Abstract
Low-income, racial/ethnic minorities are often nonadherent to diabetes medications, have uncontrolled glycemia, and have high rates of diabetes-related morbidity. Cell phones provide a viable modality to support medication adherence, but few cell phone-based interventions have been designed for low-income persons, a population with more feature phone penetration than smartphone penetration. In an effort to reach the broadest range of patients, we leveraged the voice and text messaging capabilities shared by all cell phones to design the MEssaging for Diabetes intervention. We specifically advanced and adapted an existing tailored text messaging system to include interactive voice response functionality and support the medication adherence barriers of low-income, diverse adults with type 2 diabetes mellitus. We report on the design process and feasibility testing results (i.e., technical use patterns and subjective user experiences) from patients from the target population who used the intervention in one of three user-centered design iterations. The types of challenges encountered in design were related to providing text message content with valued information and support that engages patients. The design process also highlighted the value of obtaining mixed methods data to provide insight into legitimate versus illegitimate missing data, patterns of use, and subjective user experiences. The iterative testing process and results outlined here provide a potential template for other teams seeking to design technology-based self-care support solutions for comparable patient populations.
Keywords: cell phone, design, diabetes, interactive voice response, medication adherence, mobile, socioeconomic status, text message
Introduction
Type 2 diabetes mellitus (T2DM) affects over 25 million people living in the United States, with annual health care costs exceeding $174 billion.1 Among persons with T2DM, suboptimal adherence to diabetes medications is common2–4 and is associated with poor glycemic control2,5–7 and an increased risk of hospitalization8–10 and mortality.9,10 Low-income, racial/ethnic minorities have high rates of T2DM1 and are more likely than higher-income, non-Hispanic Whites to be suboptimally adherent to diabetes medications2,5,6,11 and experience worse glycemic control2,5–7,12,13 and more diabetes-related mortality.14
Interventions to promote medication adherence should address underlying reasons for nonadherence15 and have traditionally involved one-on-one, resource intensive counseling procedures that have limited sustainability in routine clinical care.16 Mobile devices such as cell phones can address reasons for nonadherence17,18 and offer an alternative to face-to-face intervention delivery and support. Cell phones have been used to educate, motivate, prompt, and assess various diabetes self-care activities,17,19,20 including medication adherence,21,22 and, in doing so, have improved glycemic control.19,23,24
Approximately 85% of U.S. adults are cell phone users,25 and cell phone ownership does not differ by income or race/ethnicity.25,26 However, persons with low incomes are more likely to own feature phones25 [i.e., a basic cell phone that can be used for voice communications and short messaging service (SMS) text messaging] than smartphones (i.e., cell phones that include feature phone functionality coupled with Internet access for Web browsing, instant messaging, and email and that require an operating system). While the availability and adoption of smartphones is growing significantly, as is the availability and downloading of health applications,27 application access is limited by both the affordability of smartphones and a phone’s operating system, whereas feature phone functionality (e.g., SMS and voice communications) is not phone type or operating system dependent and provides access to the broadest range of users (i.e., persons of all ages and socioeconomic strata use both voice and SMS text messaging communications in their everyday life25,26,28).
While cell phone technology has been harnessed to disseminate intervention content to promote a wide range of health behaviors to different patient populations,29,30 very little information is available about the design decisions scaffolding the development of cell-phone-based interventions for low-income, racial/ethnic minorities. For instance, we were unable to identify documentation on the rationale, decisions, and thought processes coupled with iterative patient input during the initial stages of technical, content, and protocol development of cell-phone-based interventions with this patient population. Moreover, because innovations have the potential to widen health disparities, it is even more important that we understand the design feasibility of these innovative interventions in underserved racial/ethnic groups.31
To address this noted gap in the literature, we discuss the processes associated with advancing and adapting a tailored text messaging system for this population. In an effort to reach the broadest range of patients, we leveraged the voice and text messaging capabilities shared by all cell phones to design the MEssaging for Diabetes (MED) intervention. Here we report on the design process and feasibility testing results from patients from the target population who used the intervention in one of three usability and experiential feedback iterations.
Methods
System Development
Our development decisions were informed by (1) national usage data and prior research with the target population,25,26 (2) evidence on the importance of tailored communication and feedback,23 and (3) the complimentary nature of the three communication channels we leveraged. The first communication was a daily, automated, one-way, tailored text message that addressed a user’s barriers to medication adherence. No response was requested for this message. The content for these messages were tailored to each individual based on his/her barriers to medication adherence reported in a survey prior to using the system. The second communication was a daily two-way text message that assessed medication adherence performance for the day. In this message, we simply asked the user if he/she had taken all of his/her diabetes medication for the day and requested a “yes” or “no” response. These responses were subsequently used as personalized adherence feedback within a weekly interactive voice response (IVR) phone call. The third communication was a weekly IVR call that gave the user weekly adherence feedback based on aggregated responses to the aforementioned daily two-way adherence text message question, provided an automated reinforcing/encouraging message based on changes in adherence from prior weeks, and requested qualitative information about adherence successes and failures to promote problem solving and future adherence. The IVR technology uniquely allowed this type of adaptive communication and qualitative data collection.
The core of the text messaging system used here is called SuperEgo, which provided text messages tailored (timing and content) to a user’s behavioral barriers to adherence and had been previously designed for and tested with adolescents with type 1 diabetes mellitus.17 In order to integrate voice—which is readily available, provides more personal and adaptive communication, and allows qualitative data gathering—we utilized Twilio©. Twilio also allows for integrated administration of text messaging and voice communication data.
In addition to leveraging the mobile communication modalities accessible to the target population, we integrated behavioral feedback and person-to-person communication known to be valuable in engaging patients within low-touch mobile interventions.23 To achieve this, we included a research assistant (RA)-administered motivational interview protocol with goal setting32 and planned for regular person-to-person calls with the RA to reassess and update barriers to medication adherence and provide problem solving support, recognizing that problem solving and goal setting are two of the most studied behavior change processes in diabetes.33
Figure 1 shows the procedures and intervention components utilized in each usability and experiential feedback iteration. Although created to be a weekly experience, the initial IVR call had to be scheduled for the end of the second week in order to provide the planned adherence feedback comparing the current week with the previous week. Moreover, because participants involved in the usability/feedback testing were exposed to the system for 2 weeks, they received one IVR call during that time frame.
Figure 1.
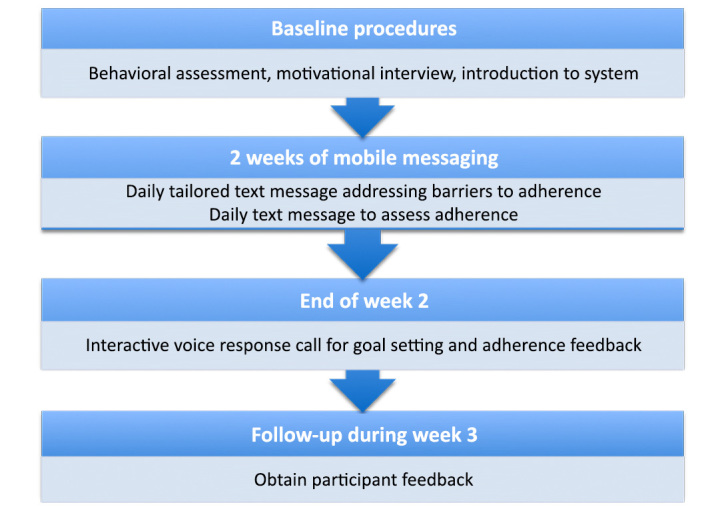
Flow diagram of participant procedures and intervention components.
Content Development
We used the research literature15,34,35 to generate a comprehensive list of 34 barriers to medication adherence. Two behavioral psychologists and three clinicians, including a diabetes educator and a practitioner who provides clinical care for the target population, iteratively reviewed and reduced this list, prioritizing barriers most relevant to the target population and those that could be addressed with text messaging content. The list was iteratively reduced to 24, 22, and, finally, 17 barriers (see Table 1). These same experts created a minimum of 10 text messages (range 10–13) to address each barrier, with the ultimate goal of providing a user with a unique daily text message to address his/her top three barriers over the course of a 1-month time frame (see Table 1 for examples of these text messages).
Table 1.
Barriers to Medication Adherence with a Corresponding Tailored Text Message to Address Each Barrier
| Barriers to medication adherence | Examples of a tailored text message to address each barrier |
|---|---|
| 1. Barriers to accessing medication(s) | If you have a hard time getting to the pharmacy, ask your doctor to prescribe a 90-day supply of your medications. |
| 2. Believing medication(s) are not important | It’s important that you take your diabetes medications as prescribed. Doing so might keep you healthy longer. |
| 3. Believing medication(s) are harmful | If you think your diabetes medications are harmful, ask your doctor if you can reduce the dosage amount and still get the same benefits. |
| 4. Complexity of regimen | Taking more than one medication can be hard. Get yourself on a schedule, so that taking your medications becomes just another habit. |
| 5. Cost of medication(s) | If you are having trouble paying for your diabetes medications, ask your doctor if there are more affordable medications you could take. |
| 6. Decision to omit doses | If you feel that you don’t need your diabetes medications, remember that they are preventing more serious health problems down the road. |
| 7. Experiencing side effects | If you’re experiencing a side effect from your diabetes medications, talk with your doctor about how you can manage it. |
| 8. Fear of dependence | If you’re afraid of being dependent on your diabetes medications, please talk with your doctor about your concerns. |
| 9. Fear of side effects | If you have a side effect from your diabetes medications, ask your doctor if you can take a different medication that doesn’t have this side effect. |
| 10. Feeling stigmatized | If you are uneasy with taking diabetes medications in front of others, do it anyway and set an example of how important it is to take care of yourself. |
| 11. Forgetfulness | If you have a hard time remembering to take your diabetes medications, try to take your diabetes medications at the same time every day. |
| 12. Lack of belief in the benefits of medication(s) | If you don’t think your diabetes medications are necessary, think about the fact that this medication controls your diabetes. |
| 13. Lack of information about medication(s) | Knowledge is power. Diabetes medications work best when used with meal planning and exercise. |
| 14. Lack of motivation to take medication(s) | If you don’t feel motivated to take your diabetes medications, think about the people who want you to be healthy. |
| 15. Lack of social support | Everyone needs someone to talk to about their diabetes. Do you know someone who has diabetes? |
| 16. Lack of diabetes symptoms | It’s important that you take your diabetes medications even when you feel good. Doing so will help keep your blood sugar under control. |
| 17. Tired of taking medication(s) or burn out | If you’re tired of taking diabetes medications, talk to someone you know and trust who also has diabetes. |
Assessing Barriers to Medication Adherence
We assessed barriers to medication adherence using items derived from the Diabetes Medication Knowledge Questionnaire (all 5 items used),36 the Medicines for Diabetes Questionnaire (10 out 14 items used),37 the Barriers to Diabetes Adherence measure (4 out of 21 items used),18 and the Medication Adherence Self-Efficacy Scale (19 out of 26 items used).38 Responses to these items were standardized to create scoring consistency across instruments or sets of items used to assess each barrier and, in turn, prioritize a user’s top three barriers according to his/her scale scores. A tagging system associated tailored text messages with each barrier, allowing a single text message without replacement to be sent daily to address one of the user’s three barriers.
Administering Tailored Text Messages
Within SuperEgo, the tailored text messaging component,17 a self-report assessment provided an automated selection of text messages based on a user’s top three barriers scale scores. As the majority of patients from the target population do not own computers, let alone computers with Internet access,26 we did not utilize the SuperEgo patient-facing Web site designed to allow Web-based user assessment and user control of text messaging. Instead, an RA administered the barriers assessment in a face-to-face interview and then input the user’s responses into a computer.
Sample
The MED intervention was designed for low-income, diverse patients receiving care at a federally qualified health center (i.e., a facility that provides primary care services to underserved, underinsured, and uninsured Americans, including migrant workers and non-U.S. citizens) in Nashville, Tennessee. Over two-thirds of the patients receiving care at this clinic are uninsured or are receiving public insurance, and the majority of patients are at or below the federal poverty level. Study personnel worked with clinic staff to recruit 20 eligible patients for one of three usability and experiential feedback iterations. Eligible patients were English-speaking adults diagnosed with T2DM who were currently taking diabetes medications, owned a cell phone with an active plan and text messaging capabilities, and had a glycated hemoglobin A1c (A1C) value in the medical record.
Procedures
The goal was to complete as many iterative usability and feedback cycles as was necessary to obtain a successful test of the MED intervention in terms of data integrity, technical stability, and user satisfaction. We ultimately performed three rounds of iterative testing.39 Each round of testing included baseline procedures in which a trained RA performed informed consent to eligible and interested patients, administered the barriers to medication adherence assessment, and performed a brief in-person motivational interview to discuss patients’ medication adherence challenges and to help them formulate a realistic, adherence-related goal.32 The RA inputted patients’ responses to the barriers to medication adherence assessment items into the SuperEgo Web-based form and had patients interact with the MED intervention for a 2-week time frame. During this time, patients received a unique daily one-way text message that addressed one of their top three barriers to medication adherence (i.e., identified by the barriers to medication adherence assessment), a daily two-way text message that asked for adherence performance data, and an IVR call after 2 weeks that provided adherence performance feedback and a reinforcement/encouragement message and asked a series of questions to facilitate problem solving strategies and future adherence success. Finally, the RA performed a follow-up phone interview to obtain patients’ feedback on their experience during the testing period. Questions asked pertained to opinions about the MED intervention’s functionality, content, and technical problems encountered during that time. During each round of testing, study investigators and research staff met weekly to identify and resolve any technical, content, and implementation issues prior to initiating another round of testing.
Results
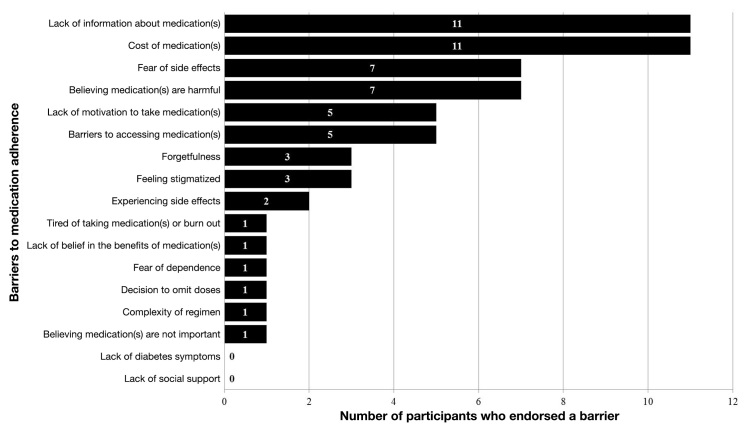
The average age of the 20 participants was 51.6 ± 8.8 years, 65% were female, 45% were African American, average years of education was 13.6 ± 2.4 years, 25% had an annual household income <*10,000, 35% did not have health insurance, and the average A1C was 7.6% ± 1.8%. Participant characteristics for the overall sample and for the participants in each testing round are presented in Table 2. Figure 2 presents each participant’s (N = 20) top 3 barriers to medication adherence out of the 17 barriers that were assessed.
Table 2.
Participant Characteristicsa
| Iterative Testing Round | ||||
|---|---|---|---|---|
| Total N = 20 | 1 n = 5 | 2 n = 9 | 3 n = 6 | |
| Age, years | 51.6 ± 8.8 | 53.0 ± 4.0 | 50.9 ± 12.9 | 51.6 ± 8.8 |
| Gender | ||||
| Male | 7 (35.0) | 1 (20.0) | 3 (33.3) | 3 (50.0) |
| Female | 13 (65.0) | 4 (80.0) | 6 (66.7) | 3 (50.0) |
| Race/ethnicity | ||||
| Caucasian/white | 10 (50.0) | 2 (40.0) | 4 (44.4) | 4 (66.7) |
| African American/black | 9 (45.0) | 3 (60.0) | 5 (55.6) | 1 (16.7) |
| Hispanic/Latino | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) |
| Education, years | 13.6 ± 2.4 | 13.4 ± 1.9 | 13.0 ± 2.6 | 14.8 ± 2.4 |
| Annual household income | ||||
| <*10,000 | 5 (25.0) | 0 (0.0) | 4 (44.4) | 1 (16.7) |
| *10,000–20,000 | 7 (35.0) | 1 (20.0) | 3 (33.3) | 3 (50.0) |
| *20,000–35,000 | 6 (30.0) | 3 (60.0) | 1 (11.1) | 2 (33.4) |
| >*35,000 | 2 (10.0) | 1 (20.0) | 1 (11.1) | 0 (0.0) |
| Insurance status | ||||
| Private | 2 (10.0) | 1 (20.0) | 1 (11.1) | 0 (0.0) |
| Public | 11 (55.0) | 2 (40.0) | 5 (55.5) | 4 (66.7) |
| None | 7 (35.0) | 2 (40.0) | 3 (33.3) | 2 (33.3) |
| Diagnosed diabetes duration, years | 7.7 ± 4.8 | 9.0 ± 4.6 | 8.7 ± 5.2 | 5.1 ± 4.2 |
| Number of medications | ||||
| All | 8.3 ± 4.7 | 11.6 ± 5.8 | 7.2 ± 3.7 | 7.3 ± 4.7 |
| Diabetes | 1.9 ± 0.8 | 2.0 ± 0.7 | 1.9 ± 0.9 | 2.0 ± 0.9 |
| Type of diabetes medications | ||||
| Oral agents only | 11 (55.0) | 2 (40.0) | 7 (77.8) | 2 (33.3) |
| Oral agents and insulin | 9 (45.0) | 3 (60.0) | 2 (22.2) | 4 (66.7) |
| Glycemic control, A1C | 7.6 ± 1.8 | 7.4 ± 1.2 | 7.0 ± 1.7 | 8.7 ± 1.9 |
Mean ± standard deviation or n (%).
Figure 2.
Participants’ top three barriers to medication adherence (N = 20).
Iterative Testing Round 1
Tailored Text Messages
All five participants in round 1 completed the follow-up interview. Most participants (four out of five) said receiving a daily text message that addressed their barriers to medication adherence were helpful and motivated them to take their medications. One reported, “These messages really helped me, and they also helped my family understand how important taking care of diabetes really is.” One participant did not think these messages were helpful, stating, “I always take my medications, so these messages didn’t really help me.” Mapping onto these data were endorsements from four out of five participants for how often these messages were sent. One participant did not endorse this frequency because she said the tailored text messages and adherence assessment text messages came at the same time, and it was difficult to tease them apart. Based on this feedback, we added questions to later versions of the follow-up survey that distinguished between experiences receiving tailored text messages versus adherence assessment text messages, and we also changed the baseline protocol to (a) better prepare the participant for the two different types of daily text messages they would be receiving and why and (b) better specify and inquire on an ideal timing to receive these messages.
Adherence Assessment Text Messages
All five participants reported receiving daily adherence assessment text messages and that these messages were delivered at the time of day they had requested. One participant did not receive a message for 5 out of 14 days, and one participant who received a message every day did not respond to any of these messages. Of the participants who did respond to messages (n = 4), the average days of responding was 10.25 ± 2.36 (range 7–12). Based on participant feedback that the two types of text messages were not easily distinguished, we changed the adherence assessment message to, “On [day of week, date], did you take ALL of your diabetes meds? Reply with ‘Yes’ or ‘No’,” in subsequent rounds. We also changed the baseline protocol and the follow-up survey to ask if participants understood how to respond to daily assessment messages, to ask about their duration of willingness to respond to these messages, and to ask if these messages also helped the participant remember to take their diabetes medications.
Interactive Voice Response Call
At the end of 2 weeks, all five participants were called, but only four out of the five answered the call, suggesting the participant who did not answer the call did not know she had been called. The participants who answered the call said the adherence feedback during the call was easy to understand and useful. Participants were generally able to identify reasons for one successful adherence day (e.g., “I’m pretty good when I take my medication with me, and I did that every day last week.”) and one unsuccessful day (e.g., “I did have a couple of days where I was out of my medications and I wasn’t able to get it refilled until my payday.”). In the follow-up interviews, when asked, “On a scale from 1–10, with 1 being the least helpful and 10 being the most helpful, how helpful was it to get a call with feedback on how you were doing?,” the average rating was high for these participants, 8.75 ± 1.83.
Iterative Testing Round 2
Tailored Text Messages
All nine participants in round 2 completed the follow-up interview. All participants (nine out of nine) in round 2 said they received a daily text message with a helpful tip or suggestion to help them take their diabetes medications, eight out of nine participants said they read these messages every day over the course of 2 weeks, and no one thought the messages were difficult to understand. When asked how these messages helped them address their barriers to medication adherence, participants thought the messages provided viable options: “They motivated me to take my medications,” or “They made me think about how my diabetes could affect me in the long run.” The average “helpfulness” rating was highly favorable, 8.33 ± 2.12 out of 10. Finally, two participants said the adherence assessment text messages were more helpful than the tailored text messages.
Adherence Assessment Text Messages
According to system data and participant self-report, all nine participants reported receiving a daily text message every day for 14 days. All participants responded to at least 11 out of 14 text messages (mean 13.55 ± 1.01). The majority of the participants (six out of nine) understood how to respond to these messages. Of the three participants who did not understand how to respond, two participants reported a system problem, in which they received text messages asking them to reply with a “FalseYes” or “FalseNo,” and one participant said she sometimes had not taken her medications by the time she had received this message, so she did not know how to respond in those instances. When asked, “How long you would be willing to respond to daily text messages asking if you’ve taken your diabetes medications,” the majority (six out of nine) said “indefinitely” or “however long I would need to.” Consistent with this, when directly asked, eight out of nine participants said they could respond to this type of message for up to 3 months. Finally, the majority of participants (seven out of nine) said these messages helped them remember to take their diabetes medications.
Interactive Voice Response Call
All participants were called, and the majority (six out of nine) answered the call. Of the three participants who did not answer the call, one participant did not remember receiving a call, one participant did not have his phone with him when the call came through, and one participant did not recognize the phone number and therefore did not answer it. Although participants could call the system back, these three participants did not pursue that option. Two of these participants said they might have picked up the phone if we had “sent a reminder text message that the call was coming up” or had called again. Of the participants who participated in the IVR call, all (six out of six) said they understood the adherence feedback they received and half (three out of six) said they still remembered this information. The average helpfulness rating was 6.50 ± 3.27 out of 10. Finally, when asked how often they would be willing to receive these automated calls, five participants said once per week and three participants said once every 2 weeks.
Iterative Testing Round 3
Tailored Text Messages
Four out of six participants in round 3 completed the follow-up interview. All four participants said they received a daily text message with a helpful tip or suggestion to help them take their diabetes medications. All participants read each of these messages and did not find them confusing or difficult to understand. When asked how these messages helped them address their barriers to medication adherence, participants said, “They addressed my issues very well and made me stop and think about alternative solutions,” or “All the messages were helpful.” Only one participant said, “They didn’t really seem to pertain to me because I take my medications regardless.” When asked, the average helpfulness rating was high, 8.25 ± 2.17 out of 10, which was also comparable to the rating in round 2. One participant did not find these messages helpful or relevant because she felt she knew most of the information in the messages.
Adherence Assessment Text Messages
Assessment text messages were successfully sent every day except for the day when there was an unexpected server reboot. Of the 13 days participants received adherence assessment text messages, participants responded to an average of 9.83 ± 2.40 messages. All participants who responded to the follow-up interview understood how to respond to these messages. None of them reported a system problem or problems with their phones that would have prevented receiving and responding to these messages. When asked about the frequency of the assessment messages, the majority of these participants (three out of four) thought every day was appropriate, and they all thought they could respond to these messages for 3 months. Finally, all follow-up participants said these messages helped them remember to take their diabetes medications, saying, “I anticipated the message every night, which prompted me to take my medication,” or “When I was busy and didn’t take my medications, I would get this message and then remember to take it.”
Interactive Voice Response Call
All calls were successfully sent, and three out of the four follow-up participants reported answering this call. One participant said he never received or answered a call and there was nothing we could have done differently that would have led him to answer a call if it had come through. Of the follow-up participants who answered the call, all (three out of three) said the adherence feedback they received was clear and that they still remembered this information. The average helpfulness of this call was higher than round 2, 8.33 ± 1.15 out of 10. When asked how often they would be willing to receive automated adherence feedback calls, the four participants who responded to the follow-up interview said once per week.
Participants in all three iterative testing rounds said they would recommend the intervention to people with diabetes. Two notable comments were, “You need to make a commercial and go worldwide with this system. This is a great thing to really help people. You are on to something here,” and “The system is very easy to use and understand.”
Discussion
Our goal was to design an engaging medication adherence promotion intervention for low-income patients with T2DM that can be delivered using readily available cell phone technology. Building on the SuperEgo tailored text messaging system, we performed three rounds of user-centered testing39 to identify barriers to implementation and obtain patient input on the content and experience of the mobile and in-person intervention. We have named this combination of motivational interviewing, tailored text messaging, voice response (IVR), and goal setting as MEssaging for Diabetes.
Patients provided input on the intervention’s technical features, on the tailored text messaging content that addressed specific barriers to medication adherence, and on adherence performance feedback and motivational messaging that were delivered during an IVR call. An iterative usability and feedback testing process brought to light issues about how participants understood the system to work and the purpose of each of the components, which allowed us to create a more appropriate set of instructions on how to use the system better and provide appropriate responses. The iterative testing rounds also identified acceptable levels of communication frequency and why as well as how messages impacted adherence. In all three rounds, the majority of participants provided positive feedback about the intervention’s features and content. Most participants received and read the daily one-way tailored text messages, were responsive to the daily two-way text messages assessing adherence, answered the IVR call, understood and remembered the feedback that was given during the call, and found the interpretation of that feedback to be useful. Participants in each round of testing also rated each intervention component as being very helpful in getting them to take their diabetes medications and would even recommend the intervention to others with diabetes. Finally, system problems that were identified in any given round were resolved by the subsequent round, and few participants reported instances when their phone or their ability to use their phone interfered with fully engaging with the intervention.
Although planned as a design and technical pilot study, the sample size was small. This precluded examining differences by patient factors such as race, income, duration of diabetes diagnosis, or controlled versus uncontrolled glycemia. Moreover, the sampling of participants could have taken a more purposive or stratified approach. In that way, we could have been more confident that, for example, the system would be appropriate for patients in good diabetes control and those in poor control or for a wider age range. Additionally, although we contemplated obtaining participant input on the content before testing the system, we hypothesized that it would be difficult for participants to judge the utility of the content out of context of their daily lives. Therefore, we decided to obtain participants’ feedback on the content after 2 weeks of actually experiencing the system. Finally, the labor-intensive nature of the iterative identification and review of technical problems led us to believe that we could have been more time efficient by adding a function to the administration portion of the intervention that monitored system failures or missing data.
Conclusions
Iterative usability and feedback testing was invaluable in identifying misconceptions we had about the intervention and providing insights about the methods and content that research and expertise led us to use. Although the intervention context was diabetes medication adherence promotion among low-income adults with T2DM, the development strategy and usability/feasibility testing process is generalizable to other cell phone-based behavior change interventions with other patient populations.
Acknowledgments:
The authors thank Cecilia Quintero, the Vine Hill Community Clinic, and the participants for their contributions to this work.
Glossary
- (A1C)
glycated hemoglobin A1c
- (IVR)
interactive voice response
- (MED)
MEssaging for Diabetes
- (RA)
research assistant
- (SMS)
short messaging service
- (T2DM)
type 2 diabetes mellitus
Funding:
This research was funded with support from the McKesson Foundation. Dr. Osborn is also supported by a career development award (NIDDK K01 DK087894).
References:
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed December 10, 2012. [Google Scholar]
- 2.Kim N, Agostini JV, Justice AC. Refill adherence to oral hypoglycemic agents and glycemic control in veterans. Ann Pharmacother. 2010;44(5):800–808. doi: 10.1345/aph.1M570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odegard PS, Gray SL. Barriers to medication adherence in poorly controlled diabetes mellitus. Diabetes Educ. 2008;34(4):692–697. doi: 10.1177/0145721708320558. [DOI] [PubMed] [Google Scholar]
- 4.Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, Selby JV. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egede LE, Gebregziabher M, Hunt KJ, Axon RN, Echols C, Gilbert GE, Mauldin PD. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34(4):938–943. doi: 10.2337/dc10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middleaged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853–1860. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 7.Ngo-Metzger Q, Sorkin DH, Billimek J, Greenfield S, Kaplan SH. The effects of financial pressures on adherence and glucose control among racial/ethnically diverse patients with diabetes. J Gen Intern Med. 2012;27(4):432–437. doi: 10.1007/s11606-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27(9):2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 9.Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care. 2011;49(4):378–384. doi: 10.1097/MLR.0b013e31820292d1. [DOI] [PubMed] [Google Scholar]
- 10.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 11.Osborn CY, Cavanaugh K, Wallston KA, Kripalani S, Elasy TA, Rothman RL, White RO. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(Suppl 3):268–278. doi: 10.1080/10810730.2011.604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ES, Brown SE, Thakur N, Carlisle L, Foley E, Ewigman B, Meltzer DO. Racial/ethnic differences in concerns about current and future medications among patients with type 2 diabetes. Diabetes Care. 2009;32(2):311–316. doi: 10.2337/dc08-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piette JD, Heisler M, Harand A, Juip M. Beliefs about prescription medications among patients with diabetes: variation across racial groups and influences on cost-related medication underuse. J Health Care Poor Underserved. 2010;21(1):349–361. doi: 10.1353/hpu.0.0247. [DOI] [PubMed] [Google Scholar]
- 14.Miech RA, Kim J, McConnell C, Hamman RF. A growing disparity in diabetes-related mortality U.S. trends, 1989–2005. Am J Prev Med. 2009;36(2):126–132. doi: 10.1016/j.amepre.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22(4):313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 16.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. 2012;18(2):115–118. doi: 10.1258/jtt.2011.111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvaney SA, Hood KK, Schlundt DG, Osborn CY, Johnson KB, Rothman RL, Wallston KA. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract. 2011;94(1):77–83. doi: 10.1016/j.diabres.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang X, Wang Q, Yang X, Cao J, Chen J, Mo X, Huang J, Wang L, Gu D. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28(4):455–463. doi: 10.1111/j.1464-5491.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 20.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 21.Mulvaney SA, Rothman RL, Dietrich MS, Wallston KA, Grove E, Elasy TA, Johnson KB. Using mobile phones to measure adolescent diabetes adherence. Health Psychol. 2012;31(1):43–50. doi: 10.1037/a0025543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266–271. doi: 10.1097/00001786-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Mulvaney SA, Ritterband LM, Bosslet L. Mobile intervention design in diabetes: review and recommendations. Curr Diab Rep. 2011;11(6):486–493. doi: 10.1007/s11892-011-0230-y. [DOI] [PubMed] [Google Scholar]
- 24.Chomutare T, Fernandez-Luque L, Årsand E, Hartvigsen G. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res. 2011;13(3):e65. doi: 10.2196/jmir.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox S, Duggan M. Mobile health 0.2012. Washington DC: Pew Research Center; 2012. [Google Scholar]
- 26.Osborn CY, Schlundt DG, Kripalani S, Quintero CC, Elasy TA. Disparities in using technology to access health information: race versus health literacy. Poster presentation at the NIH Summit on the Science of Eliminating Health Disparities Meeting. National Harbor, MD: 2012. October-November. [Google Scholar]
- 27.Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67. doi: 10.1186/1472-6947-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenhart A, Purcell K, Smith A, Zickuhr K. Social media and mobile internet use among teens and young adults. Washington DC: Pew Internet; 2010. [Google Scholar]
- 29.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J, Hollin I. Kachnowski S A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J Telemed Telecare. 2011;17(1):41–48. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 31.Frohlich KL, Potvin L. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health. 2008;98(2):216–221. doi: 10.2105/AJPH.2007.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogedegbe G, Schoenthaler A, Richardson T, Lewis L, Belue R, Espinosa E, Spencer J, Allegrante JP, Charlson ME. An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: rationale and design. Contemp Clin Trials. 2007;28(2):169–181. doi: 10.1016/j.cct.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Fisher EB, Thorpe CT, DeVellis BM, DeVellis RF. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33(6):1080–1106. doi: 10.1177/0145721707309808. [DOI] [PubMed] [Google Scholar]
- 34.Duru OK, Gerzoff RB, Selby JV, Brown AF, Ackermann RT, Karter AJ, Ross S, Steers N, Herman WH, Waitzfelder B, Mangione CM. Identifying risk factors for racial disparities in diabetes outcomes: the translating research into action for diabetes study. Med Care. 2009;47(6):700–706. doi: 10.1097/mlr.0b013e318192609d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 36.McPherson ML, Smith SW, Powers A, Zuckerman IH. Association between diabetes patients’ knowledge about medications and their blood glucose control. Res Social Adm Pharm. 2008;4(1):37–45. doi: 10.1016/j.sapharm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Farmer A, Kinmonth AL, Sutton S. Measuring beliefs about taking hypoglycaemic medication among people with type 2 diabetes. Diabet Med. 2006;23(3):265–270. doi: 10.1111/j.1464-5491.2005.01778.x. [DOI] [PubMed] [Google Scholar]
- 38.Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME. Development and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. J Clin Epidemiol. 2003;56(6):520–529. doi: 10.1016/s0895-4356(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 39.Atkinson NL, Saperstein SL, Desmond SM, Gold RS, Billing AS, Tian J. Rural eHealth nutrition education for limited-income families: an iterative and user-centered design approach. J Med Internet Res. 2009;11(2):e21. doi: 10.2196/jmir.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]