Abstract
Background
B-type natriuretic peptide (BNP) is a hormone with pleiotropic cardio-protective properties. Previously in our non-placebo controlled, un-blinded pilot study (BELIEVE) in human ST-elevation anterior acute myocardial infarction (AMI), a 72 hour intravenous infusion (IV) of recombinant human BNP (nesiritide) at a dose of 0.006 ug/kg/min suppressed plasma aldosterone and reduced cardiac dilatation while improved left ventricular ejection fraction (LV EF) at 1 month compared to baseline.
Methods and Design
The BELIEVE II study is a phase II, randomized, double-blind, placebo-controlled, single center clinical trial to assess the efficacy of 72 hour IV infusion of nesiritide therapy (0.006 ug/kg/min), in humans with first time ST-elevation anterior AMI and successful reperfusion, in preventing adverse LV remodeling and preserving LV function. A total of 60 patients will be randomized to placebo or nesiritide therapy. The primary efficacy endpoint is LV end-systolic and end-diastolic dimensions determined by MUGA scan between placebo and nesiritide group at 30 days; secondary endpoints include 30 day LV EF, diastolic function, infarct size, LV mass and combined total mortality and heart failure hospitalization.
Conclusion
This will be the first randomized, double-blind, placebo-controlled clinical trial that will assess the clinical efficacy of nesiritide in human ST-elevation anterior AMI.
Introduction
The burden of coronary artery disease (CAD) and acute myocardial infarction (AMI) remains exceedingly high in the United States, with over one million new or recurrent myocardial infarctions and over 500,000 deaths that are attributable to CAD annually.1 Importantly, AMI is known to lead to a loss of contractile function as a result of adverse left ventricular (LV) remodeling which includes LV hypertrophy, dilatation, myocyte necrosis and apoptosis, collagen deposition and fibrosis.2, 3 Despite significant advancements in early revascularization and beneficial pharmacological therapies for AMI, the expected one year mortality and progression to heart failure (HF) remains high, resulting in considerable efforts to reduce adverse post-AMI remodeling with novel therapies which include cells, devices, small molecules and/or peptides.4-7
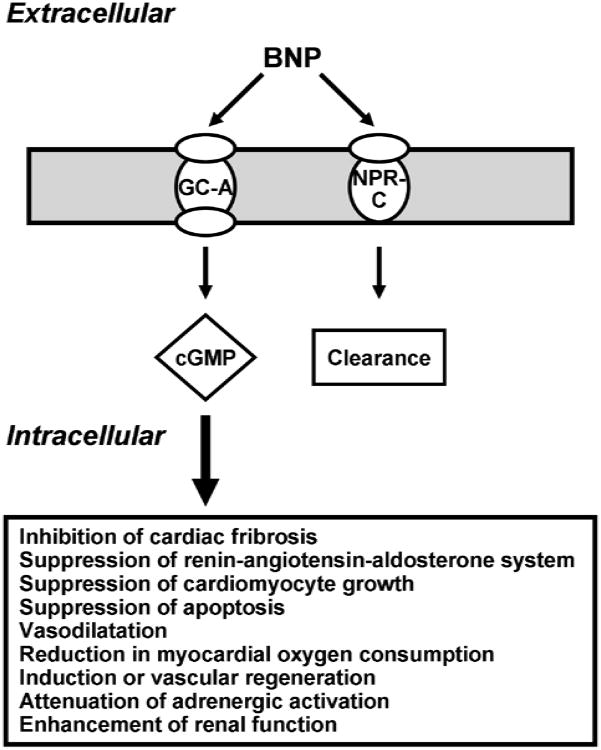
B-type natriuretic peptide (BNP) is a small endogenous cardiac peptide that possesses beneficial pleuri-potent properties that may protect the heart from injury and prevent unfavorable LV remodeling after AMI.8 Studies have demonstrated that BNP activates the particulate guanylyl cyclase receptor A (GC-A) and generates the second messenger 3′,5′-cyclic guanosine monophosphate (cGMP) leading to a reduction in myocardial oxygen consumption through coronary artery vasodilation,9 suppression of the renin-angiotensin-aldosterone system (RAAS),10 attenuation of adrenergic activation,11 induction vascular regeneration,12 inhibition of cardiac fibroblast collagen synthesis and proliferation,13-15 suppression of cardiomyocyte growth and apoptosis,16, 17 and enhancement of renal function (Figure 1).18-20 Importantly in 2001 and 2008, the United States Food and Drug Administration (FDA) and Health Canada respectively, approved human recombinant BNP, nesiritide, for the management of acute decompensated HF (ADHF).21, 22
Figure 1. Simplified BNP Signaling Pathway.
BNP specifically binds to the membrane bound particulate guanylyl cyclase receptor A (GC-A) and exerts it's biological actions through the activation of its second messenger, cGMP. BNP is removed from the circulation by a clearance receptor, which is a non-particulate GC receptor termed NPR-C.
Recently, we reported the results of a translational open label proof-of-concept investigation of the use of low dose nesiritide, that established safety and cardio-protection in humans with first time AMI and was named the BELIEVE (B-TypE natriuretic peptide and post myocardiaL Infarction LEft Ventricular rEmodeling) study.8 Here we demonstrated that 72 hour intravenous (IV) infusion of nesiritide in human AM activated plasma cGMP and suppressed plasma aldosterone during the infusion. Further, there was a reduction LV end-systolic volume and an improvement in LV ejection fraction (LV EF) one month after the three day infusion in comparison to baseline. Moreover Hillock et al.23 reported that BNP infusion at conventional clinical doses in human AMI with delayed or failed reperfusion and moderate LV dysfunction, was safe with trends towards favorable LV remodeling. Thus laboratory based research of BNP, together with these previous studies of BNP in human AMI, has laid the foundation for the BELIEVE II study. Specifically, BELIEVE II represents a novel protein therapeutic strategy for the inhibition of adverse post-AMI remodeling. Importantly, BELIEVE II represents the first, double blind, randomized, placebo controlled, single center clinical trial of BNP in human AMI. As supported by the National Heart, Lung and Blood Institute (NHLBI), this trial is designed to assess the efficacy of 72 hour IV infusion of nesiritide therapy in humans, with first time anterior AMI and successful reperfusion, in preventing adverse LV remodeling and preserving LV function.
Methods
Study Design and Setting
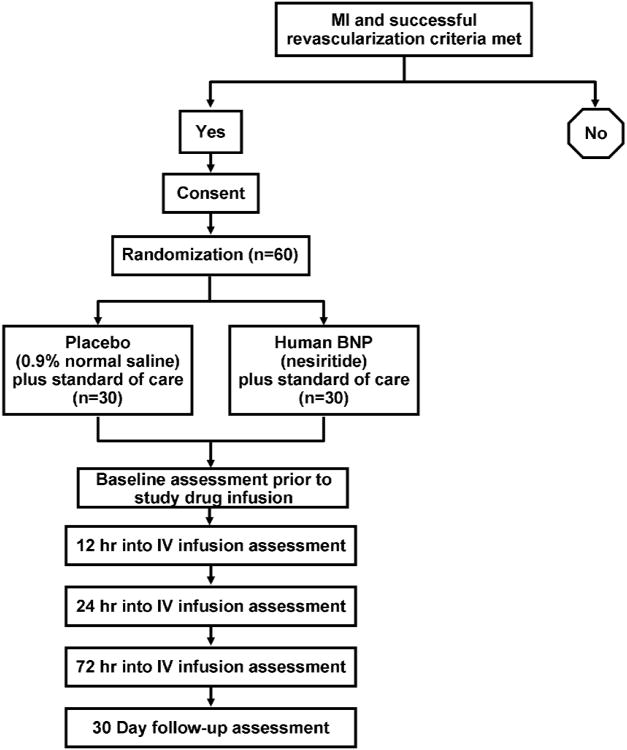
BELIEVE II is a phase II, randomized, double-blind, placebo-controlled trial conducted at Mayo Clinic, Rochester, Minnesota. The trial is supported by peer-reviewed grants (R01 HL83231 and P20 HL101439) from the NHLBI and has been registered at www.ClinicalTrials.gov (registration identifier: NCT00573144). A schematic of BELIEVE II study design is shown in Figure 2. A total of 60 patients will be randomized equally into one of two arms, receiving either 0.006 ug/kg/min IV infusion, without bolus, of nesiritide or placebo (0.9% normal saline), in addition to standard of care therapy.
Figure 2. Schematic outline of the BELIEVE II trial.
Patient Population
The study population will include 60 patients admitted to St. Mary's Hospital Coronary Care Unit (CCU) at Mayo Clinic, Rochester, Minnesota, with first time ST-elevation acute anterior Ml and successful revascularization either by thrombolytics or percutaneous coronary angioplasty (PTCA) as documented by TIMI III flow on coronary angiogram. The subject eligibility criteria are provided in Table 1. Study protocol patients will be recruited in the CCU within 24 hours of the coronary angiogram confirming TIMI III flow in the left anterior descending artery. Upon identification of a patient, the study will be explained in detail by the investigators or study coordinator, the consent form reviewed questions answered and the consent form signed.
Table 1. Patient Eligibility Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Randomization Procedure
After informed consent is obtained, they will be randomized to receive either IV infusion of nesiritide or placebo for 72 hours. The randomization schedule will be provided by Mayo Clinic's Division of Biomedical Statistics and Informatics and implemented by the Mayo Clinic Pharmacy and administered by the CCU staff. The investigators, patients and the CCU staff will not be aware of the randomization arm the patient is assigned to. The dose of nesiritide for this study will be 0.006 ug/kg/min via IV infusion, without bolus, based on the results from the BELIEVE study.8 Baseline assessment will occur after randomization, prior to study drug infusion, including a multiple gated acquisition (MUGA) scan within 24 hours and transthoracic echocardiography (TTE) within 72 hours.
Ethical Consideration
We have obtained an investigational new drug (IND; # 65,131) from the U.S. FDA for the use of nesiritide in our study. Further, this study is approved by Mayo Clinic's Institutional Review Board (IRB). All patients will receive other standard medical therapies as determined appropriate by the physician and in accordance to the ACC/AHA guidelines.24, 25 In addition, all patients will receive angiotensin converting enzyme inhibitor (ACEi) that will be started 12 hours after the start IV infusion. Lisinopril will be started at 12 hours after the start of IV infusion at an initial dose of 2.5 mg and will be titrated up by the participant's physician according to their clinical status and blood pressure (BP) to a target dose of 10 mg per day or highest dose tolerated before discharge. This is in accordance to the ACC/AHA guidelines for ACE inhibition in the management of acute anterior ST-elevation Ml (STEMI) which states that: “ An angiotensin-converting enzyme inhibitor should be administered within the first 24 hours to all patients with STEMI with anterior location, HF, or ejection fraction less than or equal to 40%, unless contraindicated.” Therefore following the ACC/AHA guidelines, in our current protocol, we are mandating that all patients be started on the ACEi, lisinopril, at a low dose of 2.5 mg and thereafter be titrated to a goal of 10 mg/day or highest dose tolerated. This target dose is based on the GISSI-3.26 Monitoring of vital signs which include BP, heart rate and respiration rate will be carried out every 15 minutes during the first 2 hours after the start of the infusion, every 30 minutes for the next 4 hours, every hour for the next 6 hours and thereafter as per standard clinical practice. During the 72 hours of infusion, if the systolic BP decreases to less than 90 mmHg for 5 minutes, the CCU service will be notified and if the systolic BP has not increased to greater or equal than 90 mmHg within 45 minutes, the infusion will be stopped for 6 hours. If the systolic BP is > or equal than 90 mmHg after 6 hour, the infusion will be restarted. If after 6 hours, the systolic BP is still less than 90 mmHg or is the patient has a second episode of hypotension (systolic blood pressure less than 90 mmHg) during the 72 hour period, the patient will be terminated from the study.
Two previous recent publications regarding nesiritide raised concern of increased risk of worsening renal failure in subjects with ADHF27 and increased short term risk of death after treatment with nesiritide for ADHF.28 However the ASCEND-HF clinical trial, which was designed to address these safety and efficacy concerns, reported that nesiritide was indeed safe and not associated with increased mortality or worsening renal function.29 All protocols carried out at Mayo Clinic will have a Data Safety and Monitoring Plan incorporated into the protocol that will be reviewed and approved by the IRB at Mayo Clinic
Outcome Assessment
The schedule of assessment is illustrated in Table 3. Each patient will be followed up at 30 days. Clinical data, laboratory data and physical exam will be performed at baseline and day 30 (± 5 days). Blood draws for determination of plasma BNP and aldosterone will be carried out at baseline prior to the start of the infusion, at 12 hours prior to the initiation of ACEi, at 72 hours before the termination of the infusion and at day 30 (± 5 days).
Table 3. Clinical End Points.
| End Point | Main Study |
|---|---|
| Primary |
|
| Secondary |
|
LV = left ventricular; MUGA = multiple gated acquisition scan; EF = ejection fraction; TTE = transthoracic echocardiography; MRI = magnetic resonance imaging; CHF = congestive heart failure; BNP = B-type natriuretic peptide
A MUGA scan will be performed within 24 hours after the initiation of the IV infusion and at the 30 day follow-up to assess systolic and diastolic volumes as well as LV EF. The MUGA scan will be performed at rest using previously reported techniques and modified in vivo red cell labeling with 30 mCi of technetium-99m. Anterior, left lateral and left anterior oblique projections will be obtained and the data acquisition will be gated to the R-wave on the electrocardiogram (ECG). LV EF and LV end-systolic and end-diastolic volumes will be calculated by a single nuclear medicine technician from the background-corrected LV counts versus time curve by use of an operator-interactive program and a commercially available dedicated computer (Medasys Pinnacle). Briefly, LV EF will be measured using LV activity independent of LV volumes. LV volumes will be based on the LV end-diastolic and end-systolic activity and corrections will be made to generate LV volumes as LV activity is proportional to volume. We will measure the activity in a blood sample obtained from the patient and subsequently, relate activity in the left ventricle to activity in the blood sample. Ten milliliters of blood will be obtained from the patient after the isotope equilibration and the activity in this sample will be determined using a gamma camera by a standard method. The LV activity will be then normalized (corrected) using the blood sample activity. This normalized LV activity will be used to generate LV diastolic or systolic volumes using a regression equation (this will be generated in comparison with LV angiography). All calculations will be reviewed and interpreted by a nuclear cardiologist, who will be blinded to the treatments.
A TTE will be performed within 72 hours after the initiation of the IV infusion and at the 30 day follow-up to assess LV diastolic function. All TTE studies will be performed by a certified cardiac sonographer from the Mayo Clinic echocardiography laboratory and will be reviewed by a single blinded cardiologist with level III echocardiography certification. Echocardiographic images will be obtained from standard acoustic windows according to the recommendations of the American Society of Echocardiography. Apical 4 and 2 chamber views and the long-axis view will be obtained from the apical window. Pulsed wave doppler measurements of mitral inflow velocity, pulmonary venous inflow velocities and doppler tissue imaging of the mitral annulus (septal aspect) and continuous wave doppler of the tricuspid regurgitant signal will be obtained from the apical views. Color doppler and 2D imaging of the mitral, tricuspid and aortic valves will be performed to screen for insufficiency or stenosis. In the subcostal window, imaging of the inferior vena cava and hepatic veins will be performed and used to estimate right atrial diastolic pressures.
Assessment of LV diastolic function and LV filling pressures
Pulsed wave Doppler examination of mitral (before and with Valsalva maneuver) and pulmonary venous inflow as well as Doppler tissue imaging of the mitral annulus will be performed. Diastolic function is categorized according to the progression of diastolic dysfunction (DD) as: normal; impaired relaxation without evidence of increased filling pressures (mild DD); impaired relaxation associated with moderate elevation of filling pressures (“pseudonormal filling” - moderate DD) and advanced reduction in compliance (“reversible or fixed restrictive filling” – severe DD). Subjects are required to have two Doppler criteria consistent with moderate or severe DD to be so classified. Subjects with one criterion for moderate-severe DD or those whose parameters are borderline and suggestive of, but not definitive for DD, will classified as indeterminate rather than normal. In subjects with atrial fibrillation, the deceleration time alone will be used to assess diastolic function. If the deceleration time is < 140 ms, the subject is considered to have severe DD. Those with atrial fibrillation and a deceleration time of > 140 ms, the diastolic function is considered indeterminate. This diastolic function classification scheme has been validated against invasive measurement of diastolic function and filling pressures by our group and has been shown to have independent prognostic value in the general population, controlling for age, sex and EF.
Atrial volume
Atrial volume is a marker of chronic pressure and volume overload. The effect of therapy on atrial volume will be assessed. Left atrial volume will be calculated using two orthogonal short axis (SA) and one long axis (LA) measurements of left atrial dimension using the formula of an ellipse (LA volume = π/6 (SA1*SA2*LA) and indexed to body surface area.
Cardiac magnetic resonance imaging (MRI) will be performed at the 30 day follow-up assessment for measurement of LV end diastolic volume, LV end systolic volume and LV mass. LV volumes and LV mass will be calculated according to Simpson's rule on traced endocardial and epicardial short axis LV images. LV EF = [(LV end diastolic - end systolic volume)/LV end diastolic volume] × 100%. All exams will be performed on GE 1.5 Tesla Twin Speed EXCITE or HD systems. After localizing scouts, functional assessment of the ventricles will be performed by pre-contrast cine images obtained using ECG-gated steady state free precession imaging. An initial 4 chamber view obtained from the scout views will be followed by short axis slices (8mm slice thickness, 1mm gap) covering the entire ventricle from base to apex. Scan parameters include TR 3.5ms, TE 1.6ms, bandwidth 125 kHz, flip angle 45, temporal resolution 40ms, matrix 192 × 160-192. Three short axis slices at the base, mid ventricle, and apex will also be acquired using a grid tagging ECG-gated gradient echo sequence, with parameters including TR/TE 6.8/3.7 ms, flip angle 30, bandwidth 32 kHz, 8 mm slice thickness, matrix 224 × 128, tag spacing 7 mm.
Contrast enhanced myocardial delayed enhancement images will be obtained approximately 10 minutes after the injection of IV gadolinium-DTPA (0.2mmmol/kg) in the same location used for the short axis cine images, using a segmented inversion recovery prepared fast gradient echo sequence for detection of infarction. Typical scan parameters include TR 6.5ms, TE 3.1ms, inversion time (adjusted for optimal myocardial nulling) 175-225ms, matrix 256 × 192. Images will be transferred to a Windows workstation (GE advantage windows) and a software analysis package (Mass analysis +) will be used be used to analyze images at the Mayo Clinic MRI laboratory.
Short axis cine images will be used to calculate left and right ventricular EF, end-diastolic and end-systolic volumes, and LV mass based on endocardial contours traced using computer-assisted planimetry at end-diastole and end-systole (Mass Analysis, GE Medical Systems). LV mass will be determined based on endocardial and epicardial contours traced at end-diastole.
Myocardial delayed enhancement corresponds to infarcted myocardium, and this will be quantified by tracing the area of delayed enhancement in the left ventricle on each short axis slice in the delayed enhancement sequence. The total volume is then determined using the Mass Analysis software, and multiplied by the specific gravity of myocardium (1.05) to obtain the mass of infarcted tissue.
Patients will return at 30 days for repeat blood draws for determination of neurohumoral profile, MUGA scan, TTE and cardiac MRI as stated above. The primary and secondary endpoints of BELIEVE II are summarized in Table 3.
Sample Size and Statistical Analysis
The sample size calculation and statistical analysis have been designed in collaboration with Mayo Clinic's Division of Biomedical Statistics and Informatics and a lead statistician. The primary analyses will be 2-sample comparisons (2-sample t-test) of the effects of interest: Changes in LV end-systolic and end-diastolic volumes, LV EF, plasma BNP and plasma aldosterone. Also, main effects model with/without interaction included. BNP will be expressed as median and IQ ranges and all other values will be express as the mean + SE. A statistically significant difference will be considered to be present when p<0.05. Patients who developed ischemia and requiring revascularization of the coronary arteries during the 30 day follow-up will be excluded form the final analysis.
Sample size calculation
Preliminary data were obtained on 11 treated subjects with 0.006 μg/kg/min in our pilot study. These data were used to estimate the standard deviation (SD) of the change in each parameter in the treated group.
Based on our pilot study, for LV end-systolic volume the conservative estimate of delta is 20 ml/m2 and the SD is 27.88 (SD in pre/post =36, with r=0.70 between time points), therefore the power for detecting such a significant change between placebo and treatment group is 80% when we study a total of 60 patients, controlling for type I error at 5%.
Based on our pilot study, for LV end-diastolic volume the conservative estimate of delta is 30 ml/m2 and the standard deviation (SD) is 43.37 (SD in pre/post =56, with r=0.70 between time points), therefore the power for detecting such a significant change between placebo and treatment group is 80% when we study a total of 60 patients, controlling for type I error at 5%.
Based on our pilot study, for LV EF the conservative estimate of delta is 10 % and the SD is 13.94 (SD in pre/post =18, with r=0.70 between time points), therefore the power for detecting such a significant change between placebo and treatment group is 80% when we study a total of 60 patients, controlling for type I error at 5%.
Based on the sample size calculations above, with 30 subjects in each group and a total of 60 patients we will have 80% power to dectect a significant change in LV volumes and EF.
Discussion
BELIEVE II will be the first study to assess the efficacy of a 72 hour IV infusion of nesiritide, with early intervention, to preserve myocardial structure and function in humans with first time anterior AMI. This strategy, to be tested in a single center, randomized, double blind, placebo controlled trial, is designed to complement myocardial revascularization, with the hypothesis that the cardiac hormone BNP will prevent adverse post-AMI LV remodeling when combined with interventional and conventional pharmacologic therapy.
Despite the seminal advances achieved with acute revascularization at the time of AMI, the late effects of acute AMI often are characterized by progressive dilatation and fibrosis of the LV myocardium, often leading to HF and increased mortality. Animal and human studies at the molecular level have established the pleiotropic properties of BNP, secondary to its activation of the GC-A receptor and the generation of the second messenger, cGMP. These protective actions include a reduction in myocardial oxygen consumption through coronary artery vasodilation,9 suppression of the RAAS,10 attenuation of adrenergic activation,11 induction vascular regeneration,12 inhibition of cardiac fibroblast collagen synthesis and proliferation,13-15 suppression of cardiomyocyte and apoptosis growth,16, 17 and enhancement of renal function.18-20 Indeed, the findings from our initial proof of concept, un-blinded, non-placebo controlled pilot study in human AMI, demonstrated that a three day infusion of nesiritide initiated shortly after revascularization, reduced LV end-systolic volume and suppressed plasma aldosterone, one month following AMI.8 This strategy is also strongly supported by key studies using atrial natriuretic peptide in human AMI, as well as in a separate study of AMI using BNP with delayed or failed revascularization.23, 30
BELIEVE II therefore lays the foundation for a paradigm shifting concept which includes the combined use of acute revascularization to regain myocardial perfusion, which may limit infarct size, coupled with a 72 hour IV infusion of nesiritide. Notably, this combination therapeutic strategy in BELIEVE II may enhance the protection and integrity at the cellular level of myocytes, fibroblasts and endothelial cells, so as to prevent unfavorable LV remodeling, preserve LV function and ultimately reduce the burden of HF.
Table 2. Schedule of Assessment.
| Assessment | Baseline | 12 hrs | 24 hrs | 72 hrs | 30 days |
|---|---|---|---|---|---|
| Demographics | × | ||||
| Medical History | × | ||||
| Physical Exam | × | × | |||
| Vital Signs | × | × | × | × | × |
| 12-Lead ECG | × | × | |||
| Plasma BNP | × | × | × | × | |
| Plasma Aldosterone | × | × | × | × | |
| MUGA scan | × | × | |||
| TTE | × | × | |||
| Cardiac MRI | × | ||||
| Assessment of Adverse Events | × | × | × | × | × |
ECG = electrocardiogram; BNP = B-type natriuretic peptide; MUGA = multiple gated acquisition scan; TTE = transthoracic echocardiography; MRI = magnetic resonance imaging.
Acknowledgments
The authors acknowledge the outstanding contribution of cardiac catheterization laboratory on-call team, coordinators for recruitment, sonographers and technical staff at Mayo Clinic, Rochester, MN.
Disclosures: This work is support by research grants from the NHLBI (P20 HL101439 and R01 HL83231), Scios Inc. and the Mayo Foundation. Drs. Burnett and Chen and Mayo Clinic have patented designer natriuretic peptides. Drs. Burnett and Chen have received royalties from Nile Therapeutics Inc. and Anexon Inc., and are the co-founders of Zumbro Discovery Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108(11):1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 4.Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53(24):2262–9. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Chung ES, Dan D, Solomon SD, Bank AJ, Pastore J, Iyer A, et al. Effect of peri-infarct pacing early after myocardial infarction: results of the prevention of myocardial enlargement and dilatation post myocardial infarction study. Circ Heart Fail. 2010;3(6):650–8. doi: 10.1161/CIRCHEARTFAILURE.110.945881. [DOI] [PubMed] [Google Scholar]
- 6.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53(6):501–10. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Parikh NI, Gona P, Larson MG, Fox CS, Benjamin EJ, Murabito JM, et al. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart study. Circulation. 2009;119(9):1203–10. doi: 10.1161/CIRCULATIONAHA.108.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HH, Martin FL, Gibbons RJ, Schirger JA, Wright RS, Schears RM, et al. Low-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept study. Heart. 2009;95(16):1315–9. doi: 10.1136/hrt.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaels AD, Klein A, Madden JA, Chatterjee K. Effects of intravenous nesiritide on human coronary vasomotor regulation and myocardial oxygen uptake. Circulation. 2003;107(21):2697–701. doi: 10.1161/01.CIR.0000070547.88378.EA. [DOI] [PubMed] [Google Scholar]
- 10.Cheung BM, Kumana CR. Natriuretic peptides--relevance in cardiovascular disease. JAMA. 1998;280(23):1983–4. doi: 10.1001/jama.280.23.1983. [DOI] [PubMed] [Google Scholar]
- 11.Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol. 2001;37(5):1221–7. doi: 10.1016/s0735-1097(01)01172-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamahara K, Itoh H, Chun TH, Ogawa Y, Yamashita J, Sawada N, et al. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc Natl Acad Sci U S A. 2003;100(6):3404–9. doi: 10.1073/pnas.0538059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209(3):943–9. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- 14.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91(12):1127–34. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 15.Huntley BK, Ichiki T, Sangaralingham SJ, Chen HH, Burnett JC., Jr B-type natriuretic peptide and extracellular matrix protein interactions in human cardiac fibroblasts. J Cell Physiol. 2010;225(1):251–5. doi: 10.1002/jcp.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenkranz AC, Hood SG, Woods RL, Dusting GJ, Ritchie RH. B-type natriuretic peptide prevents acute hypertrophic responses in the diabetic rat heart: importance of cyclic GMP. Diabetes. 2003;52(9):2389–95. doi: 10.2337/diabetes.52.9.2389. [DOI] [PubMed] [Google Scholar]
- 17.He JG, Chen YL, Chen BL, Huang YY, Yao FJ, Chen SL, et al. B-type natriuretic peptide attenuates cardiac hypertrophy via the transforming growth factor-beta1/smad7 pathway in vivo and in vitro. Clin Exp Pharmacol Physiol. 2010;37(3):283–9. doi: 10.1111/j.1440-1681.2009.05281.x. [DOI] [PubMed] [Google Scholar]
- 18.La Villa G, Fronzaroli C, Lazzeri C, Porciani C, Bandinelli R, Vena S, et al. Cardiovascular and renal effects of low dose brain natriuretic peptide infusion in man. J Clin Endocrinol Metab. 1994;78(5):1166–71. doi: 10.1210/jcem.78.5.8175974. [DOI] [PubMed] [Google Scholar]
- 19.Mentzer RM, Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr, Luber JM, Jr, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49(6):716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116(11 Suppl):I134–8. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 21.Howlett JG. Current treatment options for early management in acute decompensated heart failure. Can J Cardiol. 2008;24(Suppl B):9B–14B. doi: 10.1016/s0828-282x(08)71023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett JC, Jr, Korinek J. The tumultuous journey of nesiritide: past, present, and future. Circ Heart Fail. 2008;1(1):6–8. doi: 10.1161/CIRCHEARTFAILURE.108.776294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillock RJ, Frampton CM, Yandle TG, Troughton RW, Lainchbury JG, Richards AM. B-type natriuretic peptide infusions in acute myocardial infarction. Heart. 2008;94(5):617–22. doi: 10.1136/hrt.2006.110239. [DOI] [PubMed] [Google Scholar]
- 24.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54(23):2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 25.O'sGara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–55. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 26.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'sinfarto Miocardico. Lancet. 1994;343(8906):1115–22. [PubMed] [Google Scholar]
- 27.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 28.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–5. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 30.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370(9597):1483–93. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]