Abstract
More research effort needs to be invested in antimicrobial drug development to address the increasing threat of multidrug-resistant organisms. The enzyme DHPS has been a validated drug target for over 70 years as the target for the highly successful sulfa drugs. The use of sulfa drugs has been compromised by the widespread presence of resistant organisms and the adverse side effects associated with their use. Despite the large amount of structural information available for DHPS, few recent publications address the possibility of using this knowledge for novel drug design. This article reviews the relevant papers and patents that report promising new small-molecule inhibitors of DHPS, and discuss these data in light of new insights into the DHPS catalytic mechanism and recently determined crystal structures of DHPS bound to potent small-molecule inhibitors. This new functional understanding confirms that DHPS deserves further consideration as an antimicrobial drug target.
Overuse of antibacterial drugs and poor patient compliance have led to the development and spread of drug resistance to virtually all anti-bacterial agents used in the clinic today. Infectious diseases are currently responsible for up to a third of deaths worldwide, causing a major healthcare crisis [1–3]. Mono-, multi- and pan-resistant microbial strains are appearing at an alarming and increasing rate, and it is clear that there is a critical need for the development of new effective antimicrobials to sustain our modern day quality of life and maintain the steady reduction in worldwide mortality rates. In addition to treating active infections, antibiotics are used prophylactically in many medical procedures, including surgeries and transplants, to prevent secondary infections. The absence of a durable and viable antimicrobial developmental pipeline that can foresee and address evolving resistance means that this need will not be met in the near future. Indeed, we are close to beginning a ‘post-antibiotic era’ in which there is the real danger of being unable to treat common infections [2,4,5]. The limited number of new antibiotics currently being developed is unlikely to meet the ever-growing medical need. Furthermore, most of these new drugs do not represent novel classes of compounds with the ability to overcome known mechanisms of resistance [5–7].
Alarmingly, only two new antibacterial drug classes have been approved in the past 20 years, despite the urgent need for them [6,8]. One way to adeptly move forward is to identify drug-like inhibitors against known and validated targets but which have distinct mechanisms of action from the antibiotics currently available. With unique modes of action against validated targets, they will probably be effective but not prone to existing mechanisms of target-based resistance. This article summarizes recent literature on small-molecule inhibitors of the bona fide drug target DHPS, and discusses published patents and articles that focus on inhibitors with novel mechanisms of inhibition. A recent review by Swarbrick et al. discusses new small-molecule inhibitors of the various enzymes in the folate biosynthetic pathway [9]. This article will focus solely on DHPS and emphasize the impact on drug discovery of recent structural insights on the mechanism of action of this enzyme.
Folates are crucial cofactors in the synthesis of thymidine, purines, certain amino acids, and pantothenic acid, and generally play critical roles in one-carbon transfer reactions in cell metabolism [10]. Higher eukaryotes obtain folic acid and reduced folate polyglutamates from dietary sources by uptake through membrane-associated folate transport proteins. In contrast, prokaryotes and some lower eukaryotes are obligated to synthesize folate de novo [11, 12]. The enzymes of the folate biosynthetic pathway are thus unique to those microorganisms and make the pathway an excellent target for anti-infective agents. In 2002, Derrick and Bermingham summarized the structural and mechanistic information available at the time for the enzymes of the folic acid biosynthetic pathway and evaluated each enzyme as a potential target for antibiotic research [13]. Two enzymes are current clinical targets for antimicrobial therapy, DHPS and DHFR (Figure 1). DHPS catalyzes the condensation of p-aminobenzoic acid (pABA) with 7,8-dihydropterin-pyrophos-phate (DHPPP) to form 7,8-dihydropteroate, a key convergent step in the pathway. DHPS is the target of the first synthetic antimicrobial agent Prontosil rubrum, a prodrug for sulfanilamide, which was found by Gerhard Domagk to have antibacterial efficacy in 1932 [14]. Soon thereafter, research in the laboratory of Ernest Fourneau identified sulfanilamide as the active compound in prontosil [15,16]. Many thousands of derivative compounds, sulfonamides and sulfones, have since been synthesized and tested, leading to the discovery of a variety of potent ‘sulfa drugs’ that are now used in modern medicine.
Figure 1. Later stages of the folate biosynthetic pathway and related antibacterial drugs that target the pathway.
PPi: Pyrophosphate.
Sulfa drugs have been successfully used to treat a broad range of bacterial and protozoal infections. They are close structural analogs of pABA and exhibit their antimicrobial activity by competing with pABA at the DHPS active site, forming sulfa drug–pterin adducts instead of dihydrofolic acid, and critically depleting cellular folate levels [17–20]. Sulfonamides can be classified according to their duration of action; ‘short acting’, such as sulfamethizole and sulfisoxazole; ‘intermediate acting’, such as sulfasalazine and sulfamethoxazole; and ‘long acting’, such as sulfadoxine and sulfamethoxine [21]. Sulfone drugs are not as prominent as sulfonamides, and the most widely used sulfone is dapsone. Dapsone is frontline therapy for the treatment of leprosy and a second-line agent for the treatment of Pneumocystis carinii pneumonia, as well as an antimalarial agent [22]. Emergence of resistance, particularly for some key indications, such as the treatment of malaria [23], and the introduction of antibiotics with fewer adverse side effects and more rapid killing, have decreased the clinical utility of sulfa drugs. However, they still represent a cost-effective alternative and are especially useful in combination therapy [24]. Since its introduction in the 1960s, the trimethoprim–sulfamethoxazole combination drug that simultaneously targets DHFR and DHPS has been successfully used to treat a variety of common, as well as specific, clinical infections. The use of both drugs in combination has a synergistic effect in vitro, and it was believed that this synergy would occur in vivo while decreasing the risk of the development of drug resistance [24,25]. Trimethoprim–sulfamethoxazole continues to be used as a first-line therapy in the prophylaxis and treatment of HIV-associated secondary Pneumocystis carinii pneumonia infections [26], for urinary tract infections and as an oral therapy for methicillin-resistant Staphylococcus aureus. However, these current uses of sulfa drugs remain limited by their ongoing potential to cause drug allergies. For example, in HIV-infected patients undergoing prolonged prophylaxis therapy, studies have demonstrated adverse reactions to trimethoprim–sulfamethoxazole in 65% of patients where the sulfonamide component is believed to be largely responsible [21,24]. Despite this long and successful history of using sulfonamides that clearly validate DHPS as a premier drug target, no new drugs that target DHPS have been developed for use in the clinic.
DHPS structure & mechanism
The clinical significance of DHPS has prompted many structural studies of the enzyme, and these have resulted in numerous publications that report structures of the free enzyme as well as complexes with substrates, product analogs and inhibitors [19,27–37]. To date, crystal structures of DHPS from ten different organisms have been determined: Escherichia coli [19]; S. aureus [27]; Mycobacterium tuberculosis [28]; Bacillus anthracis [29]; Saccharomyces cerevisiae [31]; Streptococcus pneumonia [32]; Thermus thermophilus HB8 [37]; Francisella tularensis [33]; Burkholderia cenocepacia [35]; and Yersinia pestis [36]. The structure is highly conserved and comprises a classical (α8/β8) TIM barrel with the active site at the ‘C-terminal’ end of the β-barrel. The active site can be subdivided into three conserved subsites: the pterin-binding pocket deep within the β-barrel; the pABA binding subsite, which largely comprised two flexible, but conserved, loops 1 and 2; and the anion-binding pocket that accommodates the pyrophosphate moiety [36]. Due to the inherent flexibility of the two loops that contribute to the pABA-binding site, it has been poorly resolved in all of the early DHPS structures. To facilitate structure-based drug discovery, the authors have invested much effort in determining the structure of DHPS in its active liganded conformation, and recently succeeded by using DHPS from Y. pestis (YpDHPS). The key to this study was finding that loops 1 and 2 are unconstrained by crystal contacts in the substrate-free structure of YpDHPS and the recognition that they are potentially free to adopt their functional conformations in the crystalline state. The authors also discovered that YpDHPS and B. anthracis DHPS (BaDHPS) are both able to perform catalysis in the crystalline state.
By performing catalysis in crystalline BaDHPS and solving the structure of YpDHPS near the transition state, the catalytic mechanism of DHPS has been elucidated and the key inter-mediates along the pathway have been identified [36]. The condensation of pABA with DHPPP to form 7,8-dihydropteroate occurs via an SN1 reaction mechanism instead of the previously postulated SN2 reaction. The reaction proceeds via an ordered mechanism in which DHPPP first binds DHPS, which then eliminates its pyrophosphate group (PPi) prior to pABA binding by stabilizing the cationic intermediate DHP+, which could be observed in the crystal (Figure 2). The released PPi, together with a Mg2+ ion, induce the formation of a complex loop1–loop2 substructure within which pABA binds. As the reaction nears the transition state, pABA, DHP+, PPi and the Mg2+ ion are all bound within a highly organized and complex active site prior to product formation (Figure 3a). The Mg2+ ion is essential to DHPS catalysis, and its role is to help order the loop 1–2 substructure and to stabilize the leaving PPi group. The authors have demonstrated that the Mg2+ ion is not required for the cleavage of PPi from DHPPP, but it does accelerate PPi release from the enzyme [36]. The key findings from this study are that DHPS catalysis proceeds via an SN1 reaction and that the conserved pterin-binding pocket is specifically designed to stabilize the DHP+ intermediate by charge delocalization.
Figure 2. Stable cationic SN1 reaction intermediate DHP+ found to reside in crystals.
PPi: Pyrophosphate.
Figure 3. Pre-transition state of Yersinia pestis DHPS.
(A) The fully formed active site with bound pABA (yellow), DHP+ (blue), pyrophosphate group (pink), and a Mg2+ ion (green). (B) The molecular envelope of DHPS into which inhibitors should be designed to reside to avoid the development of resistance. pABA: p-aminobenzoic acid.
Sulfa drug-resistance mechanism
The authors’ crystal structure of sulfamethox azole (SMX) bound to YpDHPS confirms that sulfa drugs bind in the pABA-binding pocket [36]: SMX fits into the tight pABA-binding pocket perfectly; the sulfonyl group mimics the inter-actions of the pABA carboxyl group; and their common phenyl groups make almost identical interactions within the hydrophobic pocket created by loop 1–2. The methoxazole ring of SMX and the thiazole ring of sulfathiazole in the BaDHPS:sulfathiazole complex both protrude out of the active site in such a way that the common sulfa drug resistant mutations in loops 1 and 2 are able to uniquely disrupt sulfa drug binding but not pABA binding, which lacks these extra rings (Figure 3b) [36]. It has been observed that drug resistance commonly develops in response to parts of the drug that extend beyond the molecular envelope of the actual substrate [38]. This is clearly the case with the sulfa drugs, and future DHPS inhibitors can now be designed to remain within the substrate envelope that has recently been characterized by the authors (Figure 3b).
Small-molecule inhibitors of DHPS
Pterin site inhibitors
Mutations within the highly dynamic pABA-binding site are unlikely to affect the essential structure of the enzyme, which helps to explain why sulfa drug resistance occurs so rapidly. In contrast, DHPPP binds in a deep and highly conserved structured cleft at the center of the TIM barrel, which has been visualized in all published crystal structures of DHPS. The pterin ring interacts with hydrophilic residues and a structured water molecule via nine hydrogen bonds, and the complementary donor/acceptor groups result in a high degree of specificity. Any mutations causing drug resistance in this site will most probably affect the structure and function of DHPS making it an ideal pocket to target with small-molecule inhibitors. As predicted, mutations that confer resistance to sulfonamide drugs have no effect on DHPPP binding [32] and pterin-binding site inhibitors will not be affected by the existing target-based resistance mechanisms that have evolved against sulfa drugs. Mechanistically, pterin site inhibitors should also behave synergistically with DHFR inhibitors in a manner similar to that of the sulfa drugs. With the wealth of structural information available and the recent mechanistic information obtained by the authors, it is now possible to target the pterin-binding pocket with novel chemistry.
Aminopterin, methotrexate and trimethoprim are known inhibitors of DHFR inhibitors that interact with the pterin site [39]. However, their 2,4-diaminopteridine core engages the pterin pocket in a different pose compared with how the pterin ring of the substrate dihydrofolate normally binds and is rotated by <60° (see PDB ID 1RX2 vs 1RX3). The highly structured and relatively narrow pterin-binding pocket of DHPS is extremely selective towards the hydrogen bonding pattern and shape of pterin. It is speculated that in DHPS, steric clashes prevent the 2,4-diamonopteridines from binding within the pterin site in a manner such as in DHFR, which may explain why there are no reports of these molecules inhibiting DHPS.
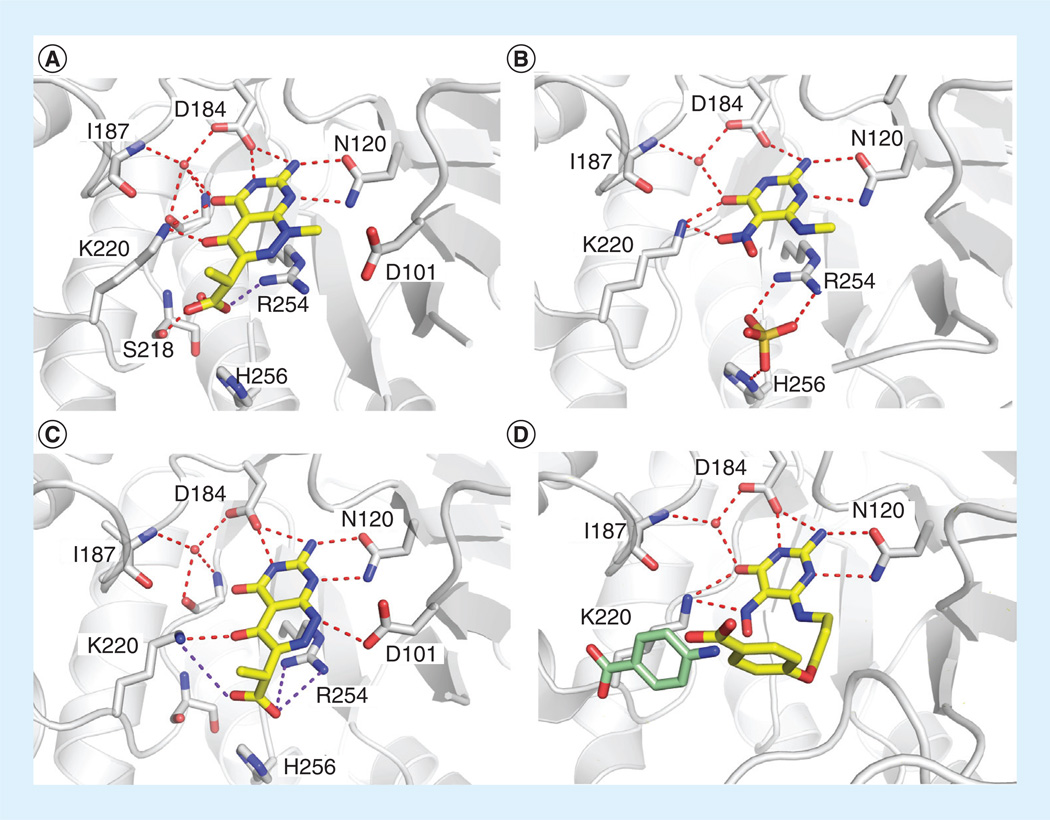
Targeting the DHPPP binding site of DHPS with small molecules is not a new idea – it has been recognized for some time that DHPS must contain a pterin-binding pocket distinct from that of pABA [23,40–42]. Various pterin-like bacteriostatic derivatives of pyrimido[4,5-c]pyridazines were reported to be potent inhibitors of DHPS and to exhibit antimicrobial activity in the 1970s by researchers at Burroughs Wellcome Co. (London, UK), and their synthesis, use in pharmaceutical preparations and process for preparations were patented at that time [50]. Approximately 30 compounds were reported to display a range of DHPS inhibition, with IC50 values ranging from two-digit micromolar to sub-micromolar. Recently, the pyrimido[4,5-c]pyridazines were revisited in an attempt to design more potent inhibitors with the help of high resolution crystal structures [34]. Starting from a potent inhibitor discovered by Burroughs Wellcome Co. (I; Figure 4), a series of 4,5-dioxo-1,4,5,6-tetrahydropyrimido[4,5c]pyridazines were designed and tested for their ability to inhibit BaDHPS in vitro. The authors sought to obtain a comprehensive understanding of the binding interactions from structural, kinetic and thermodynamic perspectives. It was found that compounds lacking the N-methyl substitution on the ring are higher affinity binders, as well as more potent DHPS inhibitors in vitro, due to their ability to form the hydrogen bond interaction with Asp101 (Figure 5a & b). This interaction occurs with the natural pterin substrate and stabilizes the essential DHP+ intermediate. The co-crystal structure with compound I also suggested that the length of the methylene side chain is crucial for correctly positioning the terminal carboxyl group in the anion binding site and allowing the undistorted docking of the pyridazine ring within the pterin pocket (Figure 5A). However, comparing compounds 6 & 7 reveals that a one-carbon extension actually reduces activity. This may be due to the increased flexibility of the longer side chain and the increased entropic cost of its binding. Replacing the flexible methylene side chain with anionic rigid ring structures is currently being investigated as a way to overcome this problem.
Figure 4. Pyrimido[4,5c]pyridazines as reported by Zhao et al.
Inhibition was tested against Bacillus anthracis DHPS, percentage inhibition values reported were determined at a test compound concentration of 250 µM [34].
Figure 5. Details of the interactions between DHPS and the pyrimido[4,5-c]pyridazines reported by the authors.
(A) Compound I, (B) compound 6, (C) compound 10, and (D) compound 21 bound in the pterin-binding site of Bacillus anthracis DHPS. Modeled into the structure in pale green is p-aminobenzoic acid.
In 1985, Lever et al. reported several mono-cyclic 6-(alkylamino)-5-nitrosoisocytosines with high in vitro potency which compete with pterin for binding to DHPS [41]. One of the reported compounds (12; Figure 6) contains a methylamino at the C-6 position and inhibited DHPS with an IC50 value of 1.6 µM. However, despite their ability to potently inhibit DHPS in vitro, none of the nitrosoisocytosines displayed significant antibacterial activity [40,41]. The authors speculate that this lack of antibacterial activity may be related to poor cellular penetration or rapid breakdown once inside the cell. At the time the Burroughs Wellcome researchers performed their studies, no structural information was available for DHPS.
Figure 6. Monocyclic DHPS inhibitors reported by Lever et al.
In vitro inhibition was tested against Escherichia coli. DHPS for all compounds – except compound II, which was tested against Bacillus anthracis DHPS [30].
In 2004, Babaoglu et al published the crystal structure of 6-methylamino-5-nitroisocytosine (MANIC; 10) in complex with BaDHPS [29]. It engages the pterin-binding pocket in a similar fashion to the substrate analogs 6-hydroxymethyl-pterine-pyrophosphate and 6-hydroxymethyl-pterine-monophosphate (Figure 5C), and interacts with five out of the six pterin-binding residues [29]. In a later study, the close analog 6-amino-5-nitrosoisocytosine was shown to be a more potent inhibitor of BaDHPS, and this result was rationalized by the crystal structure which revealed that the free amine binds to Asp101 via an electrostatic/ hydrogen bond interaction [30]. In the previous study by Lever and co-workers [41], substituting the nitroso moiety for a nitro group resulted in a slightly decreased affinity to the enzyme, and this result is supported by the crystal structures of compounds 10 & 13 [30].
Pterin/pABA site binders
In the preceding study, several compounds with elongated substituent side chains at the C-6 position were generated such as the 6-(ω-phenylalkyl)amino substituted analog 14. These displayed good inhibitory activity and suggested that the side chain may reach into the pABA-binding site. In 1986, the Lever group further optimized the 6-(alkylamino)-5-nitrosoisocytosines class of inhibitors by linking various substituted phenoxy moieties via a C-3 linker to the amino group at C-6 of the nitrosoisocytosine. These optimized small molecules inhibited DHPS with IC50 values in the sub-micromolar range (Figure 7) [40]. In 2010, the authors obtained a co-crystal structure of the DHPS:21 complex and showed that the phenyl group extends towards, but does not engage, the pABA binding site (Figure 5D) [30]. In this study, compound 21 was soaked into BaDHPS crystals in which loops 1 and 2 are constrained by crystal contacts and are not free to adopt the functional conformation that contains the complete pABA-binding pocket. The potency of these dual site binders is slightly increased over the corresponding pterin-only binders such as MANIC, but the ligand efficiency is much lower suggesting that there is still much room for improvement of these dual-site binders [43]. These dual-site binders largely remain within the substrate envelope and will probably not rapidly generate resistance.
Figure 7. A selection of the most potent inhibitors reported by Lever et al.
In vitro inhibition was tested against Escherichia coli DHPS [40,41].
Transition state mimics
Several years before obtaining the near transition state structure of DHPS, the authors attempted to design transition-state analog inhibitors that simultaneously contact the pterin-, pABA- and PPi-binding pockets [44]. Two of the four compounds synthesized display in vitro activity against purified BaDHPS and show evidence of slow-binding kinetics (Figure 8). When these compounds were originally designed, it was generally believed that DHPS operates via an SN2 mechanism, and these molecules incorporated features that were consistent with an SN2 reaction coordinate. If designed success-fully, transition state mimic inhibitors should display nanomolar inhibition [45], which is not the case with compounds 22 & 23. The subsequent discovery that DHPS catalysis proceeds via an SN1 mechanism explains the inactivity of these compounds [36]. However, knowledge of the presence of the DHP+ SN1 intermediate and the active site loop geometries in the near transition state crystal structure now offers new avenues for improving these molecules. This structure does indeed contain three independent substrate-binding pockets. The spatial orientation of the substrate-like moieties on the transition state analogs can now be optimized for engaging the pockets. Poor solubility was also noted as a problem for this compound series despite the presence of several ionizable groups, and this problem can also now be addressed in a structure-based fashion.
Figure 8. Two transition state mimics (22 & 23) and para aminosalicylic acid.
DHPS as a prodrug activator
Chakraborty and co-workers recently demonstrated that the antitubercular agent para-aminosalicylic acid (Figure 8), a close structural analog of pABA, binds DHPS and acts as a replacement substrate [46]. The generated product goes on to act as an inhibitor and/or replacement product for subsequent enzymes in the folate biosynthetic pathway, which ultimately leads to its inhibition. It is important to note that there have also been previous reports of aminopterin and sulfonamide precursors that are activated by DHPS into larger molecules that, in turn, inhibit different steps in folate biosynthesis [47,48]. These studies demonstrate that catalysis by DHPS can be used as a novel prodrug strategy for drug discovery, and it also demonstrates that sulfonamide antibacterials are prone to intracellular metabolism and/or modification in M. tuberculosis as well as in other microorganisms.
Conclusion
DHPS has been a validated drug target for over 70 years with a highly successful track record of inhibition by sulfonamide drugs. However, adverse reactions to the sulfonamide class of compounds and widespread resistance to these agents has severely compromised the use of these drugs. The focus of this review has been on the literature concerning recent efforts to create non-sulfa drug DHPS inhibitors, with an emphasis on molecules that bind at the pterin site deep within the enzyme. One important aid to this process has been the significant amount of new high-resolution structural data for DHPS in combination with substrates and pterin-targeted inhibitors. These data have created ripe opportunities to apply modern structure-based drug-discovery strategies, including the optimization of previously discovered pterin binders, available in the older patent literature, and the discovery of new inhibitory scaffolds, using methods such as fragment-based lead discovery (FBLD) [49]. Furthermore, recent insights into the mechanism of DHPS catalysis and the associated high-resolution pre-transition state structure of DHPS should now enable the design of novel and potent transition-state analogs and pterin/pABA dual-site binders.
One area of caution in future medicinal chemistry efforts against this target relates to the fact that the pterin-binding pocket inhibitors discovered, thus far, are similar in structure to pterin, presumably due to the highly specific nature of the DHP+–DHPS interaction. Pterin-like compounds tend to be flat and have high crystal energies resulting in poor solubility. This was a recurring problem noted in many of the studies described in this review because poor solubility is highly undesirable in drug candidates. It is for this reason that FBLD approaches are now favored to identify unbiased and completely novel scaffolds that engage the pterin pocket. A second challenging area relates to the poor activity of these pterin-based inhibitors in MIC assays, which is probably due to a lack of defined uptake transporters, poor general permeability into bacteria and/or active efflux out of the cell, and insufficient target affinity to generate a pharmaco dynamic effect. These important issues will need to be addressed in order to move towards the identification of the nanomolar inhibitors that will be necessary to generate viable lead candidate molecules for future antibacterial agents.
Future perspective
At a time when antimicrobials are urgently needed, DHPS has not been given the consideration it deserves as a validated and historically successful antibacterial drug target. This is evident from the limited recent literature reporting small-molecule inhibitors of this enzyme. It is predicted that the recent highly significant and important discoveries relating to the structure and mechanism of DHPS will now accelerate the near future identification of novel and potent inhibitors for this bona fide drug target. Most significantly, it is now known that the cationic SN1 reaction intermediate DHP+ is stabilized by the pterin-binding site, meaning that this key feature can now be exploited in future drug discovery attempts. Furthermore, the emergence of novel inhibitory scaffolds of DHPS from expanding FBLD screening efforts is fully expected.
Executive summary.
Background
-
▪
Widespread resistance to all available antibiotics together with the absence of new drugs threatens our ability to treat infectious diseases.
-
▪
The bona fide drug target DHPS has been neglected in recent literature and deserves further consideration in light of new mechanistic and structural data.
DHPS structure & mechanism
-
▪
A wealth of high-resolution structural data on DHPS from ten different species is available.
-
▪
Recent structures at the near transition state, in which key loop residues are resolved, can now be exploited for structure-based drug design.
-
▪
The catalytic reaction of DHPS proceeds via an SN1 mechanism in which DHP+ is stabilized by the pterin-binding pocket of DHPS.
Sulfa drug-resistance mechanism
-
▪
Common sulfa drug-resistant mutations in DHPS have developed to prevent the binding of part of the sulfa drugs that protrude from the molecular envelope of the substrate.
Small-molecule inhibitors of DHPS
-
▪
Pyrimido[4,5-c]pyridazines and nitrosoisocytosines are potent inhibitory scaffolds that bind to the pterin site of DHPS.
-
▪
Thus far, inhibitors targeting the pterin-binding site display poor antibacterial activity, which will probably improve upon the development of nanomolar inhibitors that are structurally optimized to contact other binding pockets.
-
▪
Although past attempts to synthesize transition-state analogs were designed based on an SN2 reaction mechanism, new mechanistic and structural insights can now be exploited based on an SN1 reaction mechanism to optimize these compounds and generate high-affinity inhibitors.
DHPS as a prodrug activator
-
▪
Catalysis by DHPS can be exploited for drug discovery of prodrug inhibitors that act on the later enzymes in the folate biosynthesis pathway.
Future perspective
-
▪
The emergence of novel inhibitors for DHPS as a result of fragment-based drug discovery efforts is expected.
Acknowledgments
This work was supported by NIH Grant AI070721 (SW White and RE Lee), Cancer Center Support Grant CA21765, and the American Lebanese Syrian Associated Charities, St Jude Children’s Research Hospital.
Key Term
- Synergy
Ability of two antibiotics, when used in combination, to provide increased killing than that achieved by the antibiotics when used individually. Targeting two enzymes within the folate biosynthetic pathway provides a synergistic double hit that is very difficult for bacteria to overcome.
- Molecular envelope
Volume of space occupied by natural substrates of the enzyme. Drugs that remain within this volume are less prone to the development of resistance because of the fitness cost to the activity of the enzyme
- Transition state mimic
Important concept in medicinal chemistry design that takes advantage of the fact that enzymes have evolved to stabilize high-energy reaction intermediates. An enzyme typically has the highest affinity to the transition state, and molecules that mimic the transition state are often very potent
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- 1.WHO. The World Health Report 2004 – changing history, Annex Table 2: death by cause, sex and mortality stratum in WHO regions, estimates for 2002. J. Adv. Nurs. 2004;48(5):542–542. [Google Scholar]
- 2.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 2005;36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Namba K, Zheng XX, Motoshima K, et al. Design and synthesis of benzenesulfonanilides active against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus . Bioorg. Med. Chem. 2008;16(11):6131–6144. doi: 10.1016/j.bmc.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum PC. 2012 and beyond: potential for the start of a second pre-antibiotic era? J. Antimicrob. Chemother. 2012;67(9):2062–2068. doi: 10.1093/jac/dks213. [DOI] [PubMed] [Google Scholar]
- 6.Chopra I. The 2012 Garrod Lecture: discovery of antibacterial drugs in the 21st century. J. Antimicrob. Chemother. 2012;68(3):496–505. doi: 10.1093/jac/dks436. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J. Am. Geriatr. Soc. 2002;50(7 Suppl):S226–S229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 8.Silver LL. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011;24(1):71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swarbrick J, Iliades P, Simpson JS, et al. Folate biosynthesis – reappraisal of old and novel targets in the search for new antimicrobials. Open Enzym. Inhib. J. 2008;1:12–33. [Google Scholar]
- 10.Blakley RL. Folates and Pterins. NY, USA: John Wiley and Sons Inc; 1984. [Google Scholar]
- 11.Matherly LH. Molecular and cellular biology of the human reduced folate carrier. Prog. Nucleic Acid Res. 2001;67:131–162. doi: 10.1016/s0079-6603(01)67027-2. [DOI] [PubMed] [Google Scholar]
- 12.Henderson GB, Huennekens FM. Membrane-associated folate transport proteins. Meth. Enzymol. 1986;122:260–269. doi: 10.1016/0076-6879(86)22180-1. [DOI] [PubMed] [Google Scholar]
- 13.Bermingham A, Derrick JP. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays. 2002;24(7):637–648. doi: 10.1002/bies.10114. [DOI] [PubMed] [Google Scholar]
- 14.Domagk G. Ein beitrag zur chemotherapie der bakteriellen infektionen. Dtsch. Med. Wschr. 1935;7:250–253. [Google Scholar]
- 15.Trefouel J, Nitti F, Bovet D. Activité du p. aminophénylsulfamide sur l’infection streptococcique expérimentale de la souris et du lapin. C. R. Soc. Biol. 1935;120:756. [Google Scholar]
- 16.Trefouel J, Trefouel J, Bovet D, Nitti F. The contribution of the institut pasteur, paris, to recent advances in microbial and functional chemotherapy. Br. Med. Bull. 1946;4(4):284–289. doi: 10.1093/oxfordjournals.bmb.a072793. [DOI] [PubMed] [Google Scholar]
- 17.Then R, Angehrn P. Sulfonamide-induced thymineless death in Escherichia coli . J. Gen. Microbiol. 1973;76:255–263. doi: 10.1099/00221287-76-2-255. [DOI] [PubMed] [Google Scholar]
- 18.Roland S, Ferone R, Harvey RJ, Styles VL, Morrison RW. Characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J. Biol. Chem. 1979;254(20):337–345. [PubMed] [Google Scholar]
- 19.Achari A, Somers DO, Champness JN, Bryant PK, Rosemond J, Stammers DK. Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nat. Struct. Biol. 1997;4(6):490–497. doi: 10.1038/nsb0697-490. [DOI] [PubMed] [Google Scholar]
- 20.Wood DD. The relationship of p-aminobenzoic acid to the mechanism of the action of sulphanilamide. Br. J. Exp. Pathol. 1940;21:74–90. [Google Scholar]
- 21.Bryskier A. Washigton, DC, USA: ASM Press; 2005. Antimicrobial agents: antibacterials and antifungals; pp. 941–963. [Google Scholar]
- 22.Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J. Am. Acad. Dermatol. 2001;45(3):420–434. doi: 10.1067/mjd.2001.114733. [DOI] [PubMed] [Google Scholar]
- 23.Nzila A. Inhibitors of de novo folat enzymes in Plasmodium falciparum . Drug Discov. Today. 2006;11(19–20):939–944. doi: 10.1016/j.drudis.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-sulfamethoxazole revisited. Arch. Intern. Med. 2003;163(4):402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- 25.Bushby SRM, Hitching GH. Trimethoprim a sulphonamide potentiator. Br. J. Pharmacol. 1968;33(1):72–90. doi: 10.1111/j.1476-5381.1968.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin SI, Fishman JA, Practice AIDC. Pneumocystis pneumonia in solid organ transplant recipients. Am. J. Transplant. 2009;9:S227–S233. doi: 10.1111/j.1600-6143.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 27.Hampele IC, D’Arcy A, Dale GE, et al. Structure and function of the dihydropteroate synthase from Staphylococcus aureus . J. Mol. Biol. 1997;268(1):21–30. doi: 10.1006/jmbi.1997.0944. [DOI] [PubMed] [Google Scholar]
- 28.Baca AM, Sirawaraporn R, Turley S, Sirawaraporn W, Hol WG. Crystal structure of Mycobacterium tuberculosis 7,8-dihydropteroate synthase in complex with pterin monophosphate: new insight into the enzymatic mechanism and sulfa-drug action. J. Mol. Biol. 2000;302(5):1193–1212. doi: 10.1006/jmbi.2000.4094. [DOI] [PubMed] [Google Scholar]
- 29.Babaoglu K, Qi J, Lee RE, White SW. Crystal structure of 7,8-dihydropteroate synthase from Bacillus anthracis: mechanism and novel inhibitor design. Structure. 2004;12(9):1705–1717. doi: 10.1016/j.str.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Hevener KE, Yun MK, Qi J, et al. Structural studies of pterin-based inhibitors of dihydropteroate synthase. J. Med. Chem. 2010;53(1):166–177. doi: 10.1021/jm900861d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence MC, Iliades P, Fernley RT, Berglez J, Pilling PA, Macreadie IG. The three-dimensional structure of the bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/dihydropteroate synthase of Saccharomyces cerevisiae . J. Mol. Biol. 2005;348(3):655–670. doi: 10.1016/j.jmb.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Levy C, Minnis D, Derrick JP. Dihydropteroate synthase from Streptococcus pneumoniae: structure, ligand recognition and mechanism of sulfonamide resistance. Biochem. J. 2008;412(2):379–388. doi: 10.1042/BJ20071598. [DOI] [PubMed] [Google Scholar]
- 33.Pemble CWT, Mehta PK, Mehra S, et al. Crystal structure of the 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase dihydropteroate synthase bifunctional enzyme from Francisella tularensis . PLoS ONE. 2010;5(11):e14165. doi: 10.1371/journal.pone.0014165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Hammoudeh D, Yun MK, Qi J, White SW, Lee RE. Structure-based design of novel pyrimido[4,5-c]pyridazine derivatives as dihydropteroate synthase (DHPS) inhibitors with increased affinity. J. Med. Chem. 2012;7(5):861–870. doi: 10.1002/cmdc.201200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan RE, Batot GO, Dement JM, Rao VA, Eadsforth TC, Hunter WN. Crystal structures of Burkholderia cenocepacia dihydropteroate synthase in the apo-form and complexed with the product 7,8-dihydropteroate. BMC Struct. Biol. 2011;11:21. doi: 10.1186/1472-6807-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun MK, Wu Y, Li Z, et al. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science. 2012;335(6072):1110–1114. doi: 10.1126/science.1214641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagautdinov B, Kunishima N. Crystral structure of of dihydropteroate synthase (FolP) from Thermus thermophilus HB8. RIKEN Struct. Genomics/Proteomics Initiative. 2006 Online. [Google Scholar]
- 38.Romano KP, Ali A, Royer WE, Schiffer CA. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc. Natl Acad. Sci. USA. 2010;107(49):20986–20991. doi: 10.1073/pnas.1006370107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone SR, Montgomery JA, Morrison JF. Inhibition of dihydrofolate-reductase from bacterial and vertebrate sources by folate, aminopterin, methotrexate and their 5-deaza analogs. Biochem. Pharmacol. 1984;33(2):175–179. doi: 10.1016/0006-2952(84)90472-6. [DOI] [PubMed] [Google Scholar]
- 40.Lever OW, Bell LN, Hyman C, Mcguire HM, Ferone R. Inhibitors of dihydropteroate synthase - substituent effects in the side-chain aromatic ring of 6-[[3-(aryloxy)propyl] amino]-5-nitrosoisocytosines and synthesis and inhibitory potency of bridged 5-nitrosoisocytosine-para-aminobenzoic acid analogs. J. Med. Chem. 1986;29(5):665–670. doi: 10.1021/jm00155a014. [DOI] [PubMed] [Google Scholar]
- 41.Lever OW, Bell LN, Mcguire HM, Ferone R. Monocyclic pteridine analogs - inhibition of escherichia-coli dihydropteroate synthase by 6-amino-5-nitrosoisocytosines. J. Med. Chem. 1985;28(12):1870–1874. doi: 10.1021/jm00150a019. [DOI] [PubMed] [Google Scholar]
- 42.Lever OW, Hyman C, Ray PH, Ferone R, Kelsey JE. A galactoside derivative of a nitrosoisocytosine inhibitor of dihydropteroate synthase – synthesis and biological evaluation. J. Heterocycl. Chem. 1986;23(2):629–631. [Google Scholar]
- 43.Tanaka D, Tsuda Y, Shiyama T, et al. A practical use of ligand efficiency indices out of the fragment-based approach: ligand efficiency-guided lead identification of soluble epoxide hydrolase inhibitors. J. Med. Chem. 2011;54(3):851–857. doi: 10.1021/jm101273e. [DOI] [PubMed] [Google Scholar]
- 44.Qi J, Virga KG, Das S, et al. Synthesis of bi-substrate state mimics of dihydropteroate synthase as potential inhibitors and molecular probes. Bioorg. Med. Chem. 2011;19(3):1298–1305. doi: 10.1016/j.bmc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schramm VL. Transition states, analogues, and drug development. ACS Chem. Biol. 2013;8(1):71–81. doi: 10.1021/cb300631k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty S, Gruber T, Barry CE, 3rd, Boshoff HI, Rhee KY. Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis . Science. 2012;339(6115):88–91. doi: 10.1126/science.1228980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel O, Satchell J, Baell J, Fernley R, Coloe P, Macreadie I. Inhibition studies of sulfonamide-containing folate analogs in yeast. Microb. Drug Resist. 2003;9(2):139–146. doi: 10.1089/107662903765826723. [DOI] [PubMed] [Google Scholar]
- 48.Nduati E, Hunt S, Kamau EM, Nzila A. 2,4-diaminopteridine-based compounds as precursors for de novo synthesis of antifolates: a novel class of antimalarials. Antimicrob. Agents Chemother. 2005;49(9):3652–3657. doi: 10.1128/AAC.49.9.3652-3657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fattori D, Squarcia A, Bartoli S. Fragment-based approach to drug lead discovery: overview and advances in various techniques. Drugs R D. 2008;9(4):217–227. doi: 10.2165/00126839-200809040-00002. [DOI] [PubMed] [Google Scholar]
Patent
- 101.Morrison RW, Jr, Revill MW. EP0000383 A1 Styles LV. 1979