ABSTRACT
Introduction: Type-2 diabetes mellitus and Vitamin D deficiency are both common in Saudi Arabian population. New roles of vitamin D have emerged recently especially in the prevention of cardiovascular disease, cancer and insulin resistance.
Objective: To estimate 25-OH vitamin D deficiency in patients with type-2 diabetes mellitus in comparison to normal age-matched non-diabetic control population. Methods: A Randomized Case-Control study was done in three tertiary care hospitals in Southern Region, Saudi Arabia from June 2010 to June 2012 and 345 patients were selected; 172 in the diabetic group and 173 in the non-diabetic group. Biochemical workup and 25-OH vitamin D levels were done.
Results: The mean serum 25-OH vitamin D levels in the diabetic group were 15.7 + 7.5 ng/mL as compared healthy non-diabetic group having 11.1 + 5.9 ng/mL and a total of 340 patients (98.5%) from both groups were found to be deficient in 25-OH vitamin D which is the highest reported so far in Saudi Arabia.
Conclusion: The population in our study was generally deficient in 25-OH vitamin D irrespective of diabetes mellitus indicating a greater need for vitamin D supplementation.
Keywords: vitamin D deficiency, Saudi Arabia, sunlight exposure, diabetes mellitus
INTRODUCTION
Type-2 diabetes mellitus (T2DM) is a worldwide pandemic and World Health Organization (WHO) predicts that the current figure of 170 million affected patients with diabetes will more than double to 370 million patients by the year 2030 (1). Saudi Arabia is currently at top in the list of middle-east countries with the highest number of estimated cases of diabetes mellitus (2). The population of Saudi Arabia with changes in lifestyle, reduced physical activity and high calorie snacks have lead to increased prevalence of obesity which is related to type-2 diabetes, hyperlipidemia and infertility (in women) (3). The prevalence of type-2 diabetes in Saudi Arabia is around 23.7% of total population which is highest by percentage in Asia (4). Prevalence of obesity is 39.3% among diabetes as compared to 18.5% among non-diabetics (5). Recently vitamin D deficiency has been found to be associated with type-2 diabetes and obesity with up to 80% of obese adults being vitamin D insufficient (5). Researches have shown low vitamin D status to be associated with the development of type-2 diabetes as well as metabolic syndrome (6). It has lead to the hypothesis that vitamin D insufficiency correlates positively with insulin resistance and cardiovascular risk in obese adolescents and that 25-OH vitamin D supplementation improves insulin resistance and cardiovascular risk factors in this population (7). Up until recently, vitamin D deficiency was considered rare in those parts of the world that had plenty of sunshine all year round but WHO now estimates that globally one billion people have vitamin D deficiency or insufficiency (8,9).
Although, there is limited information on whether vitamin D supplementation in adolescents might actually result in improvement in insulin resistance and related parameters, there have been many recent studies implicating the role of vitamin D in cardiovascular disease prevention, cancer prevention, inhibiting parathyroid hormone secretion, promoting insulin secretion, inhibiting adaptive immunity while promoting innate immunity as well as inhibiting proliferation and stimulating differentiation of cells (10). Pittas et al have shown that insulin sensitivity is improved by as much as 60% when levels of 25-hydroxy vitamin D are increased from 25 to 75 nmol/L, a fact which has been reemphasized by local studies (11,12). Recent studies have shown association of 25-hydroxy vitamin D deficiency with an increased risk of stroke death in whites while other researchers have reported anticancer activities of vitamin D against many cancer types, including breast cancer as well as protection against Non-Alzheimer Dementias (13,14). To the best of author's knowledge, no study has been done to estimate the vitamin D deficiency in a specific patient population with type-2 diabetes mellitus while compared to normal population in Saudi Arabia.
The purpose of the study is to determine the degree of 25-hydroxy vitamin D deficiency in patients with type-2 diabetes mellitus as compared to non-diabetic population in the capital of Southern Region of Saudi Arabia. ❑
OBJECTIVE
To compare and assess the 25-hydroxy vitamin D deficiency between patients with type-2 diabetes mellitus and normal age-matched non-diabetic population in Southern Region of Saudi Arabia to assess if any difference exists between them. ❑
METHODS
It was a Multicentered Case-Control study done in the outpatient departments of three tertiary care hospitals in the two large cities, namely Abha and Khamis Mushyt, in the Southern Region of Kingdom of Saudi Arabia. The ethical approval of the study was given by the research and ethics committee of King Khalid University, Abha. The study was done from June 2010 to June 2012 and a total of 345 patients were selected randomly with 172 patient in the diabetic group and 173 patients in the non-diabetic control group which was age and sex matched. The patients with diagnosed type-2 diabetes for at least 1 year with recent glycosylated hemoglobin of less than 9 (either currently on oral hypoglycemic agents or insulin) and age more than 20 were included in the Diabetic group while the Control group (Non-Diabetic) consisted of age and sex matched patients from other clinics with no active or chronic problems. The patients whose serum calcium was more than 10.4 mg/dL or those taking multivitamin supplementation or having hepatic, renal or metabolic bone disorders (including parathyroid related problems) were excluded from the study. Also those patients with use of glucocorticoids or anti-seizure medications in the previous 6 months; or those patients having history of malabsorption syndromes such as celiac disease or active malignancy or with active infection were excluded from the study.
The patients fulfilling the above-mentioned criteria were selected after informed consent and their demographic data as well as data pertaining to history of present illness and positive physical signs were obtained. 25-hydroxy vitamin D levels were done for these patients and deficient patients were identified. Corresponding age and sex matched patients fulfilling the inclusion criteria were added in the control (non-diabetic) group and 25-hydroxy vitamin D (25OHD) levels were done for these patients using Chemiluminescence immunoassay (IDS Ltd., Boldon Colliery, Tyne & Wear, UK) to check for deficiency. The biochemical workup (Urea, creatinine, electrolytes liver function tests, fasting lipid profile etc) of these selected patients in both groups was done. It has been previously proposed that vitamin D deficiency should be defined as a serum 25OHD level <50 nmol/L (<20 ng/mL), however most endocrinologists have a consensus that a serum 25OHD level of <75 nmol/L (<30 ng/mL) should be taken as abnormal/insufficient (16), and this is the reason why we considered serum 25OHD level of <30 ng/mL to be as 25-hydroxy vitamin D insufficiency. All this information was collected through a specially designed proforma and the data collected was analyzed by SPSS package for Microsoft Windows version 11.0. The p-value of less than 0.05 was taken as significant, while employing t-test/Levene's test for quantitative variables and Pearson's chi square for qualitative variables. The variables of demography like age and sex, body mass index and investigations like urea, creatinine, liver function tests, fasting lipids, serum calcium and vitamin D levels etc were presented as simple descriptive statistics as illustrated in Table 1. ❑
Table 1.
Characteristics of Non-Diabetic and Diabetic Groups in the study.
| Sr # | Variable |
Non Diabetic Mean + SD N=172 |
Diabetic Mean + SD N=173 |
p value * |
|---|---|---|---|---|
| 1. | Age in years | 48.1 + 15.9 | 53.4 + 15.6 | 0.35 |
| 2. | Female patients (percentage) | 88 (51.2%) | 86 (49.7%) | 0.78 |
| 3. | Body Mass Index kg/m2 | 32.6 + 6.7 | 33.4 + 7.4 | 0.59 |
| 4. | Alanine aminotransferase IU/mL | 17.3 + 6.6 | 19.6 + 8.9 | 0.39 |
| 5. | Aspartate aminotransferase IU/mL | 19.1 + 5.1 | 16.5 + 3.2 | 0.14 |
| 6. | Urea mg/dL | 30.6 + 9.7 | 31.3 + 13.4 | 0.77 |
| 7. | Creatinine mg/dL | 0.91 + 0.18 | 0.88 + 0.22 | 0.55 |
| 8. | Fasting blood glucose mg/dL | 91.7 + 10.9 | 179.6 + 73.1 | 0.00 |
| 9. | Glycosylated Hemoglobin HbA1C | 6.0 + 0.3 | 8.6 + 17 | 0.00 |
| 10. | Total cholesterol mg/L | 185.6 + 44.2 | 170.8 + 45.5 | 0.89 |
| 11. | Triglycerides mg/L | 126.2 + 65.8 | 164.6 + 114.5 | 0.01 |
| 12. | Serum Calcium mg/dL | 8.8 + 0.5 | 9.0 + 0.6 | 0.98 |
| 13. | High density lipoproteins mg/dL | 46.7 + 12.1 | 41.1 + 13.6 | 0.70 |
| 14. | 25-OH vitamin D levels ng/ml | 11.1 + 5.9 | 15.8 + 7.5 | 0.50 |
* To calculate p value, t-test/Levene's test used for quantitative variables and Pearson's chi square used for qualitative variables.
RESULTS
The patients in the non-diabetic group had a mean age of 48.9 + 15.9 years as compared to diabetic group having mean of 53.4 + 15.6 years. There were 51.2% females in non-diabetic group while there were 49.7% females in diabetic group. The mean body mass index of non-diabetic group was 32.6 + 6.7 kg/m2, while diabetic group had a mean BMI of 33.4 + 7.4 kg/m2; the difference being not statistically significant with a p-value of 0.5. The mean glycosylated hemoglobin (HbA1c) of the diabetic group was 8.6 + 1.6 % as compared to non-diabetic group having mean HbA1C of 6.0 + 0.3 % with p-value being less than 0.001. There was no significant difference seen in the cholesterol level between the non-diabetic group (185.5 + 42.2 mg/dl) and diabetic group (170.8 + 45.5 mg/dl) with a p-value of 0.89, but the triglycerides levels were higher in the diabetic (164.5 + 114.5 mg/dl) versus the non-diabetic group (126.2 + 64.8 mg/dl) with a p-value of 0.01 which was statistically significant. The High Density Liporotein (HDL) was similar in the diabetic (46.8 + 12.1 mg/dl) versus the non-diabetic group (41.1 + 13.6 mg/dl) with p-value of 0.7 while the difference between serum calcium levels of non-diabetic group (8.8 + 0.5 mg/dL) and the diabetic group (9.0 + 0.6 mg/dL) was also not significant with a p-value of 0.98.
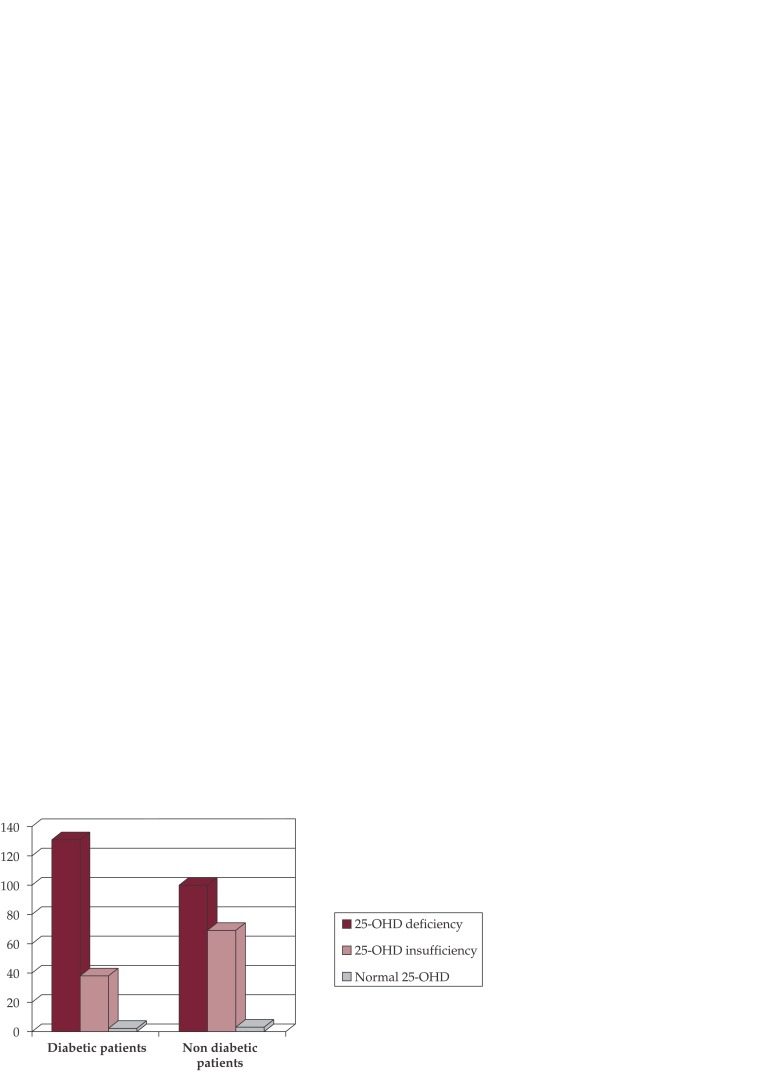
25-Hydroxy vitamin D (25OHD) levels: The mean serum 25OHD levels in the diabetic group were 15.7 + 7.5 ng/mL which was low. But surprisingly the mean 25OHD levels in the non-diabetic group were even lower, 11.1 + 5.9 ng/mL, which was not statistically significant from diabetic group with p-value of 0.5. Only 3 patients (1.7%) from non-diabetic group and 2 patients (1.2%) from the diabetic group had mean 25OHD levels more than normal cut-off value of >30 ng/mL. All in all, a total of 340 patients (98.5%) from both groups were found to be insufficient in 25OHD with no considerable statistical difference between the non-diabetic and diabetic groups. However on further evaluation, 131 (76.6%) of the patients with diabetes had 25OHD deficiency with levels less than 20 ng/mL, while 38 (22.2%) patients had 25OHD insufficiency as compared to non-diabetic population of which 100 (58.1%) patients had 25OHD deficiency (less than 20 ng/mL) while 69 (40.1%) patients had 25OHD insufficiency (less than 30 ng/mL but greater than 20 ng/mL). Comparing the two populations for 25OHD deficiency only (see Figure 1), the group with diabetes had more patients with 25OHD deficiency than non-diabetic group which was statistically significant with a p-value was 0.0003. ❑
Figure 1. Figure 1. 25 OH-vitamin D (25-OHD) deficiency and insufficiency distribution between non-diabetic and diabetic group of patients.
Key
25OH vitamin D deficiency considered when levels less than 20 ng/mL
25OH vitamin D insufficiency considered when levels between 20 and 30 ng/mL
Normal 25OH vitamin D when levels more than or equal to 30 ng/mL
DISCUSSION
Vitamin D deficiency has been reported previously in Saudi Arabia but our study shows a very significant level of 25-OH vitamin D deficiency in our population. Recent studies have shown vitamin D deficiency among healthy young Saudi women of age 25 to 35 years was 30% and 55% in women of 50 years or more, indicating that it is common in young and postmenopausal women (17). In another study on male population from Saudi Arabia, the prevalence of vitamin D deficiency was found to between 28% and 37% (18). Researchers has shown that vitamin D deficiency is highly prevalent among healthy Saudi women as well as men and largely attributed to obesity, poor exposure to sunlight, poor dietary vitamin D supplementation, sedentary lifestyle, lack of education and older age; which in turn affects BMD and bone turnover markers (19,20). New studies have shown Vitamin D deficiency among young and middle age Saudi Arabian males may lead to serious health consequences as high prevalence of a vitamin D deficiency occurs in Saudi Arabians despite having adequate exposure to sunlight and reported adequate intake of dairy products (21). Supplementing with high-dose vitamin D have shown benefits in many medical conditions including fibromyalgia in our population where 61% of diagnosed fibromyalgia women had 25OHD insufficiency (22,23). The recent studies implicating vitamin D deficiency in various illnesses like insulin resistance, allergic condition, multiple sclerosis and cancers and its possible role in the treatment of these conditions are in evolution (24), and more research is needed to ascertain these findings.
Vitamin D deficiency has been implicated in decreased insulin secretion and increased insulin resistance, and more recently with development of type 2 diabetes mellitus (25). However due to the presence of 25OHD insufficiency in up to 98.5% of our study population, which is the highest reported so far in Saudi Arabia to the best of the author's knowledge, we could not ascertain any significant difference in vitamin D status between our patient with diabetes and without diabetes. However specifically comparing only the deficiency of 25OHD with levels less than 20 ng/mL, 76.6% of the patients with diabetes as compared to 58.1% patients from non-diabetic population had 25OHD deficiency which was statistically significant difference with a p-value less than 0.0005.
We found that there was no significant difference in vitamin D status of males and female patients in our study population which is contrary to the previous studies from Saudi Arabia that shows a much higher degree of vitamin D deficiency in females in comparison to males. The high number of 25OHD insufficiency does demand larger scale studies and aggressive supplementation of vitamin D in our population.
Our study was somehow limited by a small number of patients. Also the control (non-diabetic) group although fulfilling the selection criteria of having no active diseases, was not entirely an ideal healthy control group as 76.5% patients from this group were found to have chronic medical conditions in the past, which nevertheless were controlled at the time of the study but may still have contributed towards to vitamin D deficiency/insufficiency. The common medical conditions in the non-diabetic group included hypothyroidism with maintained euthyroid status (33.8%) and euthyroid multinodular goiter (23.5%). Also up to 30 patients (17.4%) in non-diabetic group did complain of non-specific aches and pains but these patients could not be followed up specifically for the presence of fibromyalgia. Besides the non-diabetic group also had glycosylated hemoglobin in the range of 5.7% to 6.3% which meant that some of them could have had prediabetes, which may have affected our results. The authors believe that presence of these medical conditions in the control group may have contributed to lower 25-OH vitamin D levels in non-diabetic population. ❑
CONCLUSION
The population in Southern Region of Saudi Arabia is generally insufficient in 25OH vitamin D irrespective of presence of type 2 diabetes mellitus and a there is greater need for supplementation of vitamin D in this population. Although the presence of medical conditions such as euthyroid multinodular goiter and hypothyroidism in our control group may have affected our data but the general inference of 25OH vitamin D deficiency in 98.5% of population cannot be ignored.
We recommend larger scale studies for detecting vitamin D deficiency in our population especially in patients with type-2 diabetes mellitus and suggest planning aggressive strategies to supplement our population with vitamin D. An interesting avenue in this aspect would be to see if supplementing with vitamin D can help improve glycemic control in diabetic population.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.World Health Organisation. Diabetes Program 2004. Available at:http//www.who.int/diabetes/en/. September 21, 2004. [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Al-Nuaim AR. Population based epidemiological study of the prevalence of overweight and obesity in Saudi Arabia, regional variation. Ann Saudi Med. 1997;17:195–9. doi: 10.5144/0256-4947.1997.195. [DOI] [PubMed] [Google Scholar]
- 4.El-Hazmi MAF, Warsy AS. Prevalence of overweight and obesity in diabetic and non-diabetic Saudis. East Med Health J. 2000;6:276–82. [PubMed] [Google Scholar]
- 5.El-Hazmi MA, Warsy AS. Obesity and overweight in Type II diabetes mellitus patients in Saudi Arabia. Saudi medical journal. 1999;20:167–72. [PubMed] [Google Scholar]
- 6.Michos ED. Vitamin D deficiency and the risk of incident Type 2 diabetes. Future Cardiology. 2009;5:15–8. doi: 10.2217/14796678.5.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab. 2005;31:318–25. doi: 10.1016/s1262-3636(07)70200-8. [DOI] [PubMed] [Google Scholar]
- 8.Ultraviolet radiation and human health. Fact sheet N° 305: http://www.who.int/mediacentre/factsheets/fs305/en [Google Scholar]
- 9.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–6. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 10.McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med. 2011;155:820–6. doi: 10.7326/0003-4819-155-12-201112200-00004. [DOI] [PubMed] [Google Scholar]
- 11.Pittas AG, Harris SS, Stark PC, et al. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 12.Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med. 2010;30:454–8. doi: 10.4103/0256-4947.72265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao T, Klein P, Grossbard ML. Vitamin D and Breast Cancer. Oncologist. 2012;17:36–45. doi: 10.1634/theoncologist.2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28:367–71. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annweiler C, Rolland Y, Schott AM, et al. Serum Vitamin D Deficiency as a Predictor of Incident Non-Alzheimer Dementias: A 7-Year Longitudinal Study. Dement Geriatr Cogn Disord. 2012;32:273–278. doi: 10.1159/000334944. [DOI] [PubMed] [Google Scholar]
- 16.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009;89:1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 17.Al-Turki HA, Sadat-Ali M, Al-Elq AH, et al. 25-Hydoxyvitamin D levels among healthy Saudi Arabian women. Saudi Med J. 2008;29:1765–8. [PubMed] [Google Scholar]
- 18.Sadat-Ali M, Al-Elq AM, Al-Turki H, et al. Vitamin D levels among Healthy Saudi Arabian Men. Annals of Saudi Med. 2009;29:378–82. doi: 10.4103/0256-4947.55168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardawi MS, Qari MH, Rouzi AA, et al. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos Int. doi: 10.1007/s00198-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 20.Ardawi MS, Sibiany AM, Bakhsh TM, et al. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 2012;23:675–86. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 21.Elsammak MY, Al-Wossaibi AA, Al-Howeish A, et al. High prevalence of vitamin D deficiency in the sunny Eastern region of Saudi Arabia: a hospital-based study. East Mediterr Health J. 2011;17:317–22. [PubMed] [Google Scholar]
- 22.Abokrysha NT. Vitamin D Deficiency in Women with Fibromyalgia in Saudi Arabia. Pain Med. 2012 Jan 5; doi: 10.1111/j.1526-4637.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 23.Matthana MH. The relation between vitamin D deficiency and fibromyalgia syndrome in women. Saudi Med J. 2011;32:925–9. [PubMed] [Google Scholar]
- 24.Hart PH. Vitamin D supplementation, moderate sun exposure, and control of immune Diseases. Discov Med. 2012;13:397–404. [PubMed] [Google Scholar]
- 25.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-Hydroxyvitamin D and Risk of Type 2 Diabetes: A Prospective Cohort Study and Meta-analysis. Clin Chem. 2012 doi: 10.1373/clinchem.2012.193003. 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]