Abstract
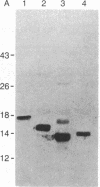
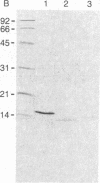
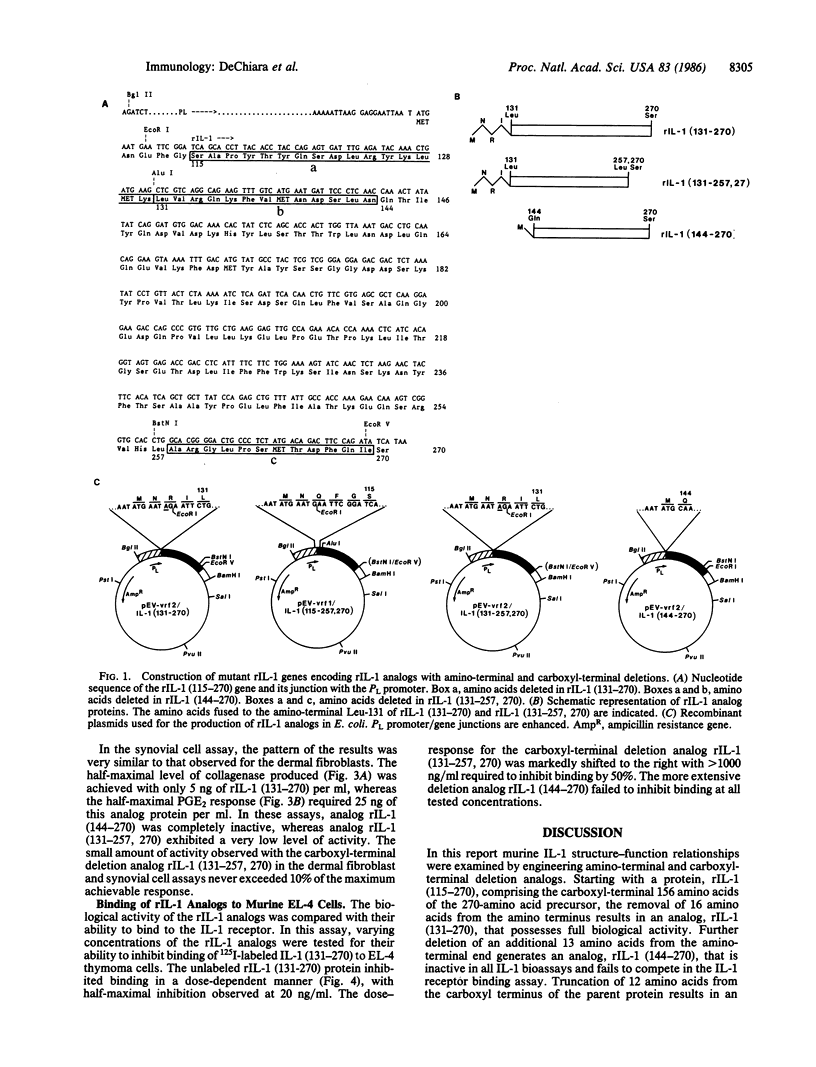
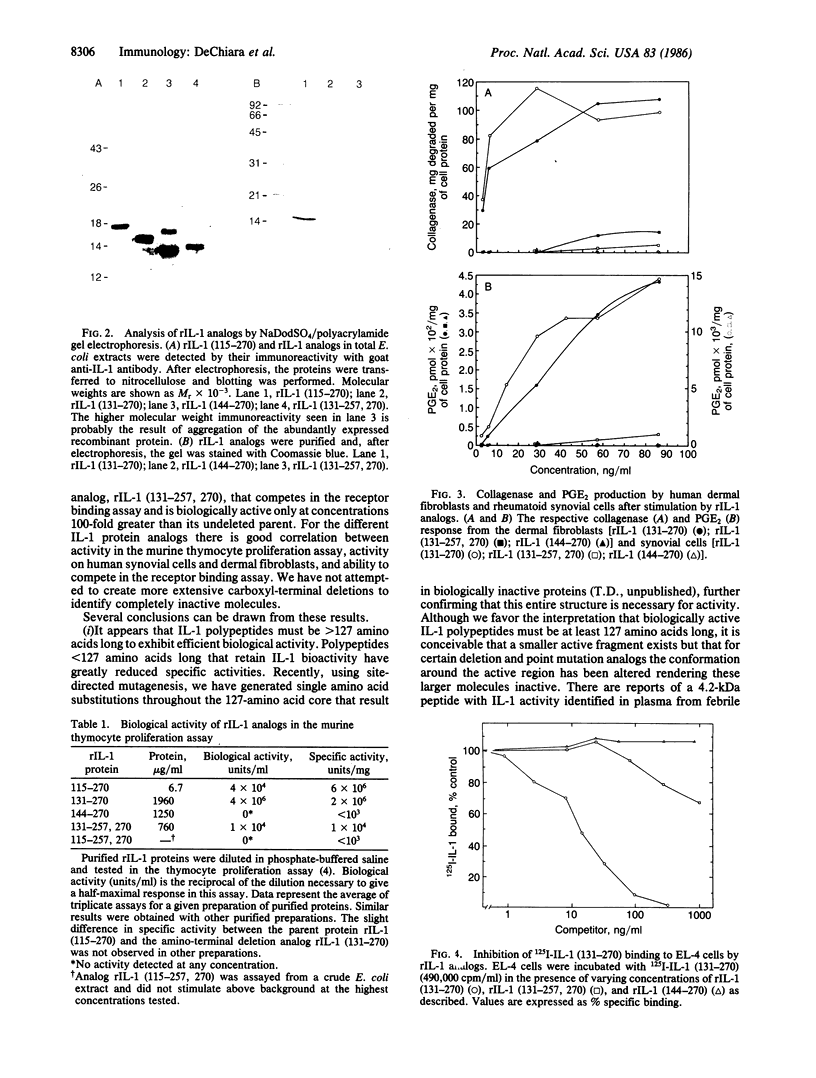
Murine interleukin 1 (IL-1) is initially synthesized as a 270-amino acid precursor protein. Guided by amino-terminal end sequence analyses of mouse macrophage-derived IL-1, it was shown that expression of the carboxyl-terminal 156 amino acids (i.e., amino acids 115-270) of this precursor in Escherichia coli yields biologically active recombinant IL-1 (rIL-1) protein. To answer questions about precursor processing and the size of the smallest biologically active IL-1 fragment, we have engineered deletions of the rIL-1 (115-270) gene to encode two amino-terminal deletion analogs, rIL-1 (131-270) and rIL-1 (144-270), and a carboxyl-terminal deletion analog, rIL-1 (131-257, 270). The analogs were produced in E. coli, purified to homogeneity, and assayed for biological activity on murine thymocytes, human rheumatoid synovial cells, and human dermal fibroblasts and for their ability to bind to IL-1 receptors on murine EL-4 thymoma cells. The amino-terminal deletion analog rIL-1 (131-270) possessed a specific activity in the murine thymocyte proliferation assay equivalent to that of the 115-270 parent protein and exhibited significant biological activity in stimulating the production of collagenase and prostaglandin E2 by synovial cells and fibroblasts. The more extensive amino-terminal deletion analog rIL-1 (144-270) was inactive in all biological assays and failed to compete in the receptor binding assay. The carboxyl-terminal deletion analog rIL-1 (131-257, 270) competed less efficiently (by a factor of 100) in the receptor binding assay, retained weak biological activity on synovial cells and fibroblasts, and only demonstrated full intrinsic activity in the thymocyte proliferation assay when 100-200 times more protein was assayed. These results suggest that biologically active murine IL-1 polypeptides are at least 127 amino acids long and are derived from the carboxyl terminus of the 270-amino acid precursor. Furthermore, it appears that the integrity of the carboxyl terminus of the 270-amino acid precursor is important for activity but that different amino termini can be utilized to generate molecules with equivalent specific activities. This amino-terminal end flexibility supports a processing model for IL-1 maturation that partially explains IL-1 polypeptide heterogeneity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Clowes G. H., Jr, Gordon A. H., Saravis C. A., Wolff S. M. Cleavage of human interleukin 1: isolation of a peptide fragment from plasma of febrile humans and activated monocytes. J Immunol. 1984 Sep;133(3):1332–1338. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Lomedico P. T., Mizel S. B. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985 Jan;134(1):343–349. [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Stern A. S., Hellmann C. P., Vitek M. P., DeChiara T. M., Benjamin W. R., Collier K. J., Dukovich M., Familletti P. C. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [PubMed] [Google Scholar]
- Kilian P. L., Kaffka K. L., Stern A. S., Woehle D., Benjamin W. R., Dechiara T. M., Gubler U., Farrar J. J., Mizel S. B., Lomedico P. T. Interleukin 1 alpha and interleukin 1 beta bind to the same receptor on T cells. J Immunol. 1986 Jun 15;136(12):4509–4514. [PubMed] [Google Scholar]
- Lacal J. C., Santos E., Notario V., Barbacid M., Yamazaki S., Kung H., Seamans C., McAndrew S., Crowl R. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5305–5309. doi: 10.1073/pnas.81.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dukovich M., Rothstein J. Preparation of goat antibodies against interleukin 1: use of an immunoadsorbent to purify interleukin 1. J Immunol. 1983 Oct;131(4):1834–1837. [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Opdenakker G., Billiau A., De Somer P., Van Beeumen J. Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature. 1985 Mar 21;314(6008):266–268. doi: 10.1038/314266a0. [DOI] [PubMed] [Google Scholar]