Abstract
Polypeptides expressed on the surface of merozoites, the invasive stage of the asexual blood cycle, are good candidates for the development of malaria vaccines. Five synthetic peptides with predetermined specificity deduced from a genomic DNA clone coding for the NH2-terminal portion of the main merozoite surface polypeptide of Plasmodium falciparum were evaluated for their capability to raise antibodies that react with the P. falciparum merozoites. Antibodies induced by two of the peptides (3 and 5) reacted with the membrane surfaces of seven of seven isolates of P. falciparum from different geographic areas. Antibodies against peptide 4, which contains a repeated amino acid sequence (Gly-Gly-Ser and Val-Ala-Ser), reacted with six of seven isolates. Structural analysis of the deduced polypeptides suggests that peptide 3 is exposed at the surface of merozoites. When it was used to immunize monkeys, three of the four animals were partially protected from a challenge infection that induced a fulminant infection in control animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung A., Shaw A. R., Leban J., Perrin L. H. Cloning and expression in Escherichia coli of a surface antigen of Plasmodium falciparum merozoites. EMBO J. 1985 Apr;4(4):1007–1011. doi: 10.1002/j.1460-2075.1985.tb03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Enea V., Arnot D., Schmidt E. C., Cochrane A., Gwadz R., Nussenzweig R. S. Circumsporozoite gene of plasmodium cynomolgi (Gombak):cDNA cloning and expression of the repetitive circumsporozoite epitope. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7520–7524. doi: 10.1073/pnas.81.23.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Hanukoglu I. Unraveling the structure of the intermediate filaments. Cell. 1983 Sep;34(2):332–334. doi: 10.1016/0092-8674(83)90367-7. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. How good are predictions of protein secondary structure? FEBS Lett. 1983 May 8;155(2):179–182. doi: 10.1016/0014-5793(82)80597-8. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Walliker D., Morgan G. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science. 1982 Jul 16;217(4556):254–257. doi: 10.1126/science.6178159. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- McGarvey M. J., Sheybani E., Loche M. P., Perrin L., Mach B. Identification and expression in Escherichia coli of merozoite stage-specific genes of the human malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3690–3694. doi: 10.1073/pnas.81.12.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. H., Richards W. H., Butcher G. A., Cohen S. Merozoite vaccination of douroucouli monkeys against falciparum malaria. Lancet. 1977 Jun 25;1(8026):1335–1338. doi: 10.1016/s0140-6736(77)92551-x. [DOI] [PubMed] [Google Scholar]
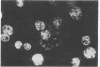
- Mrema J. E., Langreth S. G., Jost R. C., Rieckmann K. H., Heidrich H. G. Plasmodium falciparum: isolation and purification of spontaneously released merozoites by nylon membrane sieves. Exp Parasitol. 1982 Dec;54(3):285–295. doi: 10.1016/0014-4894(82)90037-6. [DOI] [PubMed] [Google Scholar]
- O'Neill P., Johnson G. D. Multispot immunofluorescence: a simple semi-automatic method of processing large numbers of tests. J Clin Pathol. 1970 Mar;23(2):185–187. doi: 10.1136/jcp.23.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Shimonishi Y., Kishida Y., Okada M., Sugihara H. Use of anhydrous hydrogen fluoride in peptide synthesis. I. Behavior of various protective groups in anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1967 Sep;40(9):2164–2167. doi: 10.1246/bcsj.40.2164. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum. Science. 1977 Jul 22;197(4301):388–389. doi: 10.1126/science.406671. [DOI] [PubMed] [Google Scholar]