DOI] [PubMed] [Google Scholar]
- 62.Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandelcorn-Monson RL, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003;121:550–556. doi: 10.1046/j.1523-1747.2003.12413.x. [DOI] [PubMed] [Google Scholar]
- 64.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciceri F, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 66.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linette GP, et al. Cardiovascular toxicity and titin cross-reactivity of affinity enhanced T cells in myeloma and melanoma. Blood. 2013:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan RA, et al. Cancer regression and neurological toxicity following anti- MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rapoport AP, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerdemann U, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Mol Ther. 2011;19:2258–2268. doi: 10.1038/mt.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson DR. Differential expression of human major histocompatibility class I loci: HLA-A, -B, and -C. Hum Immunol. 2000;61:389–396. doi: 10.1016/s0198-8859(99)00186-x. [DOI] [PubMed] [Google Scholar]
- 72.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 73.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 75.Mitchell MS, et al. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 76.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chapuis AG, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci USA. 2012;109:4592–4597. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 79.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 80.Hambach L, Goulmy E. Immunotherapy of cancer through targeting of minor histocompatibility antigens. Curr Opin Immunol. 2005;17:202–210. doi: 10.1016/j.coi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Warren EH, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quintarelli C, et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood. 2011;117:3353–3362. doi: 10.1182/blood-2010-08-300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Reilly RJ, Dao T, Koehne G, Scheinberg D, Doubrovina E. Adoptive transfer of unselected or leukemia-reactive T-cells in the treatment of relapse following allogeneic hematopoietic cell transplantation. Semin Immunol. 2010;22:162–172. doi: 10.1016/j.smim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rezvani K, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kern F, Faulhaber N, Frommel C, Khatamzas E, Prosch S, Schonemann C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 86.Haque T, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 87.Leen AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Comoli P, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004;15:113–117. doi: 10.1093/annonc/mdh027. [DOI] [PubMed] [Google Scholar]
- 89.Lucas KG, Salzman D, Garcia A, Sun Q. Adoptive immunotherapy with allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T-lymphocytes for recurrent, EBV-positive Hodgkin disease. Cancer. 2004;100:1892–1901. doi: 10.1002/cncr.20188. [DOI] [PubMed] [Google Scholar]
- 90.Sun Q, Burton R, Reddy V, Lucas KG. Safety of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for patients with refractory EBV-related lymphoma. Br J Haematol. 2002;118:799–808. doi: 10.1046/j.1365-2141.2002.03683.x. [DOI] [PubMed] [Google Scholar]
- 91.Gustafsson A, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 92.Imashuku S, et al. Unsuccessful CTL transfusion in a case of post-BMT Epstein-Barr virus-associated lymphoproliferative disorder (EBV-LPD) Bone Marrow Transplant. 1998;20:337–340. doi: 10.1038/sj.bmt.1700883. [DOI] [PubMed] [Google Scholar]
- 93.Comoli P, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7:1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 94.Leen AM, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 95.Bollard CM, et al. Multi-virus-specific T-cell therapy for patients after haematopoietic stem cell transplantation. Bone Marrow Transplant. 2011;46 (suppl):S89. [Google Scholar]
- 96.Leen AM, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong L, et al. Adoptive transfer of cytomegalovirus/Epstein-Barr virus-specific immune effector cells for therapeutic and preventive/preemptive treatment of pediatric allogeneic cell transplant recipients. J Pediatr Hematol Oncol. 2010;32:e31–e37. doi: 10.1097/MPH.0b013e3181bf5e2d. [DOI] [PubMed] [Google Scholar]
- 98.Haque T, et al. Reconstitution of EBV-specific T cell immunity in solid organ transplant recipients. J Immunol. 1998;160:6204–6209. [PubMed] [Google Scholar]
- 99.Comoli P, et al. Treatment of EBV-Related Post-Renal Transplant Lymphoproliferative Disease with a Tailored Regimen Including EBV-Specific T Cells. Am J Transplant. 2005;5:1415–1422. doi: 10.1111/j.1600-6143.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 100.Cho HI, et al. Adoptive transfer of Epstein-Barr virus-specific cytotoxic T-lymphocytes for the treatment of angiocentric lymphomas. Int J Hematol. 2006;83:66–73. doi: 10.1532/IJH97.A30505. [DOI] [PubMed] [Google Scholar]
- 101.Chua D, et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer. 2001;94:73–80. doi: 10.1002/ijc.1430. [DOI] [PubMed] [Google Scholar]
- 102.Haque T, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 103.Gandhi MK, et al. Immunity, homing and efficacy of allogeneic adoptive immunotherapy for posttransplant lymphoproliferative disorders. Am J Transplant. 2007;7:1293–1299. doi: 10.1111/j.1600-6143.2007.01796.x. [DOI] [PubMed] [Google Scholar]
- 104.Barker JN, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]