Abstract
Background
Forty million children are regularly exposed to environmental tobacco smoke (ETS) each year, increasing their risk for premature death and middle ear and acute respiratory infections. Early life exposure to ETS also is clearly associated with wheezing. However, there is no clear understanding of the influence of ETS on the development of allergic sensitization.
Objective
To determine the association of combined exposure to ETS and indoor allergens on IgE sensitization to aeroallergens in children.
Methods
This case–control study enrolled 116 cases and 121 controls from low-income families from Kansas City, Missouri. The adjusted odds ratio was calculated using a logistic model to assess the association between ETS and allergic sensitization using dust allergen levels as a covariate.
Results
Thirty-six percent of atopic children and 39% of controls were exposed to ETS (P < .05). Unadjusted analyses showed no significant influence of ETS on IgE sensitization to indoor allergens. Logistic regression analyses also showed no significant influence of ETS on sensitization when adjusted for levels of allergens in the home dust and family history of allergic rhinitis.
Conclusion
These data suggest that ETS exposure was not associated with IgE sensitization to indoor allergens, even when home allergen levels were taken into consideration. Further understanding of how components of tobacco smoke influence the immune response is necessary to interpret the disparate findings across studies.
Introduction
Nearly 40 million children are regularly exposed to environmental tobacco smoke (ETS).1 These children have an increased risk for acute respiratory infections and middle ear infections and are more likely to die prematurely.1 A causal relation between ETS exposure and asthma development has been established, supporting the idea that ETS may not be strictly immunosuppressive, but rather capable of skewing the immune system toward an atopic profile.1-3 However, the effect of ETS exposure in childhood on the development of allergic sensitization is unclear.
More than 6.5 million Americans live in homes that have moderate to severe physical problems, including leaky roofs, inadequate guttering, and cracked foundations.4 These poor housing conditions contribute to increased morbidity and mortality among the occupants by increasing the likelihood of infectious disease transmission, accidents, mental health disorders, and asthma exacerbations.4-7 Blacks and families with low incomes are most likely to live in substandard housing, creating a disparity across the population.4 Unfortunately, children at highest risk for living in physically unsafe homes are also those who are most likely to be exposed to ETS.8,9 Thus, potential exists in low-income housing environments for synergistic, deleterious interactions between chemical and microbial exposures. Because targeted home remediation is a feasible strategy for the primary prevention of environment-related health conditions, a better understanding is needed of the interactions between chemical and microbial exposures and their effect on the health of children.
The aim of this study was to determine the association of exposure to ETS and indoor allergens with IgE sensitization to aeroallergens in children. The authors hypothesized that children exposed to ETS and high levels of indoor allergens would be more likely to develop allergic sensitization to aeroallergens than those who were not.
Methods
Study Design and Aims
This retrospective case–control study was performed as a sub-analysis of the Kansas City Safe and Healthy Homes Partnership (KCSHHP). The main goal of the KCSHHP was to determine the clinical impact of environmental home assessment and targeted remediation on children with asthma and rhinoconjunctivitis (unpublished). To achieve the primary aim of this study, an odds ratio of sensitization to indoor allergens when exposed to ETS was calculated. Then, a logistic regression model was used to assess the association between indoor allergen exposure and sensitization. The KCSHHP and the present case–control study were approved by the local institutional review board.
Study Population and Data Collection
Volunteers were recruited from Kansas City through health clinics, schools, newsletters, health fairs, conferences, workshops, neighborhood events, and media broadcast from October 2008 through November 2011. The health clinics included the Allergy/ Asthma/Immunology and Primary Care Clinic at Children’s Mercy Hospital, a tertiary care center in downtown Kansas City, Missouri. Potentially eligible patients contacted the study coordinator directly by telephone. Children included were diagnosed with asthma, chronic respiratory symptoms, chronic allergy symptoms, or other chronic symptoms affected by a home environment; were living in the Kansas City area; were staying at the same home at least 4 nights per week; had lived in the same home for the past 6 months; planned to live in the same home for the next 12 months; and were from families with a total family income lower than 80% of the Kansas City median family income. Eligibility was determined through a questionnaire completed and returned before the clinic visit, which included documentation of the family income from the previous year.
Eligible families were invited to attend 1 clinic visit at the Children’s Mercy Hospital Allergy/Asthma/Immunology Clinic. Each child was accompanied to the visit by a parent or guardian. Written informed permission was obtained from the parent or guardian at that time and assent was obtained from the child when appropriate. Assistance was provided to the parent or guardian in completing a detailed questionnaire at the time of the visit. The questionnaire included medical, family, social, and environmental histories and a detailed review of symptoms. Specifically, the family was asked to self-report the child’s date of birth, gender, race, and asthma status. The family also was asked to declare whether they owned or rented their current home. Current medications were recorded on the questionnaire. Blood samples were drawn for specific IgE testing if separate permission/assent was given by the families. If specific IgE testing was completed within 1 year of study enrollment and available in the hospital electronic medical record, then the medical record was accessed and the specific IgE testing was recorded for study purposes.
At successful completion of the clinic visit, a home visit for comprehensive assessment was scheduled. The home visit was performed by a trained environmental hygienist and a trained asthma educator. While the assessment of the home was performed, the asthma educator provided home safety education and health coaching with an emphasis on interventions that would positively affect asthma. The environmental hygienist collected dust samples directly from the children’s bedrooms with a vacuum or collected dust already in the home vacuum bag.
For this study, subjects were considered atopic and therefore a “case” if their serum was positive for at least 1 tested indoor allergen (IgE >0.35 kU/L). Controls were defined as all other children enrolled in the KCSHHP who underwent or had available specific IgE testing but did not meet criteria for atopy. To determine ETS exposure, the social history from the clinic questionnaire asked if anyone living with the child smoked and if anyone living with the child smoked inside the family’s home. In addition, the number of family members who smoked or smoked inside the house was recorded. ETS exposure was analyzed as a dichotomous variable of having at least 1 family member who smoked or no family member who smoked. A separate analysis was performed in which ETS exposure was defined as having a family member who smoked inside the house (not reported). Family history was defined as having at least 1 parent or 1 sibling with a history of allergic rhinitis.
Specific IgE Analysis
Serum-specific IgE antibodies to common indoor allergens were analyzed using an ImmunoCAP 250 (Phadia, Uppsala, Sweden) according to the manufacturer’s instructions at Children’s Mercy Hospital in a laboratory certified by the Clinical Laboratory Improvement Amendments. IgEs specific to cat, dog, Dermatophagoides farina, German cockroach, Cladosporium herbarum, Aspergillus fumigatus, Penicillium chrysogenum, and Alternaria alternata were quantified. An allergen was considered positive if the IgE level was higher than 0.35 kU/L.
Dust Collection and Analysis
Dust collection protocols and immunoassay analysis protocols have been described previously.10 Dust was collected, transported, and stored according to a modified version of the Department of Housing and Urban Development protocol (http://portal.hud.gov/hudportal/documents/huddoc?id=DOC_12539.pdf). Briefly, dust was collected and transported in dust collection sample bags (X-cell 100, Midwest Filtration, Cincinnati, Ohio). Composite dust samples were collected in children’s bedrooms using special vacuum nozzles developed by the Children’s Mercy Hospital and were analyzed for Fel d 1, Can f 1, Mus m 1, Der f 1, Der p 1, and Bla g 1 using enzyme immunoassay kits (Indoor Biotechnology, Charlottesville, Virginia). The values for Der f 1 and Der p 1 were combined for inclusion in the logistic regression model. Fungal allergens, including C herbarum, A fumigatus, P chrysogenum, and A alternate, were evaluated using antibodies obtained from Greer Laboratories (Lenoir, North Carolina).11 This laboratory participated in the ongoing quality assurance program provided by the Department of Housing and Urban Development.
Statistical Analysis
Characteristics of the atopic case and control groups were compared using χ2 tests, except for age differences, which were assessed using a t test. The relation between allergic sensitization and ETS exposure was analyzed by χ2 tests. The results are presented as odds ratios and 95% confidence intervals. Then, a logistic regression model was used to assess the association of allergen exposure on the development of ETS-induced allergic sensitization. Only children who had blood allergy testing and dust analysis were included in the final model. All statistical analyses were performed with SAS 9.2 (SAS Institute, Inc, Cary, North Carolina). A P value lower than .05 was considered significant.
Results
From October 2008 through November 2011, 382 families met the enrollment screening criteria. Of these, 30 (8%) were unreachable beyond initial contact, 6 (2%) were no longer interested in participating in the study, 36 (9%) did not show up or cancelled the initial clinic visit, and 4 (1%) changed homes or the child changed homes. One family (<1%) was excluded because of cognitive impairment, and 1 (<1%) was excluded because of a language barrier. Of the remaining 304 families, blood allergy testing was performed in 237 children (78%). All 237 children were included in this study, 116 as atopic cases and 121 as controls.
The children in this study were predominantly African American and ranged in age from 1 to 18 years at time of enrollment (Table 1). More boys were enrolled than girls (P = .003); however, the difference in the percentage of boys between the 2 groups was not significantly different. As stated in the inclusion criteria, the annual income of each child’s family was lower than 80% of the median family income for Kansas City. Eighty-three percent of enrolled families had annual incomes lower than 50% of the median for the area. The difference in family income medians between cases and controls was not significant. As expected, a significantly different percentage of children in the atopic group had asthma compared with the control group (P = .002). Children in the atopic group also were older on average than those in the control group (P < .001). Further, the atopic group had a smaller percentage of children younger than 3 years, a threshold at which few children have detectable levels of specific IgE. No other significant differences were found between the case and control groups.
Table 1.
Characteristics of cases and controls
| Atopic Cases (n = 121) |
Controls (n = 116) |
P value | |
|---|---|---|---|
| Demographics, n (%) | |||
| Gender | .81 | ||
| Female | 54 (47) | 49 (41) | |
| Male | 67(53) | 67 (59) | |
| Age (y), mean ± SD | 9 ± 4.2 | 6.7 ± 4.1 | <.001 |
| Age <3 y | 2 (2) | 13(11) | .006 |
| Race/ethnicity | |||
| African American | 54 (47) | 46 (38) | .52 |
| Hispanic | 18 (16) | 29 (24) | .07 |
| White | 30 (26) | 38 (31) | .23 |
| Other | 14(12) | 8 (6) | .31 |
| Family history of allergic rhinitis, n (%) | 34 (28) | 29 (25) | .69 |
| Socioeconomic status, n (%) | |||
| <80% but >50% of MFI in KC | 35 (30) | 38 (32) | .62 |
| <50% of MFI in KC | 81 (70) | 81 (68) | .74 |
| Home ownership | 71 (59) | 77 (66) | .28 |
| Clinical, n (%) | |||
| Asthma diagnosis | 83 (72) | 63 (52) | .002 |
| Smoke exposure | 42 (36) | 47 (39) | .43 |
Abbreviations: KC, Kansas City, Missouri; MFI, median family income; SD, standard deviation.
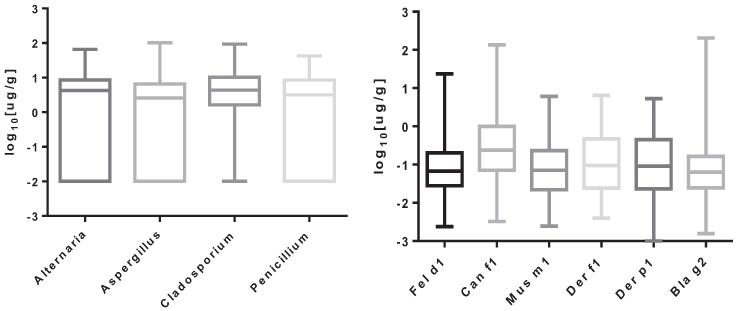
Dust allergen levels were obtained in 181 homes (n = 92 cases and n = 89 controls, P = .66). As expected, the level of furry pet allergen detected varied considerably. Although Fel d 1 (cat allergen) and Can f 1 (dog allergen) were undetectable in a subset of homes, the maximum Fel d 1 level detected was 23.5 μg/g and the maximum Can f 1 level was 135 μg/g. The maximum Bla g 2 (roach allergen) level detected was 205 μg/g, although Bla g 2 was undetectable in some homes. Similarly, mold levels varied, with the maximum detected levels of A alternata, P chrysogenum, A fumigatus, and C herbarum at 66, 42, 102, and 93 μg/g of dust, respectively. These allergens were undetectable in some homes and previously reported.12 No significant difference was seen in dust allergen levels from homes of cases vs controls for any allergen tested. These data are presented in Figure 1.
Figure 1.
Antigen levels from homes.
Table 2 presents odds ratios and 95% confidence intervals for the odds of ETS in the atopic sensitization group for specific blood allergens relative to the odds of ETS in the control group. Unadjusted and adjusted odds ratios are presented where adjustments were for home dust levels and family history. Sensitization to dust was analyzed using the combined value of Der p 1 and Der f 1. The odds of developing any IgE sensitization or IgE sensitization to individual allergens were not significantly higher for the ETS group in the unadjusted or adjusted analysis when exposed to tobacco smoke. For this analysis, as stated earlier, ETS exposure was defined as a child having any family member who smoked. A second analysis was performed in which only children with a family member who smoked inside the home were considered exposed. The analysis changed the results negligibly and is not presented here.
Table 2.
Association between exposure to environmental tobacco smoke and atopic sensitizationa
| Blood allergen | OR | 95% CI | AORb | 95% CI |
|---|---|---|---|---|
| Cat | 1.29 | 0.70–2.36 | 0.72 | 0.36–1.42 |
| Dog | 1.08 | 0.59–1.97 | 0.87 | 0.44–1.69 |
| Dermatophagoides farina | 0.79 | 0.42–1.50 | 1.40 | 0.68–2.88 |
| German cockroach | 1.38 | 0.66–2.87 | 0.84 | 0.37–1.88 |
| American cockroach | 0.62 | 0.21–1.80 | 1.87 | 0.58–6.07 |
| Penicillium chrysogenum | 1.32 | 0.65–2.68 | 0.76 | 0.34–1.69 |
| Alternaria alternata | 1.58 | 0.89–2.82 | 0.46 | 0.23–0.90 |
| Aspergillus fumigatus | 1.54 | 0.83–2.83 | 0.56 | 0.28–1.13 |
| Cladosporium herbarum | 1.31 | 0.66–2.57 | 0.70 | 0.33–1.49 |
| Overall atopic status | 0.89 | 0.53–1.51 | 0.98 | 0.53–1.79 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
OR is not significant for any allergen or for overall atopic status.
Adjusted for home dust allergen level and family history.
Discussion
This study did not find higher odds of atopic sensitization to any indoor allergen with ETS exposure. When home allergen exposure was considered, the odds remained unchanged. To the authors’ knowledge, this is the first study that considered the combined effect of ETS exposure and home allergen exposure.
Results from previous similar studies investigating the influence of ETS exposure on atopic sensitization have been mixed, with no clear answer emerging. Therefore, this study makes an important contribution to this growing body of literature. In the German Multicenter Atopy Study (MAS-90), Kulig et al13 found an association only between ETS exposure and food allergen sensitization. However, as in the present study, they failed to find an association between ETS exposure and sensitization to aeroallergens. Food sensitization was not monitored in the present study and therefore could not be analyzed. In 2008, a similar study reported discrepant findings from the BAMSE cohort.14 This 2008 study found an association not only with food sensitization but also with sensitization to cats and the mold C herbarum in a dose-dependent manner. Interestingly, in the present study, the effect of ETS exposure on sensitization to C herbarum approached, but did not reach, significance. This finding may indicate that mold exposure uniquely interacts with immune receptors to promote atopic sensitization. A recent study by Havstad et al15 concluded that the influence of ETS exposure on atopic sensitization was dependent on family history of atopic disease. Family history of allergic rhinitis was similar between the 2 groups in the present study and therefore did not significantly influence the analysis.
One reason for disparate findings among studies may be due to the different races of the studied populations. The BAMSE cohort and MAS-90 included predominantly white volunteers, whereas the present study included a large proportion of African American and Hispanic subjects. Another consideration is that the present study was the first to include allergen exposure in the model. Presumably, young children would have significant exposure to the food allergens tested in the MAS-90 and BAMSE studies in utero, through breast milk, or through diet, positively contributing to the likelihood of sensitization. Young children likely would have less exposure to indoor allergens, making sensitization to these allergens less likely. However, the present model failed to find an effect of allergen exposure on sensitization.
A strength of this study is that it is the first to investigate the effect of simultaneous exposure to home allergens and ETS on the development of allergic sensitization. Although children from low-income families are likely to have multiple, unique, microbial and chemical exposures, the interaction of these exposures on the immune response is poorly understood. In addition, this investigation enrolled a population of low-income and predominantly minority families. Not only is this population under-represented in clinical investigations, but this population also is known to have a disparate risk of asthma and atopic disease.16 Several limitations in this study also should be acknowledged. First, the smoking prevalence was determined by parent reporting, which likely underestimates the true prevalence, because children may have significant exposure outside the home environment. In addition, some parents undoubtedly conceal a smoking habit on questionnaires regarding their children’s health. To control for this limitation, an attempt was made to measure salivary cotinine levels. Unfortunately, only a small proportion of children were able to complete this method of sample collection; therefore, these data were omitted from the study. A second limitation is that this was a retrospective case–control study of pre-existing data. The primary aim of the KCSHHP was to determine the effect of home assessment and targeted remediation on asthma symptoms and severity and was not designed for analysis of the influence of ETS exposure on allergic sensitization.17,18
As the prevalence of atopic disease continues to increase, especially in industrialized countries, a greater understanding of these complex interactions of chemical and microbial exposures is necessary to provide an evidence-based framework for the development of preventive strategies in children. Because no policy exists that limits tobacco use in the home, the home environment becomes a significant source of ETS exposure for children and an important focus of investigation in the pathogenesis of atopic disease.19
In summary, these results do not support a significant association of ETS exposure with allergic sensitization or a significant additive effect of indoor allergen and ETS exposures on the development of IgE sensitization in this low-income, minority population. Because the data continue to be conflicting, further studies are needed to clarify if and under what circumstances ETS exposure influences atopic sensitization. A prospective birth cohort is needed to follow ETS exposure in children from this population from conception to clarify the issue. Because clear associations exist between ETS exposure and wheezing illness, middle ear infections, and sudden infant death, the importance of ETS avoidance in this vulnerable population should not be minimized by the results of this study.
Acknowledgments
The authors acknowledge the Center for Environmental Health at Children’s Mercy Hospital for their assistance with the completion of this study, including Ryan Allenbrand, MFS, Minati Dhar, PhD, Erica Forrest, MS, RRT, Luke Gard, Jennifer Lowry, MD, Mubeen Mohammed, MS, Freddy Pacheco, and Tanisha Webb, RRT.
Funding Sources: This work was supported by the American College of Allergy, Asthma, and Immunology Foundation, grant MOLHH0159-07 from the Department of Housing and Urban Development, and grant KL2TR000119-02 from Frontiers: The Heartland Institute for Clinical and Translational Research.
Footnotes
Reprints: Christina E. Ciaccio, MD, Children’s Mercy Hospital, 2401 Gillham Road, Kansas City, MO 64108; ceciaccio@cmh.edu.
Disclosures: Dr Ciaccio has received research funding from the American College of Allergy, Asthma, and Immunology. Dr Barnes received a research grant from Department of Housing and Urban Development. Dr Rosenwasser is president-elect of World Allergy Organization and has received financial support from the National Heart, Lung, and Blood Institute and Sanofi-Remeron.
References
- [1].US Department of Health and Human Services [Accessed June 20, 2012];The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. 2006 Available at: www.cdc.gov/tobacco/data_statistics/sgr/2006/index.htm.
- [2].Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328:1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- [3].Wang C, Salam MT, Islam T, et al. Effects of in utero and childhood tobacco smoke exposure and beta2-adrenergic receptor genotype on childhood asthma and wheezing. Pediatrics. 2008;122:e107–e114. doi: 10.1542/peds.2007-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Krieger J, Higgins DL. Housing and health: time again for public health action. Am J Public Health. 2002;92:758–768. doi: 10.2105/ajph.92.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fuller-Thomson E, Hulchanski JD, Hwang S. The housing/health relationship: what do we know? Rev Environ Health. 2000;15:109–133. doi: 10.1515/reveh.2000.15.1-2.109. [DOI] [PubMed] [Google Scholar]
- [6].Evans GW, Wells NM, Chan HY, et al. Housing quality and mental health. J Consult Clin Psychol. 2000;68:526–530. doi: 10.1037//0022-006x.68.3.526. [DOI] [PubMed] [Google Scholar]
- [7].Dunn JR, Hayes MV. Social inequality, population health, and housing: a study of two Vancouver neighborhoods. Soc Sci Med. 2000;51:563–587. doi: 10.1016/s0277-9536(99)00496-7. [DOI] [PubMed] [Google Scholar]
- [8].Datta GD, Subramanian SV, Colditz GA, et al. Individual, neighborhood, and state-level predictors of smoking among US Black women: a multilevel analysis. Soc Sci Med. 2006;63:1034–1044. doi: 10.1016/j.socscimed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [9].Diez Roux AV, Merkin SS, Hannan P, et al. Area characteristics, individual- level socioeconomic indicators, and smoking in young adults: the coronary artery disease risk development in young adults study. Am J Epidemiol. 2003;157:315–326. doi: 10.1093/aje/kwf207. [DOI] [PubMed] [Google Scholar]
- [10].Barnes CS, Kennedy K, Gard L, et al. The impact of home cleaning on quality of life for homes with asthmatic children. Allergy Asthma Proc. 2008;29:197–204. doi: 10.2500/aap.2008.29.3099. [DOI] [PubMed] [Google Scholar]
- [11].Barnes C, Tuck J, Simon S, et al. Allergenic materials in the house dust of allergy clinic patients. Ann Allergy Asthma Immunol. 2001;86:517–523. doi: 10.1016/S1081-1206(10)62899-2. [DOI] [PubMed] [Google Scholar]
- [12].Barnes C, Portnoy JM, Ciaccio CE, et al. A comparison of subject room dust with home vacuum dust for evaluation of dust-borne aeroallergens. Ann Allergy Asthma Immunol. 2013;110:375–379. doi: 10.1016/j.anai.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kulig M, Luck W, Lau S, et al. Effect of pre- and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life. Multicenter Allergy Study Group, Germany. Allergy. 1999;54:220–228. doi: 10.1034/j.1398-9995.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- [14].Lannero E, Wickman M, van Hage M, et al. Exposure to environmental tobacco smoke and sensitisation in children. Thorax. 2008;63:172–176. doi: 10.1136/thx.2007.079053. [DOI] [PubMed] [Google Scholar]
- [15].Havstad SL, Johnson CC, Zoratti EM, et al. Tobacco smoke exposure and allergic sensitization in children: a propensity score analysis. Respirology. 2012;17:1068–1072. doi: 10.1111/j.1440-1843.2012.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Forno E, Celedon JC. Health disparities in asthma. Am J Respir Crit Care Med. 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Munir AK, Einarsson R, Schou C, et al. Allergens in school dust. I. The amount of the major cat (Fel d I) and dog (Can f I) allergens in dust from Swedish schools is high enough to probably cause perennial symptoms in most chil- dren with asthma who are sensitized to cat and dog. J Allergy Clin Immunol. 1993;91:1067–1074. doi: 10.1016/0091-6749(93)90221-z. [DOI] [PubMed] [Google Scholar]
- [18].Amr S, Bollinger ME, Myers M, et al. Environmental allergens and asthma in urban elementary schools. Ann Allergy Asthma Immunol. 2003;90:34–40. doi: 10.1016/S1081-1206(10)63611-3. [DOI] [PubMed] [Google Scholar]
- [19].Marano C, Schober SE, Brody DJ, et al. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003e2006. Pediatrics. 2009;124:1299–1305. doi: 10.1542/peds.2009-0880. [DOI] [PubMed] [Google Scholar]