Abstract
Introduction:
The aim of this study was to compare, from the perspective of the statutory health insurance, resource consumption and the associated adjusted treatment costs of intensified conventional therapy (ICT) with long-acting insulins in patients with type 1 diabetes mellitus (T1DM).
Methods:
We identified patients with T1DM who started ICT with either insulin glargine or neutral protamine Hagedorn (NPH) insulin between July 2000 and February 2008 using a representative German database (IMS® Disease Analyzer). The variables age, gender, insurance status, diabetes duration, hemoglobin A1c level, body mass index, and geographic region and specialization of practice were collected. Resource consumption was evaluated over a time period of 12 months and included the quantities of applied basal and bolus insulin, blood glucose test strips, lancets and needles, physician visits (general practitioner, specialist), hospitalization, and antihypoglycemic therapy (intravenous glucose/glucagon).
Results:
A total of 2297 patients with T1DM were included; 1079 received ICT with insulin glargine and 1218 with NPH insulin. After adjustment, annual cost savings in favor of insulin glargine amounted to €423.94 compared with NPH insulin (p = .3019).
Discussion:
The adjusted results show that an ICT with insulin glargine results in lower annual costs than ICT with NPH insulin (this difference was not statistically significant). However, in the context of glucose-lowering effect and a lower hypoglycemia rate, insulin glargine is preferred to NPH insulin for patients with T1DM undergoing ICT.
Keywords: basal insulin, cost comparison, insulin glargine, neutral protamine Hagedorn insulin, resource consumption, type 1 diabetes mellitus
Introduction
Diabetes mellitus is one of the major chronic metabolic diseases. This is due to its high and steadily increasing prevalence, its chronic course, and its diabetes-specific long-term effects. According to epidemiological studies, approximately 7.5 million people in Germany currently suffer from diabetes mellitus, of which approximately 520,000 patients have type 1 diabetes mellitus (T1DM).1 The collective term diabetes mellitus denotes various metabolic disorders, the main symptom of which is hyperglycemia. Type 1 diabetes is caused by a typically immunologically mediated destruction of beta cells in the pancreas, which leads to absolute insulin deficiency. It usually manifests in childhood or early adulthood.
Treatment of T1DM includes administration of long-acting insulins to meet basal insulin requirements and administration of short-acting insulins with the main meals [intensified conventional therapy (ICT)]. Treatment goals include preventing hypoglycemia, avoiding late complications, treating concomitant risk factors, and maintaining high quality of life.2 Several prospective long-term studies have shown that near-normal metabolic control is of great importance in the prevention of diabetes-related late consequences.3–5 In particular, the protective effect of improved blood glucose control on cardiovascular risk has been shown.6
Compared with the nondiabetic population, diabetes patients have an increased risk of microvascular and macro-vascular diseases (e.g., heart attack, stroke, nephropathy, retinopathy).7 Hence, expenses of the statutory health insurance in Germany for treating a diabetes patient are, on average, nearly twice as high as those for treating a nondiabetic patient.8 The total direct cost burden of diabetes in Germany grew from €27.8 billion in the year 2000 to €42.0 billion in the year 2007 (+51.1%). Incremental per-capita costs were €2,400 in 2000 and €2,605 in 2007.9
The cost-effectiveness of insulin therapy is also gaining importance in the selection of treatment for T1DM patients. Various basal insulins are available and their effectiveness is almost comparable. Some studies have shown that insulin glargine is superior to neutral protamine Hagedorn (NPH) insulin in reducing the fasting blood glucose value, the hemoglobin A1c (HbA1c) value, and the number of nocturnal hypoglycemias in T1DM patients.10–15 Glargine appeared to be cost-effective or even cost saving among T1DM patients with basal bolus therapy from the perspective of statutory health insurance compared with NPH depending on the scenario chosen.16
The aim of this study was to compare insulin glargine and NPH insulin, in terms of resource consumption and directly associated treatment costs, in T1DM patients receiving ICT.
Methods
This historical cohort study was conducted using the IMS® Disease Analyzer database.
The Disease Analyzer database (IMS Health) compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly from the computer systems of the practices of general practitioners and specialists throughout Germany. Diagnoses (ICD-10), prescriptions [Anatomical Therapeutic Chemical (ATC) classification system], and the quality of reported data were continuously monitored by IMS based on a number of quality criteria (e.g., completeness of documentation, linkage of diagnoses and prescriptions). The data are generated directly from the computers in the physicians’ practices via standardized interfaces and provide daily routine information on patients’ diseases and therapies. A practice transmits patient data stored in the physician’s computer to IMS on a monthly basis. Before transmission, the data are encrypted for data protection. Altogether, the database includes data from roughly 3000 practices and approximately 20 million patients in Germany from at least a 10-year period. The validity of the Disease Analyzer data was previously evaluated and described.17 It has been the basis of a number of studies and peer-reviewed scientific publications in the fields of epidemiology as well as cost analyses.18,19
Data from T1DM patients who started ICT with insulin glargine or NPH insulin between July 2000 and February 2008 were analyzed (Figure 1). Patients were identified as T1DM patients if they were diagnosed according to ICD-10 before age 30 years, had not been treated with oral antidiabetic drugs in the past, and had no diagnoses indicating type 2 diabetes (ICD-10 E11). The patient population was defined by the following inclusion and exclusion criteria:
continuity of patient data at least 12 months prior to and 18 months within the treatment period;
ICT, i.e., insulin glargine/ NPH insulin + short-acting insulin (ATC A10C1) throughout the observation period;
no prescriptions of premixed insulins (A10C3) throughout the observational period;
no switch between the basal insulins glargine and NPH during the observational period;
no previous prescriptions of insulin pumps; and/or
no prescriptions of porcine/bovine insulins or U-40 insulins during the observational period.
Figure 1.
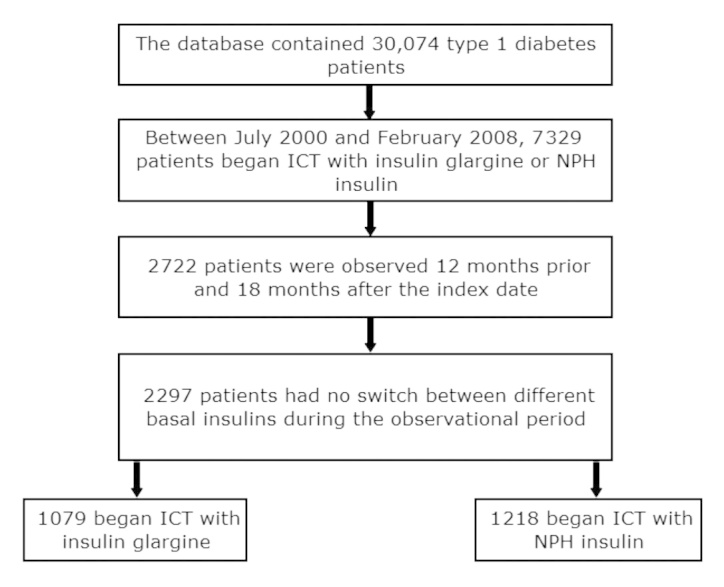
Patient selection.
The variables age, gender, insurance status (statutory or private), region (west or east), practice specialization (general practitioner or diabetologist), diabetes duration, HbA1c value (if documented), and body mass index (BMI; if documented) were recorded. The resource consumption was calculated over 12 months (months 7–18 of the observational period) and included the consumption of basal and bolus insulins, antihypotensive prescriptions (intravenous glucose/glucagon), blood glucose test strips, and the number of physician visits (general practitioner visits, referrals to specialists) and hospital admissions.
The direct treatment costs of antihyperglycemic therapy for the individual treatment groups were also determined. These included the costs for basal and bolus insulin, blood glucose test strips, and additional consumables (e.g., lancets, needles). Costs of concomitant medication for the treatment of cardiovascular risk factors and hypoglycemias were also collected, including antihypertensives (ATC codes C02, C03, C07, C08, C09), lipid-lowering agents (ATC code C10), antithrombotics (ATC code B 01), intravenous glucose/glucagon (for the treatment of hypo-glycemia), and heart drugs/anticoagulants (ATC codes C01, B02).
The identified amounts and costs were adjusted for the variables of age, gender, practice specialization, region, diabetes duration, HbA1c value, and BMI value using a multivariate Cox regression analysis. The significance level was set at p = .05.
Results
Patient Characteristics
Overall, data from 2297 patients were analyzed, including 1079 patients who received ICT with insulin glargine and 1218 patients who received ICT with NPH insulin (Figure 1).
The treatment groups exhibited differences in the recorded variables of geographic location of the practice, practice specialization, HbA1c value, and BMI. Most patients in the NPH group were treated by a practice in West Germany (p <.0001) and had a higher BMI (25.9 kg/m2) compared with insulin glargine (p = .0094). The treatment groups were comparable in other clinical and sociodemographic variables (Table 1).
Table 1.
Patient Characteristics in the Treatment Groups
| Variable | Glargine | NPH | p value |
|---|---|---|---|
| Total, n (%) | 1079 (100 %) | 1218 (100 %) | |
| Male, n (%) | 709 (66 %) | 752 (62 %) | 0.050 |
| Privately insured, n (%) | 53 (5 %) | 54 (4 %) | 0.579 |
| West Germany, n (%) | 832 (77 %) | v1042 (86 %) | <0.001 |
| Practice specializing in diabetology, n (%) | 459 (43 %) | 421 (35 %) | <0.001 |
| Age in years (average ± SD) | 31.9 ± 10.9 | 31.1 ± 11.7 | 0.262 |
| Diabetes duration in years (average ± SD) | 6.8 ± 12.6 | 7.1 ± 14.8 | 0.057 |
| HbA1c value in % (average ± SD) | 7.9 ± 1.6 | 7.7 ± 1.6 | 0.014 |
| BMI in kg/m2 (average ± SD) | 25.2 ± 4.3 | 25.9 ± 5.0 | 0.253 |
| SD, standard deviation. |
Resource Consumption
Consumption of basal insulin was lower in the glargine group (17.5 U/day). Consumption of bolus insulin was comparable in the glargine (26.5 U/day) and NPH groups (25.6 U/day). Most often, general practitioner visits took place in the NPH group. The calculated resource consumption data are shown in Table 2.
Table 2.
Annual Resource Consumption
| Variable (per 12 months) | Glargine | NPH | p value |
|---|---|---|---|
| General practitioner visits (average ± SD) | 10.5 ± 6.4 | 10.8 ± 6.9 | 0.453 |
| Hospitalizations (average ± SD) | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.774 |
| Referrals to specialists (average ± SD) | 1.6 ± 2.6 | 1.4 ± 2.5 | 0.032 |
| Basal insulin consumption in U/day (average ± SD) | 17.5 ± 8.5 | 19.7 ± 11.4 | <0.001 |
| Bolus insulin consumption in U/day (average ± SD) | 26.5 ± 14.8 | 25.6 ± 15.6 | 0.040 |
| Consumption of blood glucose test strips per day (average ± SD) | 3.4 ± 4.4 | 3.2 ± 4.2 | 0.548 |
| SD, standard deviation. |
Direct Treatment Costs
The average adjusted annual treatment costs of antihyperglycemic therapy ranged from €1,512 for ICT with glargine to €1,308 with NPH (Table 3). The costs of concomitant medication (antihypertensives, lipid-lowering agents, antithrombotic agents, cardiac drugs/anticoagulants) before the adjustment were highest for patients treated with NPH insulin and amounted to €51 per year, whereas patients treated with insulin glargine incurred costs of €47 per year (Figure 2). Annual costs for hypoglycemia treatment (glucagon/intravenous glucose) were lower for ICT with insulin glargine (€4.10) compared with NPH insulin (€5.30).
Table 3.
Direct Annual Costs of Blood Glucose Management (Pharmacy Retail Price)
| Direct annual treatment costs, unadjusted (average ± SD) | Glargine | NPH | p value |
|---|---|---|---|
| Basal insulin | €399.95 ± €190.13 | €280.10 ± €158.98 | <0.001 |
| Bolus insulin | €477.78 ± €272.50 | €415.86 ± €254.74 | <0.001 |
| Blood glucose test strips | €577.82 ± €769.82 | €556.82 ± €744.85 | 0.663 |
| Consumables (e.g., needles, lancets) | €56.63 ± €90.56 | €55.41 ± €91.87 | 0.584 |
| Total | €1,512.19 ± €950.96 | €1,308.18 ± €914.1 | <0.001 |
| SD, standard deviation. |
Figure 2.
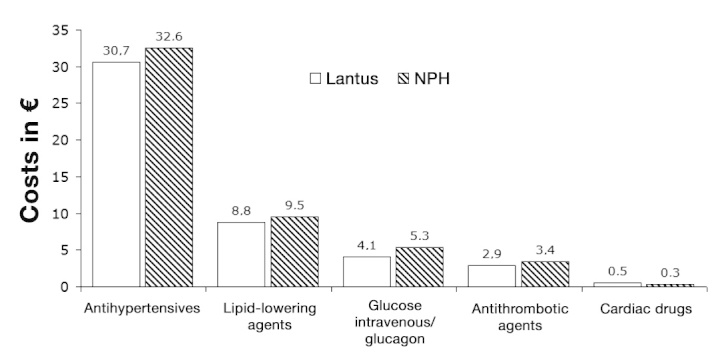
Unadjusted annual costs of concomitant medication (antihypertensives, lipid-lowering agents, antithrombotic agents, intravenous glucose/glucagon, and cardiac drugs). i.v., intravenous.
Cox Regression Analyses
The multivariate Cox regression analysis showed that ICT with insulin glargine leads to significant savings compared with ICT with NPH insulin (Table 4). Based on the annual costs of antihyperglycemic therapy, ICT with insulin glargine led to calculated savings of €423.94 (p = .3019) compared with NPH insulin.
Table 4.
Differences in Resource Consumption and Costs after Adjustment
| Glargine versus NPH | p value | |
|---|---|---|
| Antidiabetic agents (insulin, test strips, consumables) | −423.94 | 0.302 |
| Costs of basal insulin, € | 29.08 | 0.794 |
| Costs of bolus insulin, € | −86.36 | 0.572 |
| Costs of blood glucose test strips, € | −258.80 | 0.251 |
| Costs of additional consumables (e.g., lancets, needles), € | −107.86 | 0.267 |
| Number of general practitioner visits/year | −1.61 | 0.658 |
| Number of hospitalizations/year | 0.01 | 0.832 |
| Number of referrals to specialists/year | 0.16 | 0.918 |
| Consumption of basal insulin, U/day | −6.00 | 0.351 |
| Consumption of bolus insulin, U/day | −5.88 | 0.466 |
| Consumption of blood glucose test strips, U/day | −0.31 | 0.829 |
| Costs of antihypoglycemic treatment, € | −1.27 | 0.289 |
With regard to treatment of hypoglycemia, it was found that 10.6% of glargine patients and 11.4% of NPH patients received glucose or glucagon prescriptions. This was reflected in the cost calculations (Table 4).
Intensified conventional therapy with insulin glargine also showed cost benefits compared with NPH insulin in the adjusted analysis. They were not statistically significant, however. All results of the multivariate analysis are shown in Table 4.
Discussion
This analysis examined the resource consumption and associated costs of 2297 T1DM patients. Of these, 1079 patients received ICT with insulin glargine and 1218 with NPH insulin. To our knowledge, this is the first study in Germany to compare, based on a representative database, resource consumption and associated costs of T1DM patients undergoing ICT under real-life conditions. After adjustment for possible confounding factors, it became clear that ICT with insulin glargine uses fewer resources and leads to lower costs than ICT with NPH insulin. However, the differences were not significant.
Overall, the costs of T1DM to the health care system are significant. This is mainly due to the chronic course and associated complications of the disease. So far, there are no studies of the total costs solely due to T1DM in Germany. The results of this study point to potential savings for the German health care system through the use of insulin glargine for ICT for T1DM patients compared with NPH insulin. The adjusted annual costs of antihyperglycemic therapy were €423.94 lower when using insulin glargine instead of NPH insulin. There are an estimated 360,000 T1DM patients in Germany (5% of 6 million diagnosed diabetes patients), of which approximately 226,800 (63%) receive ICT, 9% conventional insulin treatment regimens, and 28% pump therapy.20 Utilizing the distributions of insulin glargine and NPH insulin identified in this analysis as a basis, an estimated 39% of T1DM patients currently receive ICT with insulin glargine, while approximately 45% are being treated with NPH insulin and approximately 16% with other basal insulins. A switch of the ICT to insulin glargine of all T1DM patients in Germany who do not yet receive insulin glargine (approximately 138,300 patients, of which approximately 102,000 take NPH and 36,000 take other basal insulins) could lead to savings of approximately €51 million per year for the statutory health insurance.
The results of this analysis are confirmed by several studies on the cost-effectiveness of long-acting insulin analogs in the treatment of T1DM in other countries. Six cost–utility analyses (CUAs) examined insulin glargine compared with NPH insulin with regard to the target value “additional costs per saved quality-adjusted life year” (QALY).21–26 The majority of these studies are based on improved metabolic control (HbA1c reduction) with insulin glargine compared with NPH insulin or a reduced rate of hypoglycemia with insulin glargine at a comparable metabolic state. The Swiss analysis includes symptomatic, severe, and nocturnal hypoglycemia and microvascular and macrovascular events with their effects on quality of life, life expectancy, and treatment costs.21 In this analysis, insulin glargine was dominant compared with NPH insulin, i.e., more effective and cost saving. Cameron and Bennett22 conducted a CUA from the perspective of the Canadian health care system. They included symptomatic and severe hypoglycemia and their effects on quality of life, including fear of hypoglycemia, and examined the treatment costs. Assuming that the compared treatments do not differ with respect to HbA1c value, in this analysis, costs per QALY amounted to Can$916,401. Another modeling study for the Canadian health care system is that of Grima and coauthors,23 based on a greater HbA1c reduction through insulin glargine. This study calculated costs of Can$20,799 per QALY gained. A British CUA considered a greater reduction of HbA1c and a greater reduction of hypoglycemia and reported costs of £31,890 per QALY gained.24 Two other studies within the British health care system that considered a lower hypoglycemia rate for treatment with insulin glargine calculated costs of between £3,496 and £4,978 per QALY, depending on how insulin was administered (insulin vial, insulin cartridge, or pen).25,26 Converting the results of these studies to Euro using Purchasing Power Parities, the additional costs per QALY gained ranged from €3,859 to €57,002. The reported costs per QALY for insulin therapy with insulin glargine are therefore within the generally accepted cost-effectiveness limits.27
When evaluating the results of this analysis, some basic structural and methodological limitations must be taken into account. The examined study population is rather young, with a comparatively short duration of diabetes. This explains the relatively low costs of concomitant medication. Further, there was no validation of diagnoses and prescriptions for reasons of data protection. Since it is a historical cohort study, randomization of patients was not possible. This is a fundamental limitation of any secondary data analysis. However, this fact was taken into account by correcting existing differences in patient characteristics in the two treatment regimens as well as their possible impacts on the target variable using accepted adjustment methods. Another limitation is that this study only examined general medical practices and diabetologic practices. In Germany, many children are monitored and treated at specialized outpatient clinics of university hospitals. These outpatient clinics are not covered by the Disease Analyzer database. Confounding by indication may have had more of an impact on the results than the underlying basal insulin used. Similarly, the type of bolus insulin used has not been accounted for in the analysis and may have had an impact on the results. The further limitation regards available variables. While adjustment has been performed based on some of the important parameters, biochemical profiles are not known, neither are the levels of comorbidity in each group, all of which may have affected the outcomes. Finally, as more subjects in the glargine arm are treated by specialists, they may well have initially been sicker but then better managed under specialist care. Despite their limitations, database analyses provide important insights into the real-life situation, offering valuable information to decision makers that is complementary to randomized controlled studies.28
Glossary
- (ATC)
anatomical therapeutic chemical
- (BMI)
body mass index
- (CUA)
cost–utility analysis
- (HbA1c)
hemoglobin A1c
- (ICT)
intensified conventional therapy
- (NPH)
neutral protamine Hagedorn
- (QALY)
quality-adjusted life year
- (T1DM)
type 1 diabetes mellitus
Funding:
This work was supported by a grant from Sanofi.
Disclosures:
Karel Kostev is an employee of IMS Health, which has received a grant from Sanofi to conduct the current database study. Franz-Werner Dippel is an employee of Sanofi.
References:
- 1.International Diabetes Federation IDF diabetes atlas. 2013. 5th ed. Accessed March 15.
- 2.Scherbaum WA, Kerner W. Evidenzbasierte Leitlinie der DDG – Therapie des Diabetes mellitus Typ 1. 2013. http://www.medical-tribune.de/uploads/media/Leitlinie_Therapie_des_Diabetes_mellitus_Typ_1.pdf . Accessed March 28.
- 3.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1:168–188. [Google Scholar]
- 5.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329(5):304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 8.Köster I, von Ferber L, Ihle P, Schubert I, Hauner H. The cost burden of diabetes mellitus: the evidence from Germany--the CoDiM study. Diabetologia. 2006;49(7):1498–1504. doi: 10.1007/s00125-006-0277-5. [DOI] [PubMed] [Google Scholar]
- 9.Köster I, Huppertz E, Hauner H, Schubert I. Direct costs of diabetes mellitus in Germany – CoDiM 2000–2007. Exp Clin Endocrinol Diabetes. 2011;119(6):377–385. doi: 10.1055/s-0030-1269847. [DOI] [PubMed] [Google Scholar]
- 10.Bolli GB, Songini M, Trovati M, Del Prato S, Ghirlanda G, Cordera R, Trevisan R, Riccardi G, Noacco C. Lower fasting blood glucose, glucose variability and nocturnal hypoglycaemia with glargine vs NPH basal insulin in subjects with type 1 diabetes. Nutr Metab Cardiovasc Dis. 2009;19(8):571–579. doi: 10.1016/j.numecd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Mullins P, Sharplin P, Yki-Jarvinen H, Riddle MC, Haring HU. Negative Binomial Meta-Regression Analysis of Combined Glycosylated Hemoglobin and Hypoglycemia Outcomes Across Eleven Phase III and IV Studies of Insulin Glargine Compared with neutral Protamine Hagedorn Insulin in Type 1 and Type 2 Diabetes Mellitus. Clin Ther. 2007;29(8):1607–1619. doi: 10.1016/j.clinthera.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Manini R, Forlani G, Moscatiello S, Zannoni C, Marzocchi R, Marchesini G. Insulin glargine improves glycemic control and health-related quality of life in type 1 diabetes. Nutr Metab Cardiovasc Dis. 2007;17(7):493–498. doi: 10.1016/j.numecd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Ashwell SG, Amiel SA, Bilous RW, Dashora U, Heller SR, Hepburn DA, Shutler SD, Stephens JW, Home PD. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with type 1 diabetes. Diabet Med. 2006;23(3):285–292. doi: 10.1111/j.1464-5491.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern Med J. 2005;35(9):536–542. doi: 10.1111/j.1445-5994.2005.00902.x. [DOI] [PubMed] [Google Scholar]
- 15.Hagenmeyer EG, Koltermann KC, Dippel FW, Schädlich PK. Health economic evaluations comparing insulin glargine with NPH insulin in patients with type 1 diabetes: a systematic review. Cost Eff Resour Alloc. 2011;9(1):15. doi: 10.1186/1478-7547-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfohl M, Schädlich PK, Dippel FW, Koltermann KC. Health economic evaluation of insulin glargine vs NPH insulin in intensified conventional therapy for type 1 diabetes in Germany. J Med Econ. 2012;15(Suppl 2):14–27. doi: 10.3111/13696998.2012.713879. [DOI] [PubMed] [Google Scholar]
- 17.Becher H, Kostev K, Schröder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):617–626. doi: 10.5414/cpp47617. [DOI] [PubMed] [Google Scholar]
- 18.Pfohl M, Dippel FW, Kostev K, Fuchs S, Kotowa W. Basal supported oral therapy with insulin glargine results in longer persistence and lower costs compared with insulin detemir in type 2 diabetics in Germany. Health Outcomes Res Med. 2011;2(1):e39–e50. [Google Scholar]
- 19.Icks A, Haastert B, Giani G, Rathmann W. Incremental prescription and drug costs during the years preceding diabetes diagnosis in primary care practices in Germany. Exp Clin Endocrinol Diabetes. 2006;114(7):348–355. doi: 10.1055/s-2006-924261. [DOI] [PubMed] [Google Scholar]
- 20.Deutsche Diabetes Union. Deutscher Gesundheitsbericht Diabetes 2009. Mainz: Kirchheim and Co. GmbH; 2008. [Google Scholar]
- 21.Brändle M, Azoulay M, Greiner R. Presented at: ADA Scientific Sessions. New Orleans, LA: 2009. Cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 1 and type 2 diabetes in Switzerland; pp. A408–A409. [Google Scholar]
- 22.Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ. 2009;180(4):400–407. doi: 10.1503/cmaj.081180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. Pharmacoeconomics. 2007;25(3):253–266. doi: 10.2165/00019053-200725030-00007. [DOI] [PubMed] [Google Scholar]
- 24.McEwan P, Poole CD, Tetlow T, Holmes P, Currie CJ. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 1 diabetes in the UK. Curr Med Res Opin. 2007;23(Suppl 1):S7–S19. [Google Scholar]
- 25.Warren E, Weatherley-Jones E, Chilcott J, Beverley C. ScHARR Rapid Reviews Group. The clinical and cost-effectiveness of long-acting insulin analogue, insulin glargine. Assessment Report. School of Health and Related Research: University of Sheffield; 2002. [Google Scholar]
- 26.Warren E, Weatherley-Jones E, Chilcott J, Beverley C. Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine. Health Technol Assess. 2004;8(45):1–57. doi: 10.3310/hta8450. [DOI] [PubMed] [Google Scholar]
- 27.Culyer A, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, Sculpher M, Brazier J. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. J Health Serv Res Policy. 2007;12(1):56–58. doi: 10.1258/135581907779497567. [DOI] [PubMed] [Google Scholar]
- 28.Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122(2):114–120. doi: 10.1016/j.amjmed.2008.09.030. [DOI] [PubMed] [Google Scholar]


