ABSTRACT
Material and methods: We prospectively evaluated 199 individuals with high pre-test clinical suspicion of OSAS. Of these, 123 patients were morbidly obese (Group A) and 76 were non-obese (Group B). We performed six channel cardio-respiratory polygraphy and assessed the correlation between the Desaturation Index (DI) and the Apnea Hypopnea Index (AHI) for both groups.
Results: In group A: 116 patients (94.3%) were diagnosed with OSAS (AHI>5/hour); mean age: 59.4±10.9 years; mean BMI: 44.8±4.9 kg/m2. The mean DI was 47.2±27.6/hour and the mean AHI: 46.5±27.6/hour. Mean average SaO2 was 88.5±6.3 %. In group B, 65 patients (85.52%) were diagnosed with SAS; mean age: 51.2 ± 12.7 years; mean BMI: 27.24±2.2 kg/m2.The mean DI was 23.12 ± 18.35/hour and the mean AHI: 28.8 ± 18.5/hour. Mean average SaO2 was 93.7±2.07 %.
A significant positive correlation (correlation index rA = 0.863 and rB= 0.877) was found between DI and AHI in both groups (p<0.001).
Conclusion: Assessment of the Desaturation Index by nocturnal pulse-oximetry maintains its utility as a screening method for OSAS in both obese and non-obese patients with high clinical pre-test suspicion, despite the fact that the basal nocturnal saturation was found to be lower in group A.
Keywords: overnight pulse-oximetry, morbid obesity, desaturation index, sleep apnea
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a disorder characterized by intermittent narrowing (hypopnea) and/or total closure (apnea) of the upper airway during sleep. The intermittent lack of air flow during these events leads to episodic oxyhemoglobin desaturations (intermittent hypoxemia). Each hypopnea or apnea is resolved by an arousal from deep sleep, resulting in sympathetic activation, sleep fragmentation and daytime sleepiness (increased risk of car crashes) (1). Due to intermittent hypoxemia and sympathetic activation, patients with severe OSAS are exposed to long term cardiovascular consequences (resistant arterial hypertension, stroke, myocardial infarction, cardiac arrhythmias) (2-5).
The prevalence of OSAS in the general population is approximately 20% if OSAS is defined by an apnea hypopnea index (AHI) of more than five events per hour. (6) The prevalence decreases to 2-9% if OSAS is defined by an AHI of more than five events per hour and daytime sleepiness (7,8). It is therefore important to recognize, adequately diagnose and subsequently treat this medical condition.
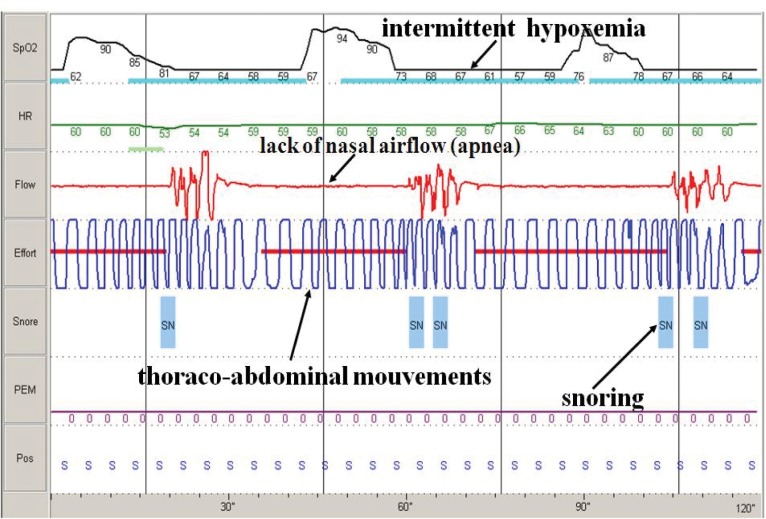
The diagnostic approach includes: patient history (snoring, witnessed apneas, daytime somnolence), clinical examination (obesity, large neck size, systemic hypertension), screening (overnight continuous pulse-oximetry) and diagnostic confirmation by cardio-respiratory polygraphy and/or polysomnography (determination of Apnea Hypopnea Index: the number of apneas and hypopneas per hour of recording or sleep time) (Figure1).
Figure 1. Poligraphy, 2 minutes aspect: obstructive apneas.
Nocturnal (overnight) pulse-oximetry is not recommended for the definitive diagnosis of OSAS, or to exclude the presence of OSAS, but it may be used as a screening method for patients with high clinical pre-test suspicion (9). The definitive diagnosis is established by polysomnography (the gold standard). Portable (ambulatory) cardio-respiratory polygraphs which record at least oxygen saturation, airflow, respiratory effort, heart or pulse rates, and body position may only be used in patients with high clinical pre-test suspicion, without pulmonary, cardiovascular, mental, neurological or neuromuscular disorders and if the procedures are supervised by trained and certified sleep medicine physicians (10).
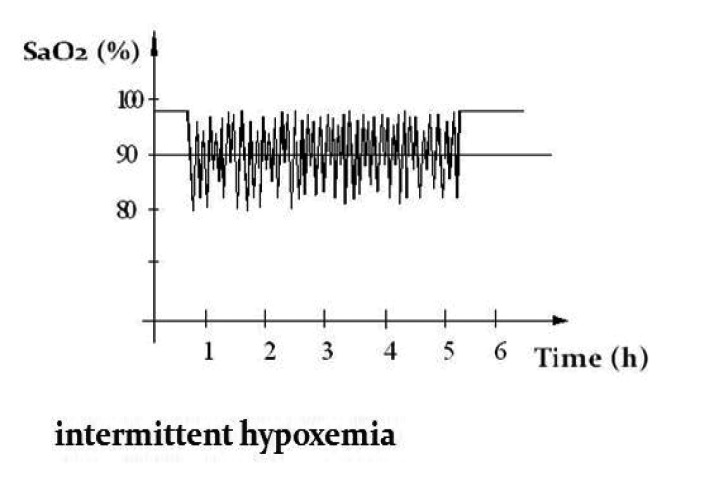
In OSAS the qualitative analysis of nocturnal pulse-oximetry curve has a distinct "sawtooth" pattern (intermittent hypoxemia) (Figure 2).
Figure 2. Intermittent hypoxemia pattern.
By calculating the Desaturation Index (the number of independent desaturations starting from the baseline per hour of recording) we could estimate the existence and severity of OSAS and refer the patient for diagnostic confirmation (10).
The Desaturation Index (DI) evaluated by overnight pulse-oximetry in patients with morbid obesity may not reflect the severity of OSAS as accurately as for patients with BMI <30 Kg/m2 (non obese), as the pattern of nocturnal pulse oximetry is different in patients with morbid obesity.
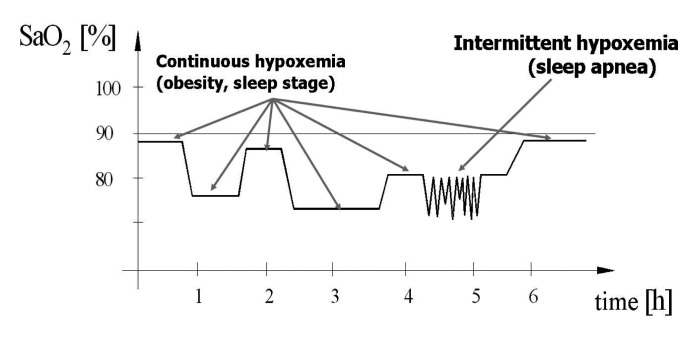
In morbidly obese (BMI >40 Kg/m2) patients with OSAS, the basal nocturnal saturation is decreased due to hypoventilation in supine position during sleep, and the aspect of the pulse-oximetry curve is often a combination between "continuous hypoxemia" and "intermittent hypoxemia" (Figure 3).
Figure 3. Continuous and intermittent hypoxemia pattern.
A similar pattern is observed in patients with overlap syndrome (OSAS and chronic obstructive pulmonary disease) (11).
The aim of this study is to evaluate the role of nocturnal pulse-oximetry as a screening method for subjects with BMI >40 kg/m2 when compared with those with BMI <30 kg/m2, by assessing the correlation between the Desaturation Index (DI) and the Apnea Hypopnea Index (AHI) for both groups. ❑
MATERIALS AND METHODS
We prospectively evaluated 123 obese (BMI>40 kg/m2) patients (Group A) and 76 non obese (BMI <30 kg/m2) patients (Group B) with a high pre-test clinical suspicion of OSAS (daytime sleepiness, snoring and witnessed apneas). A result of more than 10 out of 24 points on the Epworth Sleepiness scale was used to define significant daytime sleepiness. We performed an ambulatory nocturnal six channels cardio-respiratory polygraphy (overnight pulse-oximetry, nasal airflow, thoracoabdominal movements, body position and snoring).
The Desaturation Index (DI) was determined by automatic analysis of the recording after manually excluding the periods without pulse-oximetry signal. The cut-off (threshold) for independently significant desaturation (drop in oxyhemoglobin saturation) was set at 3%.
The Apnea Hypopnea Index (AHI) was established by visual analysis and manual validation of the recording (at least 4 hours of good quality sample). Apnea was defined as the absence of breathing (nasal/oral airflow) for at least 10 seconds associated with significant oxyhemoglobin desaturation (SaO2 drop) and hypopnea as a decrease in nasal/oral airflow signal by 50% for at least 10 seconds, associated or not with a SaO2 drop.
The diagnosis of OSAS was established if an AHI >5/hour and daytime sleepiness (Epworth Sleepiness scale >10/24) were found. The severity of OSAS was defined as mild (AHI: 5-14,9/hour), moderate (AHI: 15-30/hour) and severe (AHI >30/hour).
We assessed the correlation between the Desaturation Index (DI) and the Apneea Hypopnea Index (AHI) for both groups.
The results were expressed as mean ± standard deviation. Statistical analysis of the data was performed using SPSS version 17. A nonparametric Spearman rank correlation analysis was used. Results were considered statistically significant for p<0.05. The diagrams are Scatter/Dot plots.
Informed consent was obtained from the patients regarding the study procedures and the use of data for research purposes. ❑
RESULTS
In group A, 116 patients (94.3%) were diagnosed with OSAS (AHI >5/hour and significant daytime sleepiness). Out of these, 15% had mild, 16% had moderate and 69% had severe OSAS.
The mean age in group A was 59.4±10.9 years (range 28 to 79); mean BMI: 44.8±4.9 kg/m2 (range 40 to 67.5); 60% were men and 40% women.
44 patients were nonsmokers (38%) while 72 were active smokers (62%). 73 patients (63%) had dyslipidemia or were under hypolipemiant medication and 19 (16%) had diabetes mellitus (fasting glycemia >126 mg /dL or using antidiabetic medications). Systemic arterial hypertension (systolic pressure >140 mmHg and/or diastolic pressure >90 mmHg) was found in 68 patients (59%) and 12 of them used more than three antihypertensive drugs.
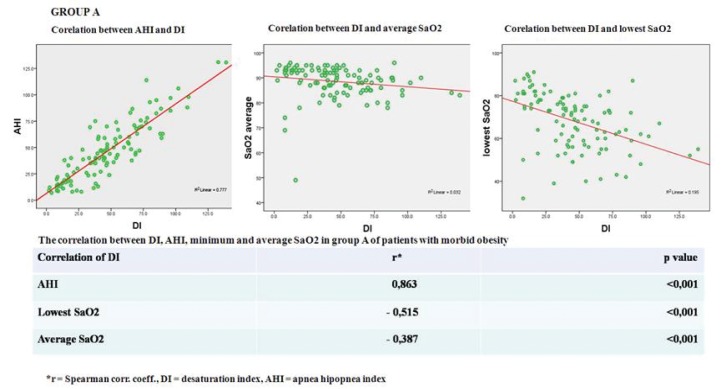
The mean Desaturation Index was: 47.2 ±27.6/hour (range 2 to 131) and the mean Apnea Hypopnea Index: 46,5±27,6/hour (range 7 to 131). Mean average SaO2 was 88.5±6.3% and mean lowest SaO2: 68.2±12.9% (Figure 4).
Figure 4. Statistical correlations Group A.
In group B, 65 patients (85.52 %) were diagnosed with OSAS (AHI >5/hour and daytime sleepiness). Out of them, 31% had mild, 29% moderate and 40% severe OSAS.
The mean age in group B was 51.2±12.7 years (range 28 to 77); mean BMI: 27.24±2.2 kg/m2 (range 21 to 30); 72% of the patients were men and 28% women.
In this group 30 patients were nonsmokers (46%) and 35 active smokers (54%). 18 patients (27%) had dyslipidemia or were using hypolipemiant medication and 8 (12%) had diabetes mellitus. Systemic arterial hypertension was found in 15 patients (23%) and 4 of them were taking more than three antihypertensive drugs.
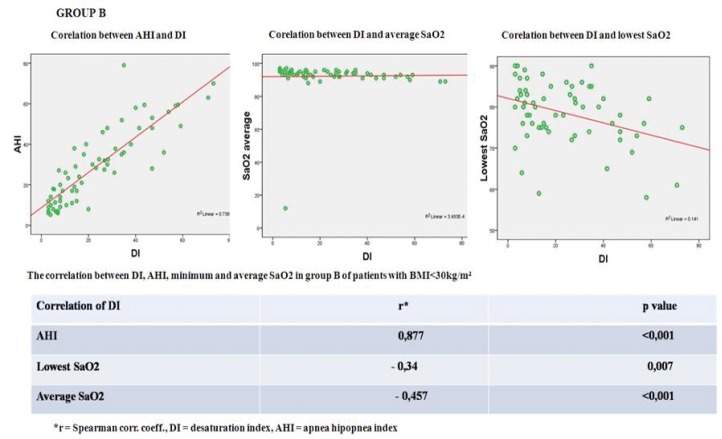
The mean Desaturation Index was 23.12 ±18.35/hour (range 3 to 73) and the mean Apnea Hypopnea Index: 28.8±18.5/hour (range 5.2 to 79). Mean average SaO2 was 93.7±2.07% and mean lowest SaO2: 78.5±7.4% (Figure 5).
Figure 5. Statistical correlations Group B.
A significant positive correlation (correlation index rA= 0.863 and rB= 0.877) was found between the Desaturation Index and Apnea Hypopnea Index in both groups (p<0.001).
Desaturation Index was inversely correlated with average SaO2 in both groups (correlation index rA= - 0.515 and rB= - 0.457; p<0.001). There was a strong negative correlation in group A (rA= - 0.515; p<0.001) and a moderate negative correlation in group B (rB= - 0.34; p=0.007) between Desaturation Index and lowest SaO2. ❑
DISCUSSION
Morbidly obese patients (BMI >40 Kg/m2) suffer from chronic hypoventilation of the lung parenchyma secondary to large abdominal volume, reduced diaphragmatic excursions and low chest wall compliance (12).
While supine during sleep, hypoventilation is more severe, leading to carbon dioxide retention with hypercapnia and secondary continuous hypoxemia, even if the lung parenchyma is normal (a medical condition known as Obesity Hypoventilation Syndrome) (13). Up to 90% of these patients associate obstructive sleep apnea syndrome, characterized by intermittent episodes of airway obstruction with secondary intermittent hypoxemia.
In this group of patients, the use of the overnight pulse-oximetry to estimate the Desaturation Index (in order to evaluate the coexistence of OSAS) could be influenced by the fact that the basal nocturnal saturation is decreased. This may lead to the underestimation of OSAS. There are no studies comparing the role of nocturnal (overnight) pulse-oximetry in recognition of obstructive sleep apnea syndrome in morbidly obese and non obese patients with high clinical pre-test probability.
In group A (123 morbidly obese patients) OSAS was diagnosed in 94.3% of cases, with a predominance of male patients (60%), as we expected. Studies have revealed that hormonal influences on the muscles of the upper airway and its ability to collapse, body fat distribution, and differences in pharyngeal anatomy could play an important role in the pathogenesis of OSAS (14). In group B (76 non obese patients) OSAS was diagnosed in 85.5% of cases, most of them men (72%). The high predominance of men (72%) diagnosed with OSAS in group B may be explained by the fact that women tend to seek medical assistance when symptoms or consequences of the disease are more severe. However, it is important to note that in our study both groups had a high clinical pretest probability of OSAS.
In group A, severe and moderate OSAS was diagnosed in 85% of cases, while in group B in 69% of cases. In non obese patients 31% were diagnosed with mild OSAS. This aspect could be explained by the fact that obesity is the major risk factor for the appearance of OSAS (14).
The major aim of this study was to compare the Desaturation Index evaluated by overnight pulse-oximetry with Apnea Hypopnea Index determined by ambulatory six channel cardio-respiratory poligraphy. Upon comparison of the values of these parameters in both groups, we found a significant positive correlation (correlation index rA= 0.863 and rB= 0.877) between the Desaturation Index and Apnea Hypopnea Index (p<0.001), despite the fact that basal nocturnal saturation is decreased in morbidly obese patients.
Analyzing the overnight pulse-oximetry curves we found that the basal nocturnal saturation (average SaO2) and the lowest nocturnal saturation are lower in group A as compared to group B, as we expected, due to hypoventilation in supine position during sleep (13). The Desaturation Index tends to vary in the opposite direction with the lowest nocturnal saturation, and the cut off of 3% for independent desaturation seems to be adequate in morbidly obese patients, as well. It is important to note that the chosen oxyhemoglobin desaturation threshold (3% or 4%), used to calculate the DI or the hypopnea index in different studies, can lead to under or overestimation of the disease severity (10,15). ❑
CONCLUSION
Desaturation Index assessed by nocturnal pulse-oximetry maintains its utility as a screening method in the recognition of obstructive sleep apnea in both morbidly obese and non obese patients with high clinical pre-test suspicion, despite the fact that basal nocturnal saturation is decreased in morbidly obese patients due to hypoventilation in supine position during sleep.
The Desaturation Index is useful in the diagnostic approach of obstructive sleep apnea in both obese and non-obese patients, and the cut off of 3% for independent desaturation seems to be adequate in morbid obese patients, as well.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 4.Baguet JP, Barone-Rochette G, Pépin JL. Hypertension and obstructive sleep apnoea syndrome: current perspectives. J Hum Hypertens. 2009;23:431–443. doi: 10.1038/jhh.2008.147. [DOI] [PubMed] [Google Scholar]
- 5.Ross SD, Sheinhait IA, Harrison KJ, et al. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23:519–532. [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, et al. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–246. [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–263. [PMC free article] [PubMed] [Google Scholar]
- 8.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33:907–907. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez D, Hornero R, Garcia M, et al. Improving diagnostic ability of blood oxygen saturation from overnight pulse-oximetry in obstructive sleep apnea detection by means of central tendency measure. Artif Intell Med. 2007;41:13–24. doi: 10.1016/j.artmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Penzel T, Blau A, Schobel C et al. Ambulatory diagnosis of OSA and new technologies. In McNicholas W, Bonsignore M. European Respiratory Society Monograph. 2010;50 (SleepApnoea):136–149. [Google Scholar]
- 11.Dumitrache-Rujinski S, Croitoru A, Bogdan M. Therapeutical approach in severe exacerbation of COPD associating obstructive sleep apnea and obesity. Pneumologia. 2012;61(2):117–120. [PubMed] [Google Scholar]
- 12.Piper A, Grunstein R. Obesity Hypoventilation Syndrome Mechanisms and Management. Am. J. Respir. Crit. Care Med. 2011 Feb 1;183(3):292–298. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 13.Ambrogio C, Lowman X, Kuo M, et al. Sleep and non-invasive ventilation in patients with chronic respiratory insufficiency. Intensive Care Med. 2009 Feb;35(2):306–13. doi: 10.1007/s00134-008-1276-4. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg E. Epidemiology of OSA. In McNicholas W, Bonsignore M. European Respiratory Society Monograph. 2010;50 (Sleep Apnoea):51–68. [Google Scholar]
- 15.Shigemi Tanaka, Masayuki Shima. Assessment of screening tests for sleep apnea syndrome in the workplace. J. Occup. Health. 2010;52:99–105. doi: 10.1539/joh.l8175. [DOI] [PubMed] [Google Scholar]