Abstract
Background
Hypertension frequently accompanies diabetes mellitus, worsening prognosis and complicating medical care for patients. Low medication adherence with multiple medications is a major factor in the inadequate achievement of blood pressure treatment goals. Widespread access to mobile phones offers a new opportunity to communicate with patients and enhance disease self-management.
Methods
We recruited 50 high-risk urban patients with hypertension, who are using at least two prescription medications for hypertension, into an open-label trial using medication reminder software on a mobile phone. Medication adherence was assessed by review of pharmacy refill rates before, during, and after availability of the medication reminder software (pre-activation, activation, and post-activation phase, respectively).
Results
Forty-eight patients completed the study. All subjects were insured by Medicaid, 96% were African-American, and the majority had diabetes mellitus. The proportion of days covered for each study phase was as follows: pre-activation phase = 0.54, activation phase = 0.58, and post-activation phase = 0.46. A significant difference was found between the activation and post-activation phases (p = .001). The increase in measured adherence between the pre-activation and activation phases approached significance (p =.057). Forty-six patients completed the pre- and post-Morisky medication adherence survey. The median score rose from 2.0 at baseline to 3.0 at study completion (p <.001). Average blood pressure and level of control during study period improved significantly after initiation of the study and remained improved from baseline through the course of the study. The 48 subjects who completed the study reported a high level of satisfaction with the medication reminder application at the final study visit.
Conclusions
A mobile-phone-based automated medication reminder system shows promise in improving medication adherence and blood pressure in high-cardiovascular-risk individuals.
Keywords: hypertension, medication adherence, mobile health
Introduction
Hypertension remains a major health problem estimated to affect over 65 million adults in the United States. Rates of awareness and treatment have increased since 2000, but rate of control remains below public health goals.1 Furthermore, individuals with low socioeconomic status and underserved populations have a higher prevalence of uncontrolled hypertension, with increased cardiovascular morbidity and mortality.2–4 Poor adherence to antihyper-tensive medications has been identified as a major barrier to adequate blood pressure control.5,6 Methods to increase adherence and improve blood pressure control utilizing self-monitoring through telephone or other electronic media have shown promise in improving clinical outcomes.7–9
We hypothesized that a mobile phone with an automated medication reminder application (Pill Phone) would be well accepted and result in increased rates of medication adherence with hypertension medication. Our specific aims were to (1) assess adherence to antihypertensive medication in the 3-month period before versus during activation of the Pill Phone provided on a personal mobile phone in Medicaid patients recruited from a university clinic in Washington, DC, (2) assess continued medication adherence in the 3-month period after withdrawal of the Pill Phone, and (3) evaluate patient usage patterns and acceptance of the Pill Phone.
Study Methods
Between December 2009 and January 2010, 50 patients with the primary diagnosis of hypertension were recruited from the internal medicine, renal/hypertension, and cardiology clinics of the George Washington University Medical Faculty Associates in Washington, DC. Using our electronic medical record system, we identified approximately 350 patients insured by DC Medicaid who had been seen at our center in the past 12 months with the ICD-9 codes 401.0, 401.1, 401.9, 402.0, 402.1, 402.10, 402.11, 403.0, 403.1, 403.10, and 404.0. These patients were placed in a random number sequence and then invited by the research nurse to participate in the program. The first 50 eligible patients who agreed to participate were enrolled after giving written informed consent. The study was approved by the institutional review board of the George Washington University.
Inclusion criteria were (1) age 18 to 80 years, (2) established essential hypertension, (3) prescribed at least two antihypertensive medications, (4) fluency in English, and (5) DC Medicaid as primary medical insurance. Exclusion criteria were (1) patients with end-stage organ disease (kidney, liver, heart, lung, pancreas), (2) patients with a terminal illness (expected survival of less than one year), (3) patients with severe dementia or serious mental illness, and (4) inability to use a mobile phone or the medication reminder software.
After obtaining informed consent and at enrollment, participants received new, identical mobile phones preloaded with the Pill Phone application, owned by VOCEL Inc. (currently no longer available). Pill Phone was a patented, Food and Drug Administration-cleared, secure wireless medication reminder software for mobile phones. It included a comprehensive drug resource, the “Pill Book,” which contained up-to-date information on more than 1800 of the most commonly prescribed medications. The software allowed the following functions: (1) visual/audible prompts to take medication, (2) tracking and storage of pill-taking records, (3) image display of pills, (4) confirmation that a dose was taken, and (5) display of potential side effects and drug interactions. The Pill Phone application was downloaded from the wireless carrier’s catalog, just like a ringtone or game. The Pill Phone used transmission control protocol/Internet protocol to communicate (TCP/IP) with the server. Any phone that is able to browse the Web (or download a ringtone) has TCP/IP enabled. Regardless of the data/voice plan that allows Web browsing, the amount of data used per month is very small. Depending on the number of alerts set up, the monthly data usage typically ranges between 30 and 800 KB per month (a ringtone uses approximately 800 KB). Using a personal identification number, the user activates the functions of the Pill Phone application. Once activated, the patient can use either a computer or the phone keyboard to enter the name and dose of each medication and the time to be reminded to take each pill. The phone automatically displays a picture and name of the medication when the reminder is set off. The patient then taps either the “taken,” “not taken,” or “snooze” button to stop the reminder. Selecting “snooze” will generate a follow-up reminder at a preset interval (e.g., 30 min). All responses were recorded in the Pill Phone server database for retrieval by authorized users.
All subjects participated in a sequential study design comprising three phases, allowing assessment of hypertension medication adherence as measured by pharmacy refill records for (1) three months prior to study entry, (2) three months while using the cell-phone-based Pill Phone medication reminder system, and (3) three months after withdrawal of the Pill Phone reminder system. Phase 1, or the pre-activation phase, was the 3-month period before a mobile phone was provided (pharmacy refill medication adherence historical control period). Subjects first met the study staff for the baseline visit at which time subjects were provided with and trained to use his/her cell phone, but without activation of the Pill Phone application. After 1 month, patients returned to start phase 2, or activation phase. The Pill Phone software was turned on, and subjects were instructed to use the medication reminder application daily. After 3 months, the subjects returned to start phase 3, or post-activation phase, during which subjects still had access to the cell phone but with the Pill Phone application turned off. A final visit occurred after 3 months without the Pill Phone system (Figure 1).
Figure 1.
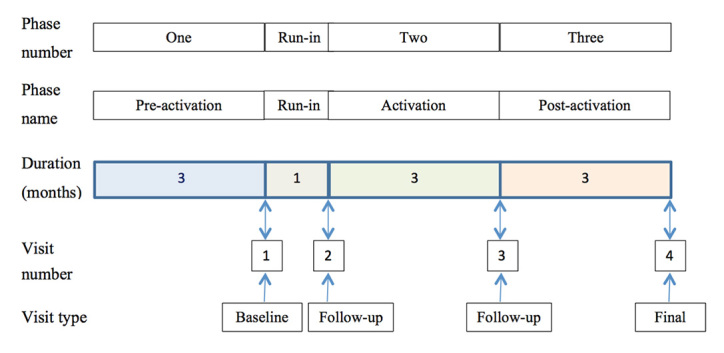
Study overview.
At the first study visit (visit 1), informed consent was obtained, and all subjects were provided with a mobile phone handset and phone contract provided from Cricket Communications Inc. for the duration of the study. Participants were provided written information on hypertension and blood pressure goals (“Your Guide to Lowering Blood Pressure” and select material from “Prevent and Control High Blood Pressure: Mission Possible”), provided free by the National Heart Lung Blood Institute Web site (www.nhlbi.nih.gov/health/public/heart). All participants were administered questionnaires to assess health attitudes and beliefs and quantify health literacy at this visit.
The second study visit (visit 2) was scheduled approximately 1 month after visit 1. At that time, the study nurse assisted the patient in downloading and activating the Pill Phone software, entering all antihypertensive medications (and any others patients requested) and setting alarm parameters. After 6 weeks using the Pill Phone, each patient was called to confirm that the phone system was functioning properly and report if there were any changes in medications, emergency room (ER) or clinic visits, or hospitalizations.
The third study visit (visit 3) occurred 3 months after activation of the Pill Phone. During visit 3, the Pill Phone medication software system was discontinued, or deactivated. However, the cell phone remained operational for another 3 months until the final study visit (visit 4).
Data Collection
Baseline clinical parameters along with a focused medical history on cardiovascular risk factors were obtained: demographics (including date of birth, gender, ethnicity, insurance status, diabetes status, alcohol and tobacco use, education level, employment status) and pharmacy information. Baseline questionnaires included (1) patient quality-of-life questionnaire (Medical Outcomes Study SF-36), (2) Morisky self-reported medication scale (SMS), 10 and (3) Rapid Estimate of Adult Literacy in Medicine. 11 The Morisky SMS is a structured four-item self-reported measure of medication adherence (alpha reliability = 0.61) that demonstrated concurrent and predicative validity for blood pressure control in a population of predominately African-Americans with hypertension followed for up to 5 years. 10 It is composed of four questions: (1) Do you ever forget to take your medicine? (2) Are you careless at times about taking your medicine? (3) When you feel better, do you sometimes stop taking your medicine? (4) Sometimes, if you feel worse when you take your medicine, do you stop taking it? A response of yes to any question is associated with significant decline in adherence.
At each visit, the research nurse documented the medication regimen and measured sitting blood pressure and heart rate by automated blood pressure monitor (oscillometric method) two times after 5 min of sitting with legs uncrossed, back against chair, and cuff at heart level. Medication adherence was assessed by pharmacy refill records. Pharmacy refill information for all blood pressure medications was obtained for the entire length of the study and analyzed for a 3-month period within each study phase (Figure 1). At visit 3 (end of using the Pill Phone system), the medication usage data were downloaded from the mobile phone. At the final visit, the Morisky SMS was repeated and a patient satisfaction survey completed. The primary outcome measure was adherence with hypertension medications as assessed by pharmacy refill rate. Data on recruitment, retention, satisfaction, usage patterns, and medication adherence with medications as assessed by Pill Phone software application were also collected.
Statistical Analysis
Prior to conducting this study, a power analysis was conducted to determine the minimum sample size required to detect the hypothesized intervention effects. Our power analysis estimates were based on the hypothesis that the intervention would result in a 10% increase in medication adherence rates, which was determined to be a clinically significant improvement. Based on this assumption, a power analysis was calculated using a simple two-tailed t-test to assess differences between the pre-intervention and intervention time points. The power analysis, which assumed 80% power and an alpha level of 0.05, estimated that a total of 44 patients would be needed to detect the estimated intervention effect. All statistical analyses were completed using Windows SPSS 19.0 software. The data analysis was performed on the 48 patients that completed the study.
Results
Recruitment and Retention
Fifty patients were enrolled in the period of approximately 30 days. Forty-eight patients completed the study to the 7-month visit, for a retention rate of 96%. Of the 2 participants who failed to complete the study, one was admitted to a chronic care facility and 1 was lost to follow-up.
Baseline Parameters
Baseline characteristics of the study population are shown in Table 1. The average age was 53 years old [standard deviation (SD) = 8.7 years], 96% were self-described African-American, 69% were female, 54% had diabetes, and the average body mass index (BMI) was 39.4 kg/m2 (SD = 8.6). Patients were prescribed an average of 8.1 medications, including an average of 3 antihypertensive medications. The levels of employment, education, and health literacy are tabulated.
Table 1.
Baseline Characteristics, n = 50
| Age | 53 years (range 33–78) |
| Male gender | 31% |
| African-American | 96% |
| Employment | 21% |
| Education | |
| High school diploma/General | 79% |
| Educational Development | |
| College graduate | 17% |
| Health literacy | |
| 3rd grade reading level or below | 6% |
| 4th–6th grade reading level | 4% |
| 7th–8th grade reading level | 33% |
| High school reading level or above | 56% |
| BMI (kg/m2) | 39.4 (range 24–62) |
| Diabetes mellitus | 54% |
| Smoking | |
| Ever used | 58% |
| Current use | 42% |
| Duration hypertension | 15.1 years (range 1–45 years) |
| Total medications | 8.1 (range 3–16) |
| Hypertension medications | 3 (range 2–7) |
| Home blood pressure monitor | 50% (respond yes to having one) |
| Never use | 8% |
| Use <1x/month | 29% |
| Use 1â4x/month | 13% |
| Use 1â4x/week | 21% |
| Use >5x/week | 29% |
Primary Outcome Measures
Adherence with Hypertension Medication as Assessed by Pharmacy Refill Rate
Adherence with hypertension medications was defined using pharmacy data to calculate the proportion of days covered (PDC), which was defined as the number of days that the patient had access to the hypertensive medication in each time period/the total number of days in each time period.
As shown in Figure 2, mean medication adherence (PDC) for each study phase was as follows: pre-activation phase (months −3, −2, −1) = 0.54 (SD = 0.27, range 0 to .99), activation phase (months 1, 2, 3) = 0.58 (SD = 0.20, range .11 to 1.0), and post-activation phase (months 4, 5, 6) = 0.46 (SD = 0.31, range 0 to 1.0). A repeated-measure analysis of variance (ANOVA) was used to assess changes in mean PDC rates between the three time periods. Results suggested significant differences between the three time points (F = 6.4, p = .003). Comparisons of mean PDC rates confirmed that there was a statistically significant difference between the second and third phases, namely, the activation and post-activation phases (p < .001). There was a nonsignificant trend toward increased adherence between the pre-activation and post-activation phases (p = .057).
Figure 2.
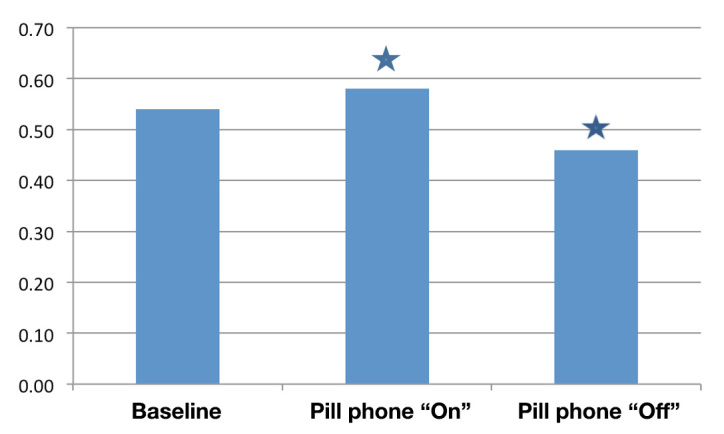
Adherence with hypertension medications using pharmacy data to calculate the PDC. The PDC was defined as the number of days that the patient had access to the hypertensive medication in each time period/the total number of days in each time period. The PDC for each study period as follows: pre-activation phase (months −3, −2, −1) = 0.54, activation phase (months 1, 2,3) = 0.58, and post-activation phase (months 4, 5, 6) = 0.46. A repeated-measure ANOVA was used to assess changes in PDC between the three time periods. Results suggested significant differences in PDC over time (F = 6.4, p =.003). Pairwise comparisons indicated significant differences between the activation and post-activation phases (p =.000) indicated by a blue star. There was no significant difference between the pre-activation and activation phases (p = .052) or the pre-activation and post-activation phases (p = .170)
Adherence with Hypertension Medication as Assessed by Morisky Self-Reported Medication Scale (Four Questions)
At baseline, the average Morisky SMS score was 2.4, and the median score was 2.0. The scale ranges from 0 to 4, with higher scores indicating better medication adherence. A total of 79% of participants scored <4 (anything less than 4 is considered nonadherent). Forty-six patients completed both a baseline survey and one conducted at the final study visit. At study completion, the mean score was 3.2, and the median score was 3.0. A paired sample t-test was used to analyze the differences between scores at baseline and the final study visit. Results indicated a significant increase in self-reported medication adherence to hypertension medication between the two time periods (t = −5.2, p = .000).
Secondary Outcome Measures
Adherence with Medication as Assessed by Pill Phone Application
Two methods to determine medication adherence by participant responses via the medication reminder software were utilized. “Nonweighted” (simple) average is the average adherence across all medications for each patient. “Weighted” average (weighted by number of pills prescribed per week) is the average number of total pills reported as “taken” across all medications/the total number of pills prescribed across all medications. Overall, the weighted percentage of “taken” responses was 60% over the 12-week activation phase. That is, across all patients and all anti- hypertensive medications, people responded via the Pill Phone application that these medications were “taken” 60% of the time that they were scheduled to take them. A comparison of “nonweighted” and “weighted” averages revealed no significant differences between the two estimation methods (t = 0.678, p = .501).
Data for Pill Phone utilization by week (number of pills indicated as “taken” in a week/number of pills prescribed for that week) was obtained and reviewed at the completion of the study (Figure 3). These data are for Pill Phone responses during the 12-week period when the medication reminder software was turned on, or activation phase. Patients indicated having “taken” an average of 63% of their pills in week 1, and this percentage declined thereafter. By week 12, this declined to 54% of their pills, despite taking into account changes in prescriptions over the Pill Phone period of the study. Interestingly, participants with more prescriptions than the mean reported taking their pills more often in the beginning of the study. In week 1, patients with nine of more medications reported taking 70% of their pills compared with 58% for patients with eight medications or less. This difference was not statistically significant (t = 1.5, p =.125) and disappeared by week 2. Over the course of the 12 weeks, the number of medications did not appear to impact the average number of “taken” responses. Analyses detected no significant differences in the percentage of “taken” responses reported by patients with nine or more medications (60%) and the average number of “taken” responses reported by patients with eight or fewer medications (58%; t = 0.282, p = 0.78. Similar results were found when using number of medications as a continuous variable.
Figure 3.
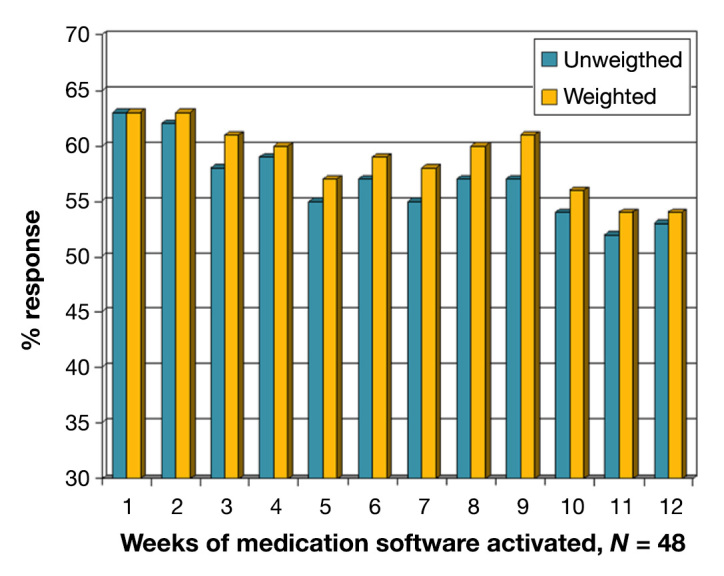
Medication adherence measured by the percentage of “taken” responses upon a reminder to the number of antihypertensives prescribed. The graph shows this for each week of the 12-week activation period. “Unweighted” refers to the average adherence across all medications for each patient. “Weighted” is the average number of total pills indicated as “taken” across all medications/the total number of pills prescribed across all medications.
Level of Blood Pressure Control by Clinic Measures
Average blood pressure and level of control during study period are displayed in Table 2. Both measures improved significantly after initiation of the study, prior to activation of the Pill Phone, and remained improved from baseline through the course of the study. Repeated-measure ANOVA was used to assess changes in blood pressure rates over time. Results suggested significant changes in systolic blood pressure (F = 4.4, p = 0.007). Comparisons of mean blood pressure rates indicated that baseline systolic blood pressure rates were significantly higher than those at each subsequent time period. Baseline systolic blood pressure rates were significantly higher than those at the one month, pre-Pill Phone visit (p = .031), the Pill Phone “use” visit (p = .004), and the Pill Phone “off” visit (p = .006). No other significant differences in systolic blood pressure were detected. Analyses examining changes in diastolic blood pressure produced similar results.
Table 2.
Blood Pressure Measurements Performed at Study Visits
| Blood pressure (mmHg mean) | p value (from baseline) | Percentage of blood pressure at goal | |
| Baseline | 144/89 | 47% | |
| 1 month visit (pre-Pill Phone) | 137/85 | p = .031 | 66% |
| Pill Phone use 3 months | 136/84 | p = .004 | 64% |
| Pill phone off 3 months | 135/85 | p = .006 | 60% |
Patient-Specific Usage of Pill Phone
The Pill Phone was activated during visit 2, just after the initial 1-month run-in phase. The application was then deactivated after approximately 3 months, at visit 3. Data on participant responses to reminders were analyzed for this 3-month period, or activation phase. Patients rarely responded that medications were “skipped” (94 times out of over 15,000 scheduled medication dosages). Delayed medication dosing (“snooze” feature utilization) was also used infrequently, only 134 times across all patients.
There was no significant correlation between pharmacy refill activity and Pill Phone use (measured by percentage of “taken” responses to scheduled medication doses) during the activation phase (r = −0.78 in month 1 of Pill Phone use, −0.12 in month 2, and 0.05 in month 3. The correlation between medication adherence (PDC) and Pill Phone use during the entire course of the study was not significant either (r = −0.025, p = .867).
Regression analyses were used to explore whether demographic and survey data collected at baseline could be used to predict Pill Phone use. The following variables were entered into a regression equation with Pill Phone use as the dependent variable: age, gender (male/female), employment status (employed/unemployed), education status (high school graduate or greater/less than high school education), and health literacy. Table 3 presents results from this analysis. Overall, the model explained only 18% of the variance associated with Pill Phone use (F = 1.8, p = .12). The only significant predictor of Pill Phone use was gender, with women reporting higher use than men (65% versus 46%). Years of education, health literacy, and employment status were not significant predictors of Pill Phone use.
Table 3.
Regression Analyses of Demographic and Survey Data Collected at Baseline to Predict Pill Phone Use
| β | t value | p value | |
| Age | 0.155 | 1.033 | 0.308 |
| Gender | −0.308 | −2.008 | 0.050 |
| Education (years) | 0.086 | 0.504 | 0.617 |
| Employment status (employed full- or part-time/unemployed) | −0.075 | −0.518 | 0.607 |
| Health literacy score | 0.252 | 1.442 | 0.157 |
Patient Survey on Satisfaction
Overall median patient satisfaction with the Pill Phone was high: 4.6 out of 5.0 (1 = disagree strongly, 2 = somewhat disagree, 3 = neutral, 4 = somewhat agree, and 5 = strongly agree). Forty-eight participants completed the survey. Average and median scores for each of the five individual questions are in Table 4.
Table 4.
Patient Survey on Satisfaction with Pill Phone Interventiona
| 1. Having Pill Phone has made it easier to keep track of my medications. | Mean 4.5 | Median 5.0 |
| 2. Keeping a medication list on my cellphone made it easier to take care of myself. | Mean 4.4 | Median 5.0 |
| 3. I think my blood pressure is better controlled now than it was just before the study. | Mean 3.9 | Median 4.0 |
| 4. I would use the Pill Phone, or similar program, in the future. | Mean 4.4 | Median 5.0 |
| 5. Being in the study has been helpful to me. | Mean 4.5 | Median 5.0 |
1 = disagree strongly, 2 = somewhat disagree, 3 = neutral, 4 = somewhat agree, 5 = strongly agree. Overall median patient satisfaction with Pill Phone application was 4.6 out of 5.0
Hypertension Medication Number and Changes during Study Period
The average number of antihypertensive medications prescribed at baseline and the last visit was virtually identical: 3.02 at baseline and 3.04 at the last visit. Over the course of the study, 51 antihypertensive medications were added or dropped, while 23 medication doses were changed, based on study visit interviews. Per protocol, study staff adjusted the medication list on the Pill Phone during scheduled study visits. However, 13 patient-initiated changes to the medication list in the application were recorded during the mid-study phone contact (at approximately 2 to 3 months) and at the 4-month visit. The majority of changes (11 of 13) were recorded at the phone contact, which was the first contact after the application was loaded.
Office Visits, Emergency Room Visits, and Hospitalizations
During the course of the study, the mean and median number of primary care provider visits was 3.2 and 3.0, respectively (range 0 to 9). Subjects visited other types of physicians on average 5.0 times during the same time course with a median of 4.0 visits (range 0–14). Seventeen patients reported at least one ER visit. Two of these dropped from the study, so from our final sample (n = 48), 15 had any ER visits (9 reported one visit, 4 reported two visits, and 2 reported three visits). Seven patients reported hospitalizations over the course of the study. For those hospitalized, the average total number of days spent in the hospital was 10, with a range of 1 to 28. Hospitalized patients reported lower Pill Phone use than nonhospitalized patients (62% versus 44%), but this difference was not statistically significant due to the small number of patients hospitalized during this study (t = 1.6, p = .108). Interestingly, patients with any ER use during the course of this study reported slightly higher rates of Pill Phone use (61% versus 56%) than patients with no ER use, but again, this difference was not statistically significant (t = 0.523, p = .603).
Discussion
In this pilot and feasibility trial, we evaluated the acceptance and usage of an automated medication reminder application for a mobile phone in 50 individuals with established hypertension, prescribed at least two antihypertensive medications, and insured by Medicaid. The study population was predominately African-American (96%) and had a high level of diabetes (54%), high BMI (over 39), low levels of health literacy, low health-related quality of life, and a high number of prescribed medications. Notably, recruitment was completed within 30 days, and the retention rate was 96%. At study completion, participants reported a high level of satisfaction with the intervention and increased medication adherence by self-reported survey. Adherence, as measured by pharmacy refill data, showed a trend toward improvement with initiation of the study and intervention, and it declined significantly after the intervention was discontinued.
Use of mobile phones to enhance health care has been studied with increasing intensity that has paralleled overall cell phone usage.12–14 With its widespread availability and simplicity of use, a mobile phone is an attractive tool to improve health care, particularly where health care disparities are the widest (National Center for Health Statistics, December 2011, http://www.cdc.gov/nchs/nhis.htm). Low socioeconomic groups such as those insured by Medicaid are at higher risk for hypertension and cardiovascular disease, 4,15–17 which may be, in part, due to lower medication adherence.18 Considering the increasing public and personal burden of chronic diseases such as diabetes mellitus and hypertension, improvements in self-care will likely be necessary to control cardiovascular disease in the future.
Adherence interventions can be broadly divided into (a) technical, (b) behavioral, (c) educational, and (d) multifaceted or complex. 19 Increasing medication adherence through a reminder system is the most common type of behavioral intervention, and one that is intuitively targeted toward patients who forget to take their medication (“unintentional nonadherence”). Automated telephone reminder systems have been successful for appointments and used in diabetes care. 20 Text messages have been used to remind patients for a wide variety of health care issues such as clinic appointments, control of asthma, taking oral contraceptives, 21–24 and even using sunscreen. A considerable amount of literature details efforts to use short message service for human immunodeficiency virus treatment and malaria control. 25,26 Despite the large public health burden of hypertension, limited data are available on the impact of cell-phone-based reminder systems for antihypertensive management.27
The intervention presented was multifaceted; beyond the Pill Phone application, subjects were provided a cell phone and educational material at initiation. Furthermore, the application had a drug reference as part of the software package. The intervention may address more than just one barrier to nonadherence. The addition of a drug reference with pictures (“Pill Book”) provides a potential educational resource that could lead to behavioral change. Furthermore, the intervention could address barriers to adherence created by a fragmented health care system by providing a mobile medication list that can be easily updated. For example, the 23 ER visits and 7 hospitalizations reported during the 7-month study period were transitions of care with the potential for miscommunication of medication prescriptions, leading to unintentional medication nonadherence. Medication reconciliation, a National Patient Safety Goal (NPSG.03.06.01) for the Joint Commission on Accreditation of Healthcare Organizations would be facilitated if patients and providers were able to access and update the list when appropriate. Additional enhancements to the intervention could reduce the system barriers further (e.g., linking medication lists to an electronic health record and pharmacy record).
As a pilot trial, we were interested in evaluating patient behavior with the use of the mobile-phone-based program. Per the study protocol, participants had to have all antihypertensive medications listed on the mobile-phone- based software program but were offered to have all of their medications listed at study initiation. However, not all participants wanted all the medications on the phone. Individual participants demonstrated variation, with a proportion answering few reminders while and some participants were very responsive. Overall, the average number of “taken” responses to reminders was between 55% and 65% over the 12-week activation phase. Since a very small fraction responded with “snooze” or “not taken,” approximately 40% of the reminders were left unanswered. For patients taking numerous medications, the number of reminders they received was perceived as an additional burden or nuisance. Participants likely became fatigued with indicating “taken” to each individual reminder for every pill taken at a certain time. This emphasizes the need to personalize reminders further to those doses that have been actually skipped and to aggregate the reminders for multiple medications when taken at the same time. There appears to be a reluctance to respond with “skipped” or “snooze” (versus “taken”) based on the very small number of these responses versus the total number of responses. Taken in the context that the average adherence as assessed by responses on the mobile phone medication reminder software was only 60%; this could be that these responses were not useful to patients. The low incidence of skipped or snooze responses may also be from a patient reluctance to confirm nonadherent behavior. Finally, very few changes to the medication list were made by the study participants themselves. We did not specifically plan on training patients to change their medication list on their phone, although many were trained at initiation. Future studies should concentrate on training and assure competence in making changes to software application to make it more interactive.
Few insights could be made from associative data from this small study. Baseline demographics did not appear to predict Pill Phone use, with the exception that women were more likely to use the mobile-phone-based medication reminder software program then men. We were unable to detect any association between the number of prescribed medications and adherence, but we did not evaluate for once-daily versus multiple-day drug adherence within individuals in our cohort.
A cost-effectiveness analysis was not performed for this study, but general costs could be outlined based on our program cost: personnel (registration staff and case manager time), mobile phone costs, and “Pill Phone” subscription costs. A cost effectiveness analysis would be appropriate for this type of study in the future. Also of interest in a larger and longer study would be evaluation of the impact of using a cell phone medication reminder system on associated health-related behavior, including home blood pressure measurements, adherence with non-hypertensive medications, number of physician office visits, and frequency of changes in medication regimen.
The limits in interpretation of the results of this pilot and feasibility trial are clear. It is an uncontrolled study with a small study population and a relatively short study period. Lack of a control group greatly reduces the ability to determine the effect of the intervention. The confounding effects of patient education in the form of nurse interaction throughout the study, literature on hypertension, and the mobile phone itself makes conclusions about the effects of the Pill Phone program more difficult. Notably, blood pressure, as measured by study visit readings, declined during the study period as compared with baseline (patients served as their own controls). However, this occurred right after initiation and before activation of the reminder system. The well-known phenomenon of improved adherence in subjects under observation likely explains this observation. As a pilot study, the intention was to determine acceptability and feasibility of the intervention.
We recognize there may be several other potential limitations to extrapolating results of this study to a wider population: (1) our gold standard for medication adherence was limited to assessing prescription refills history over a short time span from multiple pharmacies, (2) We limited our recruitment to an urban high-risk Medicaid population, (3) this study included the incentive of a free mobile phone handset and contract, and, lastly, (4) the intervention was enhanced by the study design and structure itself. Literature on hypertension and blood pressure treatment goals were distributed to subjects at baseline. Although our research nurse was not instructed to educate patients, they were available for patient questions during the study visit and also available for technical problems over the phone.
Although the simplicity of the software application may be considered a strength, other barriers to adherence could be addressed with additional features to a mobile-phone-based intervention, such as prescription refill reminders, interface with electronic health and pharmacy records, and automated text messaging from the pharmacy or health care team. Future studies utilizing mobile phones for enhancement of medication adherence in diabetes and associated cardiovascular disease should be able to accommodate for bidirectional communication between health care providers and patients. Devices “linked” or “interfaced” with the health care team would be able to personalize interventions, increasing the odds of making behavioral changes. Therefore, future studies in mobile-phone-based health interventions will not only need to include assessment of clinical outcomes and cost-effectiveness, but they should be interwoven with the health care team and medical record. Results of this study should provide many insights into this and future mobile phone applications toward improving medication adherence for patients with chronic cardiovascular disease.
Glossary
- (ANOVA)
analysis of variance
- (BMI)
body mass index
- (ER)
emergency room
- (PDC)
proportion of days covered
- (SD)
standard deviation
- (SMS)
self-reported medication scale
- (TCP/IP)
transmission control protocol/Internet protocol
Funding
Study funding was provided by a grant from Qualcomm Inc. to One Economy Corporation and the George Washington University Medical Center. Mobile phones and phone contracts were provided by Cricket Communications.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114(24):2619–2626. doi: 10.1161/CIRCULATIONAHA.106.660043. Dec 12. [DOI] [PubMed] [Google Scholar]
- 3.Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, Mensah G, Beckles GL. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med. 2006;166(21):2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 4.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the united states. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Krousel-Wood MA, Muntner P, Islam T, Morisky DE, Webber LS. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009;93(3):753–769. doi: 10.1016/j.mcna.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, Carey K. A telecommunications system for monitoring and counseling patients with hypertension. impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9(4 Pt 1):285–292. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 8.Rogers MA, Small D, Buchan DA, Butch CA, Stewart CM, Krenzer BE, Husovsky HL. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med. 2001;134(11):1024–1032. doi: 10.7326/0003-4819-134-11-200106050-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wetzels GE, Nelemans PJ, Schouten JS, Dirksen CD, van der Weijden T, Stoffers HE, Janknegt R, de Leeuw PW, Prins MH. Electronic monitoring of adherence as a tool to improve blood pressure control. A randomized controlled trial. Am J Hypertens. 2007;20(2):119–125. doi: 10.1016/j.amjhyper.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, Crouch MA. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 12.Garcia-Lizana F, Sarria-Santamera A. New technologies for chronic disease management and control: a systematic review. J Telemed Telecare. 2007;13(2):62–68. doi: 10.1258/135763307780096140. [DOI] [PubMed] [Google Scholar]
- 13.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 14.Wei J, Hollin I, Kachnowski S. A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J Telemed Telecare. 2011;17(1):41–48. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 15.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17(10):963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the multi-ethnic study of atherosclerosis) Am J Hypertens. 2011;24(2):187–193. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen NB, Diez-Roux A, Liu K, Bertoni AG, Szklo M, Daviglus M. Association of health professional shortage areas and cardiovascular risk factor prevalence, awareness, and control in the multi-ethnic study of atherosclerosis (MESA) Circ Cardiovasc Qual Outcomes. 2011;4(5):565–572. doi: 10.1161/CIRCOUTCOMES.111.960922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krousel-Wood M, Joyce C, Holt E, Muntner P, Webber LS, Morisky DE, Frohlich ED, Re RN. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011;58(5):804–810. doi: 10.1161/HYPERTENSIONAHA.111.176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollon B, Holbrook AM, Keshavjee K, Troyan S, Gaebel K, Thabane L, Perera G. Automated telephone reminder messages can assist electronic diabetes care. J Telemed Telecare. 2008;14(1):32–36. doi: 10.1258/jtt.2007.070702. [DOI] [PubMed] [Google Scholar]
- 21.Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstet Gynecol. 2010;116(3):633–640. doi: 10.1097/AOG.0b013e3181eb6b0f. [DOI] [PubMed] [Google Scholar]
- 22.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respir Med. 2010;104(2):166–171. doi: 10.1016/j.rmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong AW, Watson AJ, Makredes M, Frangos JE, Kimball AB, Kvedar JC. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145(11):1230–1236. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 24.Wei J, Hollin I, Kachnowski S. A review of the use of mobile phone text messaging in clinical and healthy behaviour interventions. J Telemed Telecare. 2011;17(1):41–48. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 25.Chi BH, Stringer JS. Mobile phones to improve HIV treatment adherence. Lancet. 2010;376(9755):1807–1808. doi: 10.1016/S0140-6736(10)62046-6. [DOI] [PubMed] [Google Scholar]
- 26.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, Jack W, Habyarimana J, Sadatsafavi M, Najafzadeh M, Marra CA, Estambale B, Ngugi E, Ball TB, Thabane L, Gelmon LJ, Kimani J, Ackers M, Plummer FA. Effects of a mobile phone short message service on antiretroviral treatment adherence in kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 27.Christensen A, Christrup LL, Fabricius PE, Chrostowska M, Wronka M, Narkiewicz K, Hansen EH. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]


