Abstract
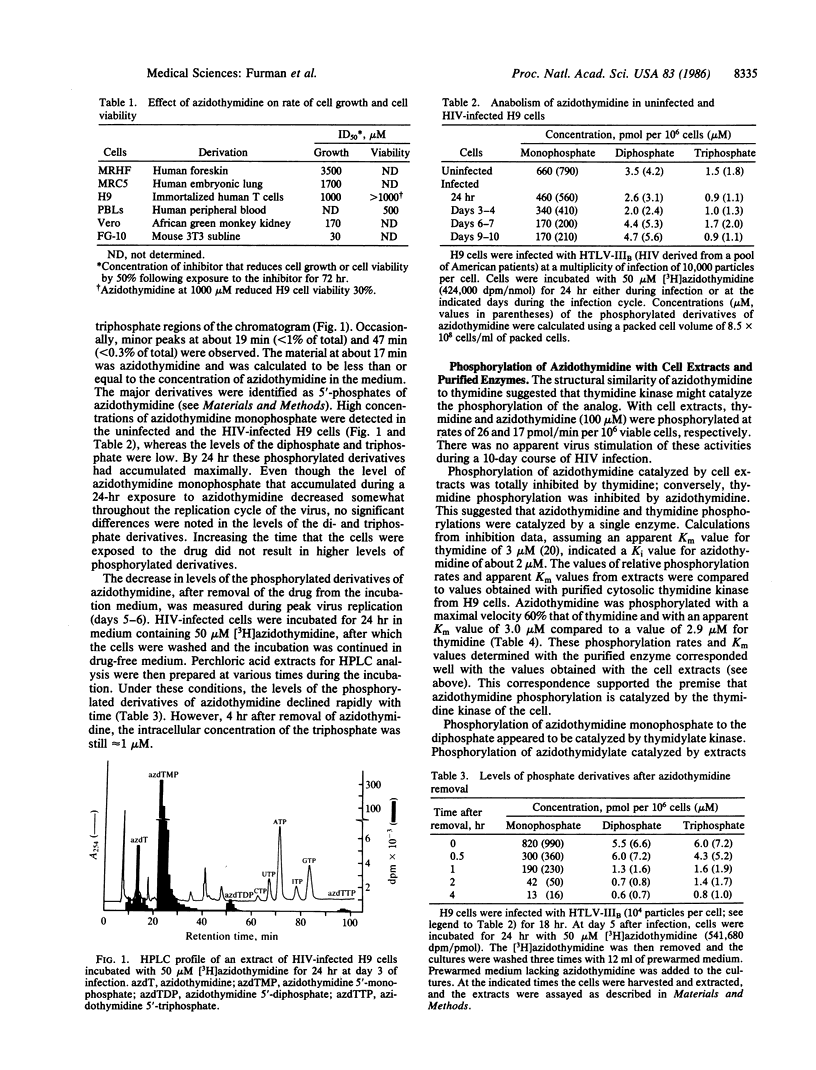
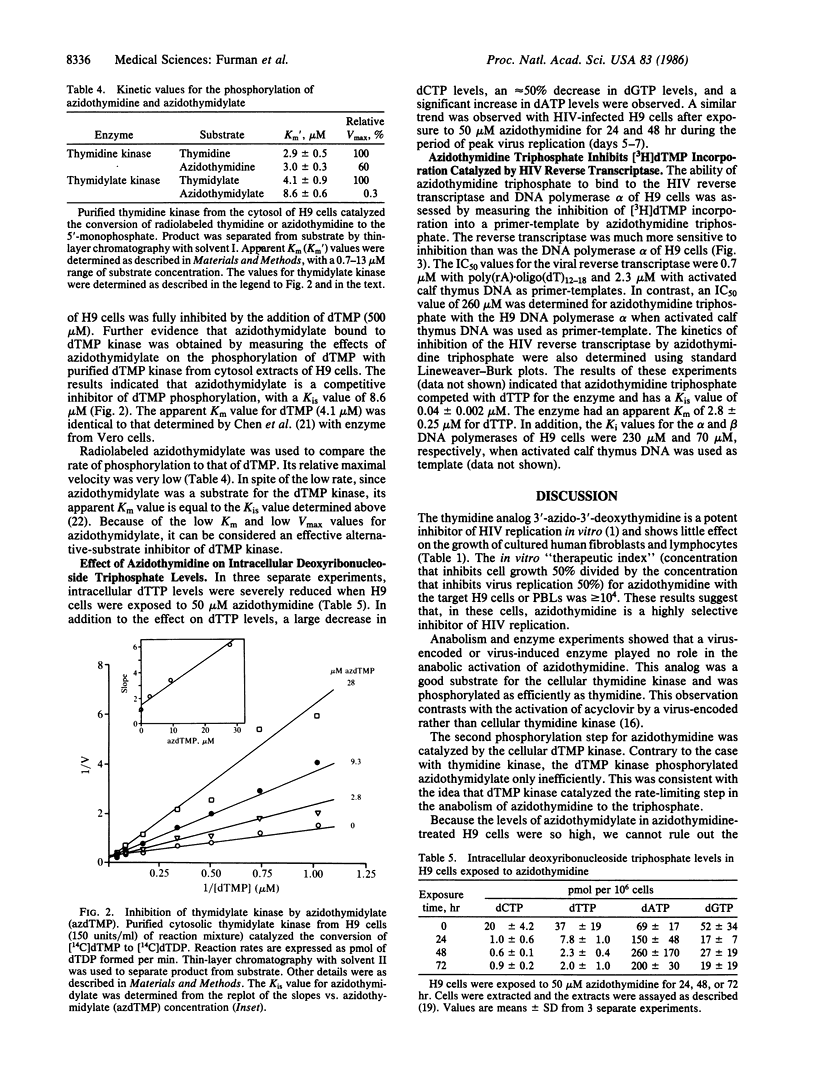
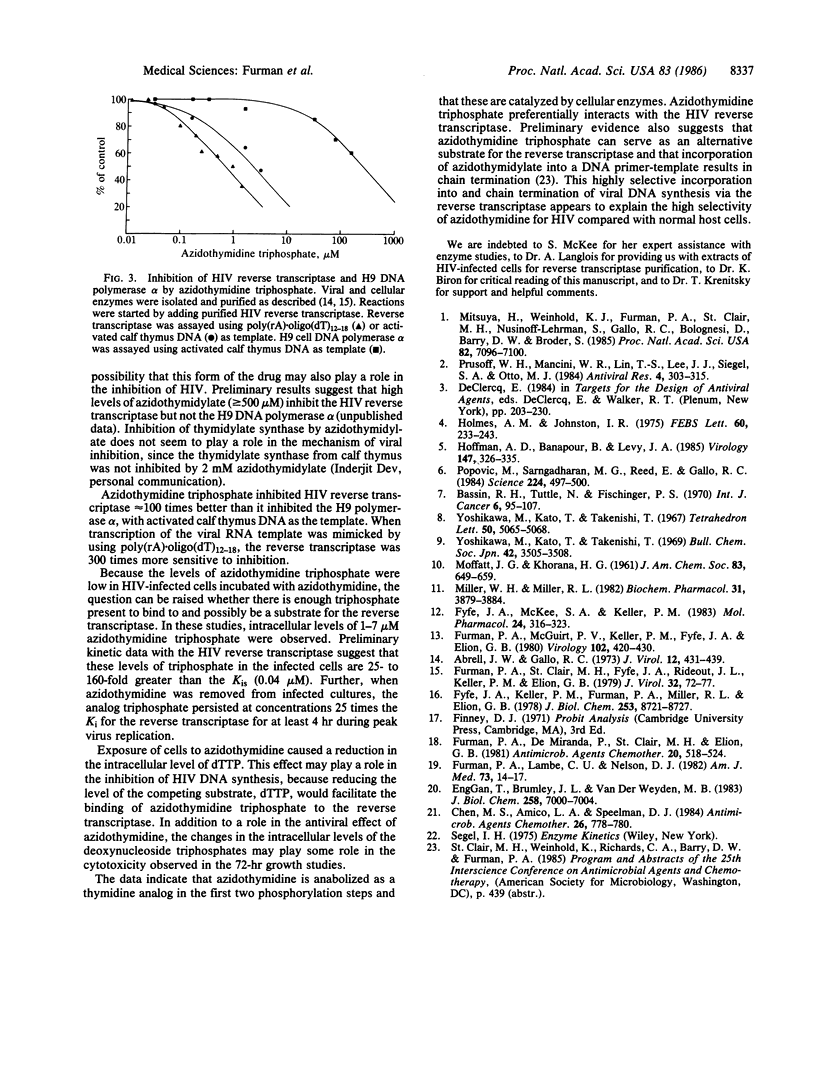
The thymidine analog 3'-azido-3'-deoxythymidine (BW A509U, azidothymidine) can inhibit human immunodeficiency virus (HIV) replication effectively in the 50-500 nM range [Mitsuya, H., Weinhold, K. J., Furman, P. A., St. Clair, M. H., Nusinoff-Lehrman, S., Gallo, R. C., Bolognesi, D., Barry, D. W. & Broder, S. (1985) Proc. Natl. Acad. Sci. USA 82, 7096-7100]. In contrast, inhibition of the growth of uninfected human fibroblasts and lymphocytes has been observed only at concentrations above 1 mM. The nature of this selectivity was investigated. Azidothymidine anabolism to the 5'-mono-, di-, and -triphosphate derivatives was similar in uninfected and HIV-infected cells. The level of azidothymidine monophosphate was high, whereas the levels of the di- and triphosphate were low (less than or equal to 5 microM and less than or equal to 2 microM, respectively). Cytosolic thymidine kinase (EC 2.7.1.21) was responsible for phosphorylation of azidothymidine to its monophosphate. Purified thymidine kinase catalyzed the phosphorylations of thymidine and azidothymidine with apparent Km values of 2.9 microM and 3.0 microM. The maximal rate of phosphorylation with azidothymidine was equal to 60% of the rate with thymidine. Phosphorylation of azidothymidine monophosphate to the diphosphate also appeared to be catalyzed by a host-cell enzyme, thymidylate kinase (EC 2.7.4.9). The apparent Km value for azidothymidine monophosphate was 2-fold greater than the value for dTMP (8.6 microM vs. 4.1 microM), but the maximal phosphorylation rate was only 0.3% of the dTMP rate. These kinetic constants were consistent with the anabolism results and indicated that azidothymidine monophosphate is an alternative-substrate inhibitor of thymidylate kinase. This conclusion was reflected in the observation that cells incubated with azidothymidine had reduced intracellular levels of dTTP. IC50 (concentration of inhibitor that inhibits enzyme activity 50%) values were determined for azidothymidine triphosphate with HIV reverse transcriptase and with immortalized human lymphocyte (H9 cell) DNA polymerase alpha. Azidothymidine triphosphate competed about 100-fold better for the HIV reverse transcriptase than for the cellular DNA polymerase alpha. The results reported here suggest that azidothymidine is nonselectively phosphorylated but that the triphosphate derivative efficiently and selectively binds to the HIV reverse transcriptase. Incorporation of azidothymidylate into a growing DNA strand should terminate DNA elongation and thus inhibit DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Amico L. A., Speelman D. J. Kinetics of the interaction of monophosphates of the antiviral nucleosides 2'-fluoro-1-beta-D-arabinofuranosylpyrimidine and (E)-5-(2-bromovinyl)-2'-deoxyuridine with thymidylate kinases from Vero cells and herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1984 Nov;26(5):778–780. doi: 10.1128/aac.26.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Lambe C. U., Nelson D. J. Effect of acyclovir on the deoxyribonucleoside triphosphate pool levels in Vero cells infected with herpes simplex virus type 1. Am J Med. 1982 Jul 20;73(1A):14–17. doi: 10.1016/0002-9343(82)90056-0. [DOI] [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., de Miranda P., St Clair M. H., Elion G. B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981 Oct;20(4):518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Fyfe J. A., McKee S. A., Keller P. M. Altered thymidine-thymidylate kinases from strains of herpes simplex virus with modified drug sensitivities to acyclovir and (E)-5-(2-bromovinyl)-2'-deoxyuridine. Mol Pharmacol. 1983 Sep;24(2):316–323. [PubMed] [Google Scholar]
- Gan T. E., Brumley J. L., Van der Weyden M. B. Human thymidine kinase. Purification and properties of the cytosolic enzyme of placenta. J Biol Chem. 1983 Jun 10;258(11):7000–7004. [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Johnston I. R. DNA polymerases of eukaryotes. FEBS Lett. 1975 Dec 15;60(2):233–243. doi: 10.1016/0014-5793(75)80721-6. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982 Dec 1;31(23):3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Mancini W. R., Lin T. S., Lee J. J., Siegel S. A., Otto M. J. Physical and biological consequences of incorporation of antiviral agents into virus DNA. Antiviral Res. 1984 Dec;4(6):303–315. doi: 10.1016/0166-3542(84)90001-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]



