Abstract
Purpose
Synergy is observed with the combination of capecitabine and docetaxel due to docetaxel mediated up-regulation of thymidine phosphorylase. A phase II trial was performed with the combination for metastatic, castrate resistant prostate cancer.
Materials and Methods
Eligible patients had metastatic, castrate resistant prostate cancer, no prior chemotherapy for metastatic disease and normal organ function. Docetaxel (36 mg/m2 per week intravenously) on days 1, 8 and 15, and capecitabine (1,250 mg/m2 per day in 2 divided doses) on days 5 to 18 were administered in 28-day cycles. The response was assessed every 2 cycles. Biomarker correlative studies were performed on blood dihydropyrimidine dehydrogenase, and the thymidine phosphorylase-to-dihydropyrimidine dehydrogenase and thymidine synthase-to-dihydropyrimidine dehydrogenase ratios in available prostate tumor tissue.
Results
A total of 30 patients with a median age of 69 years were enrolled in the study. We noted bone pain in 21 patients (70%), Gleason score 8 or higher in 18 (60%), measurable disease progression in 9, bone scan progression in 18 and prostate specific antigen progression in 22. Grade 3 or 4 neutropenia was seen in 3 patients and grade 3 hand-foot syndrome was found in 2. No treatment related deaths occurred. A prostate specific antigen response of 50% or greater decrease was observed in 22 patients (73%), of whom 9 (30%) had 90% or greater decrease. A partial response was noted in 5 of 9 patients (56%) with measurable disease. Median time to progression was 6.7 months (90% CI 4.2–7.7) and median overall survival was 22.0 months (90% CI 18.4–25.3).
Conclusions
The combination was well tolerated and it demonstrated favorable response rates with durable remission and survival outcomes.
Keywords: prostate, prostatic neoplasms, neoplasm metastasis, capecitabine, docetaxel
Docetaxel every 3 weeks with daily prednisone is the current standard treatment for metastatic CRPC based on randomized trial evidence.1,2 There was a modest median survival benefit of 3 months with the docetaxel and prednisone regimen compared to that of mitoxantrone and prednisone therapy. To improve the survival outcome in patients with metastatic prostate cancer a number of novel agents in combination with docetaxel are under evaluation. Capecitabine has demonstrated synergy with taxane therapy in vitro and in vivo.3 Capecitabine is an oral fluoropyrimidine that is selectively converted to 5-flourouracil by the enzyme TP in tumor tissue.4 Touffet et al reported higher TP activity in adenomatous and cancerous prostate tissue compared to that in normal healthy prostate tissue.5 TP has also been expressed in stromal cells surrounding prostate cancer cells at a frequency of 80% to 85%. It has shown mitogenic and angiogenic effects,6 and TP over expression directly correlates with increasing microvessel density and a poor prognosis.7 Capecitabine is metabolized by the enzyme DPD and a positive correlation between the efficacy of capecitabine and the ratio of TP to DPD has been established in multiple human cancer xenografts, including prostate cancer.8 The efficacy and the therapeutic index of capecitabine could potentially be enhanced by increasing TP activity in tumors. TS expression also has predictive value and low TS expression is predictive of a higher likelihood of a response to fluoropyrimidine therapy.9 Sawada et al noted that docetaxel induced increased TP activity in tumors.3 In this model enzyme activity increased 4 days after treatment with docetaxel and remained increased for 10 days.10 Thus, docetaxel increases TP levels and induces a favorable TP/DPD ratio in tumors, making them increasingly sensitive to capecitabine.
Phase I studies of capecitabine and weekly docetaxel were performed.11 The combination was safe and tolerable with a predominant dose limiting toxicity of hand-foot syndrome. Clinical activity of the combination demonstrated significant improvement in response and OS rates compared with those of single agent docetaxel in a randomized trial performed in pretreated patients with breast cancer.12 The rationale for evaluating the combination of capecitabine and docetaxel was based on 1) the high efficacy of docetaxel in prostate cancer, 2) documented increased activity of TP in prostate cancer tissue, providing the basis for increased 5-flourouracil formation with capecitabine use, and 3) TP induction by docetaxel, thus increasing the TP/DPD ratio in tumors, suggesting the possibility of enhanced activity of this combination.
PATIENTS AND METHODS
Eligibility
Study eligibility criteria included histologically confirmed prostate adenocarcinoma with metastasis and objective progression or increasing PSA despite androgen deprivation therapy and antiandrogen withdrawal, when applicable. Patients with progression according to increasing PSA only were required to have at least 2 consecutive increases at a minimum interval of 1 week. A minimum PSA of 5 ng/ml or new areas of bony metastasis on bone scan were required in patients with no measurable disease. There was no minimum PSA requirement in patients with measurable disease. Prior chemotherapy for metastatic disease was not allowed. All patients had to have documented castrate levels of testosterone (50 ng/ml or less). Luteinizing hormone releasing hormone agonist therapy was continued if required to maintain castrate levels of testosterone. Patients on antiandrogens had to be off for a minimum of 4 weeks for flutamide and 6 weeks for bicalutamide or nilutamide. A performance status of 0 to 2 by Zubrod criteria, and normal renal, liver and bone marrow function were required. All patients were required to provide signed informed consent. The protocol and consent were reviewed annually and approved by the Wayne State University institutional review board.
Treatment Plan
All patients received docetaxel (36 mg/m2) on days 1, 8 and 15, administered as a 60-minute infusion with capecitabine (1,250 mg/m2), administered orally and divided into 2 equal doses 12 hours apart on days 5 to 18. Cycles were repeated every 28 days. Prophylactic antiemetics and dexamethasone were administered at the discretion of the treating physician. A minimum prophylactic dose of dexamethasone (8 mg) was required before docetaxel administration. Dose adjustments were made for severe hematological and nonhematological toxicities. A maximum of 2 docetaxel dose level reductions were allowed per patient, that is first to 30 and then to 26 mg/m2. Similarly a maximum of 2 capecitabine dose reductions was allowed per patient, that is first to 1,000 and then to 800 mg/m2 daily. Patients were continued on treatment until progression, intolerable toxicity or the completion of 2 therapy cycles after the first documentation of a complete response. Treatment was discontinued when there was evidence of disease progression, unacceptable and severe grade 3 or 4 toxicity, a 4-week or greater delay in treatment, withdrawal from study at any time for any reason, or early study closure based on an unexpected high rate of toxicity or disease progression. All patients were followed until death.
Correlative Tests
Pretreatment tissue samples from blocks of prior biopsies or surgery were obtained and used to prepare slides for immunohistochemistry. Pretreatment blood samples for DPD levels were drawn on day 1 of cycle 1. Testing was performed elsewhere for DPD levels in peripheral blood, and TP, TS and DPD levels in tissue using fluorescent antibodies.
Peripheral Blood DPD and Tissue TP, TS and DPD
Mononuclear cells were separated on Ficoll® and slides were prepared using a Hettich® Universal™ 16 Cytospin Centrifuge. They were air dried and kept at −20C until stained.14 Two established breast cancer cell lines (MDA-MB-231 and ZR-75-1) and 1 bladder cancer cell line (T-24) in which DPD activity is well characterized were chosen as controls. These cell lines represent the extreme low (T-24 and MDA-MB-231) and the extreme high (ZR-75-1) TP/DPD ratios, as determined by enzyme activity measurements. Cells were harvested from cultures, washed and suspended in PBS at 105 cells per ml. Cell suspension (1 ml) was deposited on silanized slides using a cytocentrifuge. Slides were processed, prepared, air dried and kept at −20C until stained.
The stains that were used included primary antibody, followed by secondary antibody and PI. For primary labeling the antibodies used were antihuman-TP (mouse monoclonal 1C6–203, Roche, Nutley, New Jersey), mouse monoclonal anti human-TS (QED Biosciences, San Diego, California) and rat monoclonal anti human DPD (2H9-1b Roche). DPD antibody was used for tissue preparations as well as for peripheral blood cells. Appropriate species matched antibody served as an isotype control. Secondary antibody was goat antirat IgG (A11006 Invitrogen™) labeled with AlexaFluor® 488 (Molecular Probes, Eugene, Oregon).
After blocking nonspecific binding sites with Superblock (ScyTek Laboratories, Logan, Utah) slides were incubated at room temperature for 90 minutes with an optimized concentration of primary antibody (the 1:500 dilution of a stock concentration was 1 mg/ml for anti-DPD). The slides were washed 3 times with PBS, followed by 30 minutes of incubation with secondary antibodies, also at room temperature. To stain nuclear DNA the slides were washed 3 times with PBS and incubated for 10 minutes at room temperature with 0.002 µg/ml PI and 0.34 µg/ml ribonuclease A in PBS. Stained slides were analyzed using a Laser Scanning Computer II (CompuCyte, Cambridge, Massachusetts). The nuclei of cells were contoured based on the PI fluorescence detected in the red channel. Green fluorescence of the Alex-aFluor 488 DPD antibody in a peripheral contour of 10 pixels around the nucleus was used to estimate cytoplasmic DPD.
Evaluation
Toxicity was monitored every 4 weeks except for the complete blood count, which was determined weekly on days of docetaxel therapy. Toxicity was categorized according to the National Cancer Institute Common Toxicity Criteria, version 2.0. Serious adverse events were reported and monitored according to institution guidelines. All patients registered on the protocol who starting therapy with the protocol medication were considered toxicity evaluable.
All patients on the protocol who completed a minimum of 1 cycle of therapy followed by clinical, radiological or PSA assessment of disease status were considered response evaluable. Patients underwent imaging for tumor assessment at baseline and every 2 cycles thereafter. PSA level was measured on day 1 of each cycle. Response Evaluation Criteria in Solid Tumors criteria were used to categorize responses in cases of measurable disease.13 Prostate Specific Antigen Working Group criteria were used to measure the PSA response.14 A complete clinical PSA response was defined as the disappearance of all measurable and evaluable disease for a minimum of 4 weeks, no new lesions and normalization of PSA (4 ng/ml or less). If a PSA complete response was achieved, progression was defined as 2 values at least 2 weeks apart showing a 50% or greater increase in PSA over nadir with a minimum absolute increase in PSA of 5 ng/ml. A PSA partial response in patients with metastatic bone disease and PSA only progression was defined as a 50% or greater reduction in PSA sustained for 3 successive determinations performed at least 2 weeks apart. PSA progression was defined as 2 values at least 2 weeks apart with a 25% or greater increase over nadir PSA with a minimum absolute PSA increase of 5 ng/ml. Patients who did not meet these response or progression criteria were considered to have stable disease.
Statistical Methods
This single institution, phase II trial was planned with a Simon 2-stage design.15 The primary study objective was to evaluate the rate of PSA response (complete plus partial) of capecitabine and docetaxel in patients with metastatic CRPC. We wished to distinguish certain regions of the true unknown response rate, that is at least 0.65 vs 0.40 at most. The 2-stage design called for a maximum of 28 response evaluable patients, including 15 in stage 1 and 13 in stage 2. The design had a type I error of 0.042 and power of 0.812. After accruing 30 patients there were 29 response evaluable patients. With 22 PSA responders observed we concluded that the sample PSA response proportion better supported that the true unknown PSA response rate was at least 0.65.
Exact, minimum width 90% CIs for response and toxicity rates were calculated using the Casella method, as implemented in StatXact® software. TTP was measured from the treatment start date to the date of documented progressive disease (PSA or measurable disease). OS was measured from the treatment start date to the date of death from any cause. Standard Kaplan-Meier estimates of the censored TTP and OS distributions were calculated. Due to small sample sizes survival statistics, eg the median, were estimated more conservatively using linear interpolation among successive event times on the Kaplan-Meier curves. Correlative biomarker measures (enzyme variables) were compared by the PSA partial response status (yes/no) using the Wilcoxon rank sum test. Censored TTP and censored OS durations were compared by median dichotomized enzyme variables using the log rank test.
RESULTS
A total of 30 patients with a median age of 69 years were enrolled in the study. Median pretherapy PSA was 110 ng/ml (range 1.2 to 3,716.9). Table 1 lists patient characteristics. Of the patients 21 (70%) had bone pain and a Gleason score of 8 or greater was noted in 18 (60%). Most patients (67% with a doubling time of 2 months or less) had a rapid PSA doubling time before therapy. This has now been shown to be a predictor of poorer survival outcome based on TAX327 data.16 Grade 3 or 4 neutropenia was seen in 3 patients and grade 3 hand-foot syndrome was noted in 2 (table 2). No treatment related deaths occurred.
Table 1.
Patient characteristics
| Characteristic | ||
|---|---|---|
| No. pts | 30 | |
| Median age (range) | 69 | (47–80) |
| Median ng/ml pretherapy PSA (range) | 110 (1.2–3,716.9) | |
| No. performance status (%): | ||
| 0 | 8 | (27) |
| 1 | 15 | (50) |
| 2 | 7 | (23) |
| No. prostatectomy (%): | ||
| Yes | 11 | (37) |
| No | 19 | (63) |
| No. bone pain (%): | ||
| Yes | 21 | (70) |
| No | 9 | (30) |
| No. Gleason score (%): | ||
| 6 | 2 | (7) |
| 7 | 9 | (30) |
| 8–10 | 18 | (60) |
| Unknown | 1 | (3) |
| No. race (%): | ||
| White | 21 | (70) |
| Black | 9 | (30) |
| No. mos pretherapy PSA doubling time (%): | ||
| 1 or Less | 8 | (26) |
| 1–2 Mos | 13 | (44) |
| 2–4 Mos | 7 | (23) |
| Greater than 4 | 2 | (7) |
| No. prior adjuvant/neoadjuvant chemotherapy (%): | ||
| Docetaxel | 2 | (7) |
| None | 0 | (93) |
| No. progression/metastasis type (%): | ||
| Measurable disease | 9 | (30) |
| Bone metastasis progression | 18 | (60) |
| PSA progression | 22 | (73) |
Table 2.
Toxicity in 30 treated patients
| Toxicity | No. Grade 1 | No. Grade 2 | No. Grade 3 | No. Grade 4 |
|---|---|---|---|---|
| Hand-foot syndrome |
7 | 6 | 2 | 0 |
| Fatigue | 4 | 13 | 3 | 0 |
| Neutropenia | 3 | 7 | 2 | 1 |
| Anemia | 8 | 8 | 3 | 0 |
| Thrombocytopenia | 5 | 0 | 0 | 0 |
| Infection | 1 | 6 | 0 | 0 |
| Hyperlacrimation | 7 | 3 | 0 | 0 |
| Neuropathy | 5 | 3 | 0 | 0 |
| Nausea | 10 | 7 | 4 | 0 |
| Emesis | 9 | 2 | 4 | 0 |
| Diarrhea | 6 | 6 | 4 | 0 |
| Edema | 3 | 2 | 0 | 0 |
| Pleural effusion | 0 | 0 | 2 | 0 |
| Dehydration | 1 | 8 | 1 | 0 |
| Taste changes | 9 | 2 | 0 | 0 |
| Nail changes | 4 | 4 | 0 | 0 |
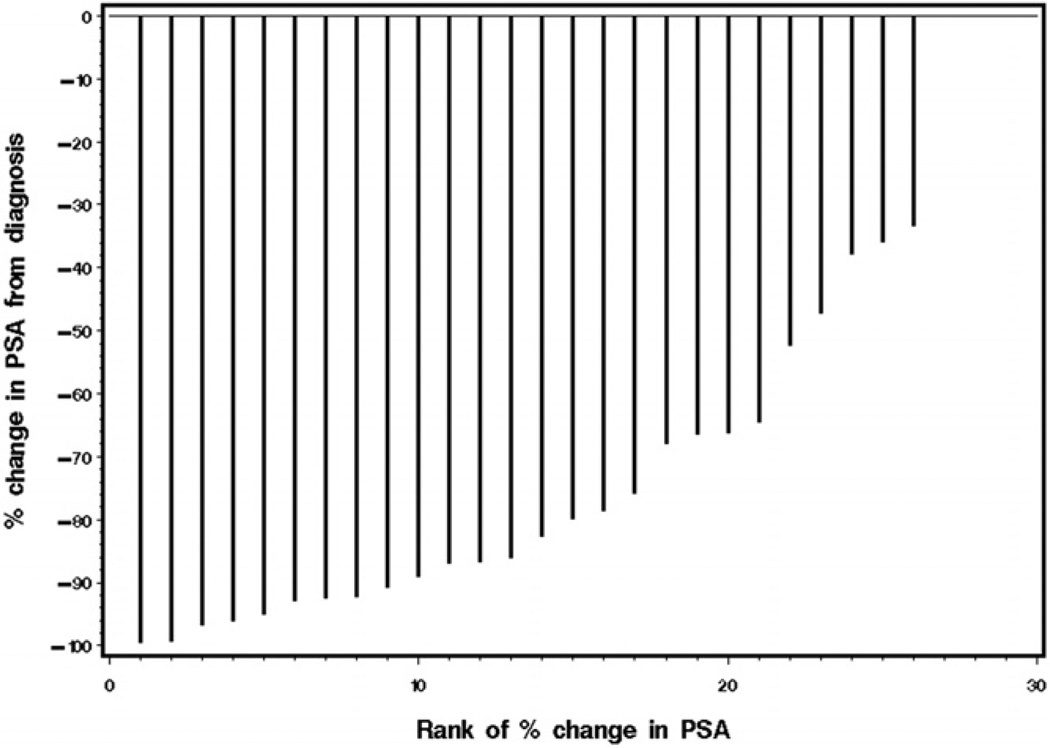
A total of 29 patients were deemed response evaluable. One patient elected to come off study even before starting oral capecitabine in cycle 1. A PSA response (50% or greater decrease) was noted in 22 patients (76%) with a 90% or greater PSA decrease in 9 (31%). A partial response was noted in 5 of the 9 patients (56%) with measurable disease. Figure 1 shows PSA response data, that is the distribution of the percent change from baseline.
Figure 1.
Waterfall plot demonstrates PSA response data, that is percent change from baseline, in all 30 patients (vertical lines). Four patients had no PSA change with 0% change. Rank 1, most negative percent change in PSA.
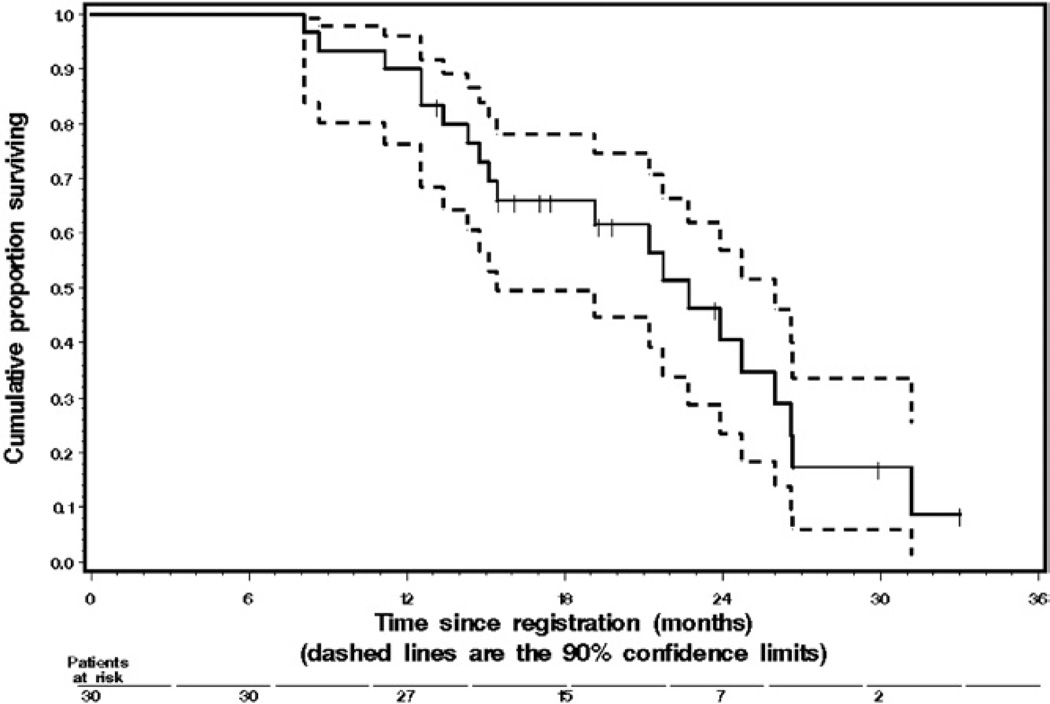
Median followup for OS in the 10 survivors was 18.3 months. The single patient who was still progression-free had been followed for 23.0 months. Median TTP was 6.7 months (90% CI 4.2–7.7) and median OS was 22.0 months (90% CI 18.4–25.3). One-year progression-free and OS rates were 12% and 90%, respectively (table 3 and fig. 2). When applied to our patients, the Halabi nomogram17 yielded a total of 146 to 147 points. The nomogram predicted a median OS of 14 months and a 1-year OS rate of 58%.
Table 3.
Response rate, TTP and OS summary statistics
| End Point | No. Pts | No. Events | Point Estimate |
90% | CI |
|---|---|---|---|---|---|
| Response: | |||||
| Measurable disease | 9 | 5 | 56% | 25 | 79 |
| 50% or Greater PSA decrease | 29 | 22 | 76% | 61 | 87 |
| 90% or Greater PSA decrease | 29 | 9 | 31% | 19 | 46 |
| TTP: | 30 | 29 | |||
| Median mos | 6.7 | 4.2 | 7.7 | ||
| % 6-Mo rate | 57 | 42 | 72 | ||
| % 1-Yr rate | 12 | 2 | 22 | ||
| OS rate: | 30 | 20 | |||
| Median mos | 22.0 | 18.4 | 25.3 | ||
| % 1 Yr | 90 | 81 | 99 | ||
| % 2 Yr | 40 | 22 | 57 |
Figure 2.
Kaplan-Meier graph shows 30 patients with metastatic CRPC treated with docetaxel and capecitabine. Tick marks represent 10 censored survivors. Dashed lines indicate 90% CI at successive time points.
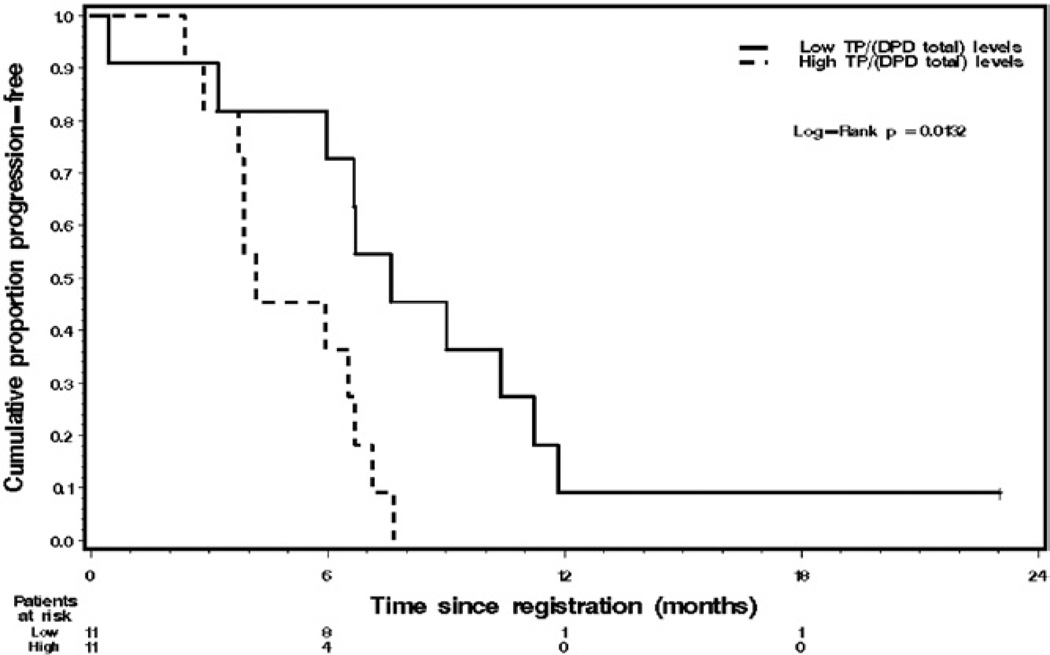
Tumor tissue was available from 22 response evaluable patients for TS, TP and DPD level testing. Samples for blood DPD analysis were available in 23 patients. Table 4 lists summary statistics on all 5 enzyme variables in all patients and Table 5 lists summary statistics on all 5 enzyme variables by PSA partial response status (yes/no). None of these 5 enzyme variables differed significantly in PSA responders vs nonresponders (each p <0.16). However, median TS was more than 10-fold higher in PSA responders compared to that in nonresponders (37.59 vs 3.45 fluorescence intensity units). Also, patients with a low tumor tissue TP/DPD ratio, ie less than 0.58, had statistically significantly longer TTP than patients with high levels of that ratio (p = 0.0132, fig. 3). None of the other 5 median dichotomized enzyme variables were significantly associated with TTP or OS (table 6).
Table 4.
Summary statistics on correlative measures (enzyme variables)
| Enzyme | Units* | No. Pts | Median | Mean | SD | Minimum | Max |
|---|---|---|---|---|---|---|---|
| Tumor tissue: | |||||||
| TS | 107 | 22 | 21.71 | 109.10 | 191.64 | 0.37 | 664.77 |
| TP | 109 | 22 | 3.80 | 7.81 | 8.02 | 0.00 | 24.45 |
| DPD | 108 | 22 | 105.82 | 131.21 | 115.95 | 5.11 | 325.82 |
| TP/DPD | Not applicable | 22 | 0.58 | 0.57 | 0.33 | 0.00 | 1.25 |
| Blood (DPD) | 106 | 23 | 0.80 | 0.83 | 0.27 | 0.00 | 1.41 |
Units are rescaled as shown (divided by a power of 10) from the original units of fluorescence intensity.
Table 5.
Summary statistics on correlative enzymes by PSA response status
| Enzyme | Units* | No. Pts | Median | Mean | SD | Range |
|---|---|---|---|---|---|---|
| Tumor tissue | ||||||
| TS: | ||||||
| PSA response | 107 | 15 | 37.59 | 128.62 | 209.49 | 1.24–664.77 |
| No PSA response | 107 | 5 | 3.45 | 84.03 | 182.46 | 0.37–410.42 |
| TP: | ||||||
| PSA response | 109 | 15 | 2.78 | 7.58 | 8.63 | 0–24.45 |
| No PSA response | 109 | 5 | 6.70 | 8.43 | 6.54 | 1.18–17.25 |
| DPD: | ||||||
| PSA response | 108 | 15 | 123.22 | 133.53 | 120.87 | 5.96–325.82 |
| No PSA response | 108 | 5 | 101.26 | 121.04 | 101.38 | 21.97–291.64 |
| TP/DPD: | ||||||
| PSA response | 100 | 15 | 0.58 | 0.55 | 0.32 | 0–1.16 |
| No PSA response | 100 | 5 | 0.59 | 0.71 | 0.34 | 0.36–1.25 |
| Blood | ||||||
| DPD: | ||||||
| PSA response | 106 | 16 | 0.79 | 0.83 | 0.18 | 0.63–1.36 |
| No PSA response | 106 | 6 | 0.87 | 0.83 | 0.48 | 0–1.41 |
Units are rescaled as shown (divided by a power of 10) from the original units of fluorescence intensity.
Figure 3.
Kaplan-Meier graph reveals TTP in 22 patients with metastatic AIPC by median dichotomized tumor tissue TP/DPD ratio. Tick mark represents 1 censored survivor who was progression free. Low, 0.58 or less. High, greater than 0.58. TTP differed significantly between these 2 subgroups (log rank test p = 0.0132).
Table 6.
TTP and OS by median dichotomized enzyme variables
| End Point + Enzyme Variable (dichotomized level) |
No. Pts |
No. Events |
End Point | Point Estimate |
90% CI |
|---|---|---|---|---|---|
| TTP | |||||
| Tumor tissue TS: | |||||
| Low (21.71 or less) | 11 | 10 | Median 6-Mo rate |
5.1 Mos 45% |
3.0–8.1 21–70 |
| High (greater than 21.71) | 11 | 11 | Median 6-Mo rate |
6.6 Mos 63% |
5.9–7.1 38–88 |
| Tumor tissue TP: | |||||
| Low (3.8 or less) | 11 | 10 | Median 6-Mo rate |
6.7 Mos 63% |
6.0–7.7 39–88 |
| High (greater than 3.8) | 11 | 11 | Median 6-Mo rate |
5.1 Mos 45% |
3.0–5.8 20–69 |
| Tumor tissue DPD: | |||||
| Low (105.82 or less) | 11 | 10 | Median 6-Mo rate |
5.1 Mos 45% |
3.0–6.2 21–70 |
| High (greater than 105.82) | 11 | 11 | Median 6-Mo rate |
6.6 Mos 63% |
5.9–7.5 38–88 |
| Tumor tissue TP/DPD: | |||||
| Low (0.58 or less) | 11 | 10 | Median 6-Mo rate |
7.1 Mos 72% |
6.2–9.9 49–96 |
| High (greater than 0.58) | 11 | 11 | Median 6-Mo rate |
4.0 Mos 36% |
3.7–6.4 12–59 |
| Blood (serum) DPD: | |||||
| Low (0.79 or less) | 11 | 11 | Median 6-Mo rate |
6.6 Mos 63% |
5.9–11.2 38–88 |
| High (greater than 0.79) | 12 | 12 | Median6 Mo rate |
6.0 Mos 50% |
3.3–7.1 26–74 |
| OS | |||||
| Tumor tissue TS: | |||||
| Low (21.71 or less) | 11 | 8 | Median 12-Mo rate |
16.7 Mos 76% |
11.8–22.2 54–98 |
| High (greater than 21.71) | 11 | 9 | Median 12-Mo rate |
15.8 Mos 83% |
8.0–21.2 64–100 |
| Tumor tissue TP: | |||||
| Low (3.8 or less) | 11 | 7 | Median 12-Mo rate |
18.0 Mos 89% |
14.0–24.9 68–100 |
| High (greater than 3.8) | 11 | 10 | Median 12-Mo rate |
13.8 Mos 82% |
12.1–26.1 63–100 |
| Tumor tissue DPD: | |||||
| Low (105.82 or less) | 11 | 9 | Median 12-Mo rate |
17.0 Mos 80% |
12.9–23.8 58–100 |
| High (greater than 105.82) | 11 | 8 | Median 12-Mo rate |
15.4 Mos 91% |
7.5–25.3 77–100 |
| Tumor tissue TP/DPD: | |||||
| Low (0.58 or less) | 11 | 7 | Median 12-Mo rate |
21.9 Mos 88% |
16.4–23.2 68–100 |
| High (greater than 0.58) | 11 | 10 | Median 12-Mo rate |
14.1 Mos 82% |
11.9–14.8 63–100 |
| Blood (serum) DPD: | |||||
| Low (0.79 or less) | 11 | 5 | Median 12-Mo rate |
23.7 Mos 83% |
19.7–* 64–100 |
| High (greater than 0.79) | 12 | 11 | Median 12-Mo rate |
15.1 Mos 78% |
14.2–25.8 58–99 |
Not available due to censoring pattern.
DISCUSSION
This phase II trial evaluated the efficacy and toxicity of the combination of capecitabine and docetaxel for metastatic prostate cancer. Along with PSA response we also noted a favorable time to progression and OS outcomes. A number of clinical prognostic markers have been established to help predict the prognosis in individuals with metastatic CRPC.17 This is likely to improve the interpretation of phase II trials of docetaxel based therapies for metastatic CRPC. The population in our study had a number of poor risk characteristics, for example two-thirds of the patients had a rapid PSA doubling time of 2 months or less and bone pain at study entry. Despite these adverse prognostic characteristics the regimen demonstrated a high chance of a PSA response and favorable survival outcomes. The observed median survival of 22 months and the 1-year OS rate of 90% far exceeded the predicted median survival of 14 months and the 1-year OS rate of 58% according to the Halabi nomogram17 after adjusting for patient specific prognostic characteristics.
Another 2 phase II trials of the combination of docetaxel and capecitabine for metastatic CRPC have also shown the tolerability and efficacy of this regimen.18,19 A PSA response rate of 41% was noted in the trial with docetaxel every 3 weeks and a 68% rate was noted in the trial using the weekly docetaxel regimen. Despite the encouraging efficacy of the regimen, in view of the small sample size the results cannot be considered conclusive, but rather suggestive and hypothesis generating.
A number of ongoing phase III trials are being done to compare the addition of another agent to the docetaxel and prednisone regimen. In randomized trials of the addition of calcitriol (DN-101) or GVAX to docetaxel further accrual was stopped due to an increased number of deaths in the experimental arm. Better tools for selecting patients for combination therapy are essential using biomarkers or patient specific tumor characteristics. It appears from the correlative studies performed in our phase II trial that an increased tumor tissue TP/DPD ratio, high TP levels and low TS levels are suggestive of shorter TTP. Currently therapeutic trials for prostate cancer tend to be inefficient and largely empirical. All patients with metastatic CRPC are treated with the same regimen despite a wide range of patient and tumor characteristics. The spectrum of response varies from immediate disease progression to the induction of remission lasting many years. Investigating tumor tissue biomarkers such as TP and TS may help identify patients at risk for a poorer outcome and enable us to target a specific subpopulation of patients for flouropyrimidine based combination therapy.
CONCLUSIONS
The combination of docetaxel and capecitabine demonstrated encouraging responses and durable remission in patients with metastatic CRPC in this phase II trial. This could be a stimulus for evaluating this regimen in a larger trial. Future investigations exploring effective tools to individualize therapy are likely to optimize outcome.
ACKNOWLEDGMENTS
Testing was performed at Asterand, Inc., Detroit, Michigan.
Supported by a grant from the Department of Internal Medicine, Wayne State University, Detroit, Michigan, Sanofi-Aventis, Inc. and National Institutes of Health Cancer Center Support Grant CA-22453.
Abbreviations and Acronyms
- CRPC
castrate resistant prostate cancer
- DPD
dihydropyrimidine dehydrogenase
- OS
overall survival
- PBS
phosphate buffered saline
- PI
propidium iodide
- PSA
prostate specific antigen
- TP
thymidine phosphorylase
- TS
thymidylate synthase
- TTP
time to progression
REFERENCES
- 1.Tannock I, DeWit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H, et al. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4:1013. [PubMed] [Google Scholar]
- 4.Schilsky RL. Pharmacology and clinical status of capecitabine. Oncology (Huntingt) 2000;14:1297. [PubMed] [Google Scholar]
- 5.Touffet S, Gayet G, Samperez S, Jouan P. Demonstration of thymidine phosphorylase activity in human healthy adenomatous and cancerous prostate. Bull Cancer. 1992;79:151. [PubMed] [Google Scholar]
- 6.Okada K, Yokoyama K, Okihara K, Ukimura O, Kojima M, Miki T, et al. Immunohistochemical localization of platelet derived endothelial cell growth factor expression and its relation to an-giogenesis in prostate. Urology. 2000;57:376. doi: 10.1016/s0090-4295(00)00907-9. [DOI] [PubMed] [Google Scholar]
- 7.Takebayashi Y, Yamada K, Miyadera K, Sum-izawa T, Furakawa T, Kinoshita F, et al. The activity and expression of thymidine phosphory-lase in human solid tumors. Eur J Cancer. 1996;32A:1227. doi: 10.1016/0959-8049(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa T, Sekiguchi F, Fukase Y, Sawada N, Ishitsuka H. Positive correlation between the efficacy of capecitabine and doxifluridine, and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res. 1998;58:685. [PubMed] [Google Scholar]
- 9.Qiu LX, Tang QY, Bai JL, Qian XP, Li RT, Liu BR, et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384. doi: 10.1002/ijc.23822. [DOI] [PubMed] [Google Scholar]
- 10.Nadella P, Shapiro C, Otterson G, Hauger M, Erdal S, Kraut E. Pharmacobiologically based scheduling of capecitabine and docetaxel results in antitumor activity in resistant human malignancies. J Clin Oncol. 2002;20:2616. doi: 10.1200/JCO.2002.22.030. [DOI] [PubMed] [Google Scholar]
- 11.Pronk LC, Vasey P, Sparreboom A, Reigner B, Planting AS, Gordon RJ, et al. A phase I and pharmacokinetic study of the combination of capecitabine and docetaxel in patients with advanced solid tumors. Br J Cancer. 2000;83:22. doi: 10.1054/bjoc.2000.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 15.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10:1. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 17.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormonerefractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 18.Ferrero JM, Chamorey E, Oudard S, Dides S, Lesbats G, Cavaglione G, et al. Phase II trial evaluating a docetaxel-capecitabine combination as treatment for hormone-refractory prostate cancer. Cancer. 2006;107:738. doi: 10.1002/cncr.22070. [DOI] [PubMed] [Google Scholar]
- 19.Kolodziej M, Neubauer MA, Rousey R, Pluenneke RE, Perrine G, Mull S, et al. Phase II trial of docetaxel/capecitabine in hormone refractory prostate cancer. Clin Genitourin Cancer. 2006;5:155. doi: 10.3816/CGC.2006.n.033. [DOI] [PubMed] [Google Scholar]