Abstract
The consensus view in mirror neuron research is that mirror neurons comprise a uniform, stable execution-observation matching system. In this article, we argue that, in light of recent evidence, this is, at best, an incomplete and oversimplified view of mirror neurons, whose activity is actually quite variable and more plastic than previously theorized. We propose an epigenetic account for understanding developmental changes in sensorimotor systems, including variations in mirror neuron activity. Although extant associative and genetic accounts fail to consider the complexity of genetic and non-genetic interactions, we propose a new Evo-Devo perspective, which predicts that environmental differences early in development, or through sensorimotor training, should produce variations in mirror neuron response patterns, tuning them to the social environment.
Keywords: Mirror neurons, Epigenetics, Development, Neonatal imitation, Sensorimotor, Plasticity
Variation and plasticity in mirror neurons' response properties
Mirror neurons have been observed in neurophysiological experiments in awake primate brains, establishing equivalence between actions of the self (by execution) and actions of others (by observation). These neurons were first discovered in the ventral premotor cortex (area F5) and subsequently in the anatomically-connected area PFG of the posterior parietal lobe [1-3]. The most striking property of mirror neurons is that they fire while monkeys are executing a goal-directed movement (i.e., grasping) and when observing the same, or a similar, actions performed by other individuals. Therefore mirror neurons are capable of mapping the visual description of biological meaningful events into the corresponding cortical motor representations. The straightforward “execution-observation matching” phenomenological interpretations of mirror-neuron function have been useful in a wide range of disciplines in proposing uniform neural mechanisms primarily in the social domain of psychological phenomena – e.g., action understanding and imitation [4], but also spoken and sign languages, mind reading [5], and social disorders, including autism [6]. Most researchers, while discussing the nature and function of mirror neurons, report what is considered the main characteristic of mirror neurons: namely, their matching mechanism. The idea that mirror neurons possess a rather restricted and uniform pattern of discharge is a widespread opinion that certainly recognizes the most apparent property of the matching mechanism. Nevertheless, it overlooks the variety of responses that were originally described and discussed in the first papers describing mirror neurons, and that are informative for understanding their nature. Recent work has in fact shown that the visual discharge of mirror neurons can vary depending on several contextual features, such as the observed agent's end-goals, the space where the action is performed, the monkey's attention, and the type of object grasped by the experimenter [3] [7-10]. This work has also demonstrated that prolonged visuomotor experience affects the tuning of mirror neurons to others' actions performed with a tool [11].
The uniformity of the properties of mirror neurons has been claimed to develop through either associative (i.e., ontogenetic adaptive learning processes) [12] [13] and/or genetic mechanisms (i.e., phylogenetic natural selection processes) [13-15]. Further, in the genetic account, canalization mechanisms [14] have been proposed to contribute to the streamlining of associative learning to form sensorimotor matching for particular sets of pre-programmed body-part actions.
In this article, we argue that the widely accepted notion of uniformity of mirror neuron properties does not take into account important properties of mirror neurons, which are evident in raw recording data, thus overlooking mirror neurons' subtle, yet crucial, variations. This is a matter for concern as it may lead to an over-generalization of the roles of mirror neurons among psychologists, and even neuroscientists, who mistakenly require too much response stability, leading many to ignore such variations as mere outliers or noisy fluctuations.
Thus, while there are indeed basic response properties of mirror neurons (i.e., visuomotor matching), at the same time they may possess critical variations and plasticity, which could be explained if mirror neuron response properties are formed through plastic biological processes during postnatal development. Here we propose recently emerging epigenetic mechanisms as strong candidates for subserving such processes, incorporating both associative and genetic accounts (including canalization). Epigenetic mechanisms refer to the modality DNA can differently express proteins depending on the environmental influences (at cellular, tissue level, whole organism). Gene expression can be switched on and off by several epigenetic mechanisms (see Box 1 and Figure 1) that, at the brain level, can ultimately affect how neurons connect to each other in order to produce and stabilize functional brain architecture. In the last few years, evidence has accumulated showing that environmental conditions influence epigenetic codes more than the influence the DNA sequence, making these codes suitable for supporting organisms' adaptations to changes in the social and physical environments, especially during development. Small differences in epigenetic patterns can produce significant impacts on the phenotype, as demonstrated by studies on cloned animals and monozygotic twins [16-18].
Box 1. Epigenetic mechanisms.
Several researchers demonstrate the importance of epigenetic effects on development and evolution of the brain. Much work in rodents reveals that interactions between infants and their pre- and post-natal environments are important for modulating gene expression and brain maturation, leading to long-lasting phenotypic traits [49] [48] [47]. Such traits involve molecular phenomena (e.g., multiple post-translational modifications of histone proteins, methylation, acetylation and phosforilation, methylation on DNA), which can alter the accessibility of DNA and the density of chromatin structure in cells, such as neurons. Interestingly, some of these molecular phenomena seem to be susceptible to cross-generational transmission [57] [45]. Studies of epigenetic mechanisms and their stabilization in populations show that epigenetic processes may be responsible for the development and evolution of some important cognitive and emotional characteristics and abilities, such as stress responsively [49] [47], maternal care [49] [47], and learning and memory skills [51].
Figure 1.
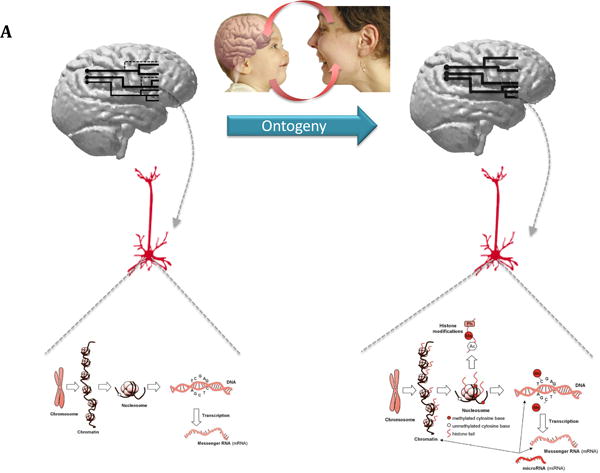
a) The figure illustrates how during ontogeny specific social experiences could produce changes in gene expression. The brains on the left and right are schematic representations of potential parietal-premotor mirror circuits, which are sensitive to facial stimuli and, over the course of development, are reshaped and refined. The effective stimuli producing such changes are represented by the mother interacting with her infant through face-to-face engagement, including facial expressions. The bottom of the figure represents hypothetical changes occurring in premotor mirror neurons in the newborn's brain during such social exchanges. On the left, the typical pattern of gene-protein expression in one of these neurons is depicted. On the right, the early social experience produces modifications in gene expression through epigenetic marks, such as DNA methylation, histone modifications, and micro-RNA production. Such epigenetic effects modify the pattern of neuronal wiring in the parietal premotor mirror circuits.
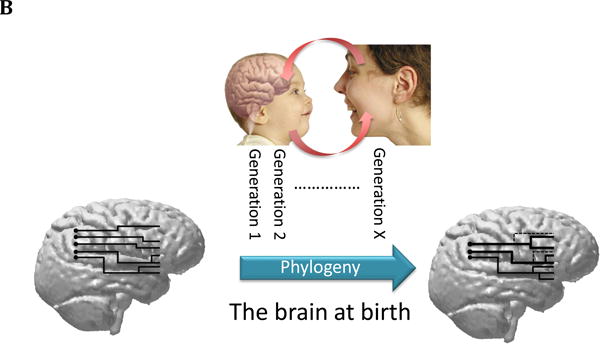
b) The figure shows hypothetical modifications that might have occurred in newborn brains if the same social environment (mothers soliciting their infants through facial expression) is present at each generation, producing a cascade of similar epigenetic events in the newborn brain. According to the epigenetic account, such plastic changes modify the neuronal wiring in the mirror circuits. The end result of these epigenetic modifications is the facilitation during the perinatal period, through yet unknown cellular and molecular modifications, of both the canalization in the construction of the underlying neuronal circuits, and their developmental trajectories. Thus, the brain on the right would be at birth better tuned to respond to a set of social stimuli (e.g., facial expressions).
In what follows, we first discuss some immediate problems that appear to derive from an over-simplified vision of mirror neurons' properties, and then propose an epigenetic account which, by giving emphasis to the adaptive developmental stages of plasticity (or Evo-Devo mechanisms), establishes mirror neurons as biologically plausible phenomena, incorporates critical aspects of associative and genetic accounts, and is consistent with the remarkable variations of mirror neurons' properties. In the final section, we provide examples that represent subtle yet crucial variations of mirror neurons' properties, that tend to be overlooked by general readers, but that are well recognized by experimentalists who directly observe raw recordings of mirror neurons.
In this opinion paper we try to provide a coherent picture of how a rather simple sensory-motor mechanism might emerge in development, and how an epigenetic view might stimulate more ‘brain-based’ realistic experiments to predict specific neurodevelopmental outcomes and evolutionary-based explanations of mirror neurons' origins and functions.
Problems with current interpretations of the development of mirror neurons
The associative account posits that mirror neurons are a product of associative learning [12] [13] [25]. Through Hebbian learning, visuomotor neurons' response to the observation of others' hand actions might emerge in the parietal and premotor cortices during an early phase of development in which infants' sight of self-reaching towards an object is systematically associated with the motor command for grasping. The simultaneous firing of these neurons can strengthen visuomotor connections. Through this mechanism, perceptual and motor experience related to own-action could produce premotor and parietal neurons that simultaneously receive specific visual input from the STS region of the temporal cortex and potentiate the motor pattern that is related to grasping execution in parietal/premotor areas. Though persuasive, this model of mirror neuron development has important limitations. One such limitation is the “correspondence problem,” which refers to the problem of how newborns link visual input of others' facial gestures to their own motor representations of the same gestures, since infants cannot see their own face. This link appears to be present prior to any experience, as evidenced by human and macaque neonatal imitation [26] [27], making it difficult to explain neonatal imitation from a purely associative learning perspective. In macaques, infants imitate even in the absence of any prior experience of contingent facial interactions with caregivers, as infants in these studies are reared in a nursery from birth [27].
A second limitation of the associative account concerns mirror neurons' plasticity. The bulk of evidence in support of this account comes from work that finds sensorimotor training modulates the mirror neuron system (e.g., [28-30]), which is interpreted as evidence that mirror neurons are not a genetically based adaptation [12]. According to this account, if mirror neurons were an adaptation then they would not be so plastic, and, instead, would be buffered from perturbations thus showing little change as a consequence of individual sensorimotor experience or modifications of the contextual/environmental conditions [12]. However, the evidence of mirror neurons' plasticity based on sensorimotor training is weak. First, in the key experiment supporting this interpretation, neuronal activity of mirror neurons was not directly assessed; instead, the excitability of the motor cortex was measured, which is only an indirect index of mirror neurons activity [31]. Moreover, recent studies replicating those by Catmur and colleagues showed that brief sensorimotor training does not reconfigure the mirror neuron system [32]. Additionally, the associative account does not consider that species-typical development of a number of fundamental genetically-based adaptations—including vision [33-35], human language [36], song in song birds' development [37], and rat copulative behaviour [38]—are context-dependent, highly plastic, and significantly influenced by experience.
A final limitation of the associative account is that it is traditionally explored with a heavy reliance on sensorimotor training paradigms in adults, and then results, often erroneously, are extrapolated to explain processes occurring earlier in development (namely, in infancy) [12] [29] [13]. It is true that general somatosensory experience in adulthood can cause temporary changes in neuronal activity without major rewiring, although, under certain circumstances, there can also be alterations of somatosensory and motor cortical maps due to increases in the strength of existing connections [39]. In contrast, experiences in infancy can cause long lasting changes in neuronal structure, particularly during critical periods of development (e.g., [40-42]). Learning is not in and of itself sufficient to indicate that there have been significant and permanent rearrangements of connections in the brain. Moreover, during early phases of development (e.g., infancy), experience has different effects on the CNS. For example, work on the development of the visual system in several vertebrate species demonstrates that preserved vision in the early postnatal period is necessary for functional binocular vision through correct synaptic connections [43] [34]. Subsequent innervation may become more specific during development through the elimination of terminals from postsynaptic neurons [42]. These synaptic changes have long lasting effects on brain structure and function, particularly during critical periods of development, reflecting experience-expectant brain organization (e.g., [42]). It is therefore important to distinguish these changes in brain organization in infancy from those occurring as a consequence of general experience in adulthood, in which molecular and structural elements are more stable and may, to a certain degree, impede plasticity. In other words, in adults, mature circuits are no longer capable of alternative wiring or drastic reorganization in response to typical/common environmental perturbations.
Given these serious limitations, some scientists are skeptical about the associative account's ability to explain the developmental origin and function of mirror neurons (e.g., [14] [44-46]). Alternatively, an evolutionary account of mirror neurons has been proposed, which hypothesizes that once mirror neurons emerged in development, individuals who possessed mirror neurons had a reproductive advantage, and therefore this system was retained and proliferated via natural selection [12].
If mirror neurons were responsible for crucial abilities for survival and reproduction, such as action understanding and imitation, mirror neurons may have become hardwired during phylogenetic history [46]. However, this model is not without limitations. One limitation of the genetic account is that it hypothesizes that mirror neurons emerged during evolution such that their previous function is the same as their current function [12]. This retained functionality, we think, is actually quite unlikely, given the common process of neural reuse, whereby neural circuits evolved for one purpose can be exapted for another purpose [47]. In human evolution it seems that several anatomical structures and cognitive mechanisms, such as language, are exaptations, which have lost their original function [48] [49]. Instead, it seems more parsimonious that mirror neurons evolved from a mechanism that monitored the own hand goal-directed movement and were then exapted to serve additional functions, especially in humans (e.g., understand others' actions and emotional states, social learning).
An additional limitation of the genetic account is that it proposes that mirror neurons are present from birth, and this could be incorrectly interpreted as meaning they are purely genetically determined. We think this interpretation arises from at least two misunderstandings regarding brain development and cognitive abilities. The first misunderstanding is that, by postulating that specific mechanisms like imitation or action understanding are innate, this account fails to acknowledge that there may be critical periods during which individuals are especially sensitive or insensitive to their environments. The second misunderstanding is that this approach suggests an all-powerful conception of evolution, with natural selection processes completely shaping the development of a phenotypic trait via genetic sequences alone (e.g., mutational change).
A novel proposal for the development of mirror neurons
In contrast to the accounts outlined above, we propose an epigenetic hypothesis, which states that mirror neurons are the result of an adaptation process involving the stabilizing selection of adaptive, environmentally-induced phenotypic traits. Unlike the genetic account, the epigenetic hypothesis supposes that mirror neurons are not the result of natural selection acting on genetic sequences that are specifically selected for the functions of goal-encoding or action understanding. In contrast to the associative account, the epigenetic hypothesis proposes that the development of mirror neurons is not only a process of associative learning, but also involves genetic and epigenetic phenomena, rendering phylogenetic and ontogenetic viewpoints critical for understanding mirror neuron development (see Figure 1).
According to this perspective, learning is central. Some authors have emphasized the importance of learning in mirror neuron development through Hebbian processes in which repeated observations of self-produced actions are coupled with motor commands to create causal sensorimotor links [13] [14] [50]. According to these authors, in phylogeny such learning, and the conditions necessary for producing these associations, was canalized. However, what remains unclear in these developmental models is the process or mechanism that produced this canalization, including how, and especially why this mechanism became fixed during evolutionary history. Secondly, the associative and genetic models fail to explain other important features of mirror neurons at the neurophysiological level, which are related to their variations and modulation in activity. We propose that an Evo-Devo perspective can bring such clarity, making testable predictions regarding the developmental emergence of mirror neurons and their variations that have been recorded in adult monkeys.
An Evo-Devo perspective
There is general agreement that infants at birth are attracted to specific sets of stimuli, including faces (e.g., [51-56] their own hands [57], and especially their own hands in motion [58-60], which may provide sensorimotor experiences that are the necessary scaffolding for mirror neuron development [14]. In the neonatal period, two important processes occur, which are relevant for mirror neuron development: Infants' neural connections between visual and motor areas are strengthened, and infants develop visuomotor coordination based on their observations of the contiguity and contingency among environmental events, such as seeing their own moving hand or synchronizing facial expressions with caregivers. It is likely that attending to sets of attractive invariant stimuli (consistently and commonly available; e.g., faces, hands) occurs from birth to develop sensorimotor control (as in the case of visually-guided hand grasping), and not specifically for producing mirror neurons [12] [13]. What is peculiar about mirror neurons, however, is the generalization process, or the link between the perception of self-movement and the perception of others' behaviors.
Despite the fact that this generalization process is one of the most critical steps in creating the mirror and in giving mirror neurons their ‘social function,’ this process has yet to be thoroughly understood. Although speculative, we hypothesize that during the evolution of mirror neurons, visual stimuli related to others' behaviors became capable of triggering activity of a specific population of visuomotor neurons. The sensitivity of these neurons to a specific set of biological stimuli—namely, social stimuli—may be mediated, in the very early stages of brain development, by several epigenetic mechanisms involving changes in gene expression in these neurons (see Box 1). These epigenetic modifications were, at the beginning, not heritable but they might have produced effects at both behavioral and cognitive levels. If this new emergent neuronal response and the related epigenetic mechanisms produced some advantages to the organism (e.g., faster or more accurate capacity to recognize others' actions through mapping others' actions onto one's own motor knowledge), natural selection would have favored their stabilization and facilitation of expression under specific environmental conditions (See Figure 1b). It is useful for the brain to be plastic early in development as this allows for the appropriate tuning of sensory motor connections into configurations appropriate for a given environment. Different developmental trajectories, thus, can be determined early in development, to help best prepare individuals for future environments. Central to this perspective is the proposal that in mirror neuron evolution, epigenetic mechanisms are sensitive to particular environmental conditions in the early stages of development. Thus, evolution supports the social and environmental conditions that contribute to specific patterns of gene expression.
As already described above, studies of neonatal imitation demonstrate a rudimentary process of visual generalization at birth [26] [27], which is sensitive to the social environmental context [61] and that is probably supported by a mirror mechanism [62]. The newborn imitation phenomenon also suggests that the coupling between visual perception (of others' mouth movements) and execution (of one's own mouth movements) is facilitated in the perinatal period through yet unknown cellular and molecular modifications that are capable of canalizing underlying neuronal circuits and their developmental trajectories (see Figure 1b). Several researchers have investigated brain plasticity during early postnatal life, and its interaction with individual experience, at the molecular level. Interestingly, recent work in rodents has demonstrated that interactions between infants and their pre- and post-natal environments (both biotic and abiotic) are important for regulating gene expression and brain maturation, leading, in several cases, to long-lasting developmental outcomes [19-24].
Studies of epigenetic mechanisms and their stabilization in populations demonstrate that epigenetic processes may be responsible for the development and evolution of some important cognitive and emotional abilities [24], such as stress responsiveness [19] [20], maternal care [19] [20] [22], and learning and memory skills [24].
Although our knowledge of epigenetic phenomena—and particularly those involving the central nervous system—is still in its early stages, there are some examples that demonstrate the stimulus-specificity of gene expression programs [63], which may be one mechanism through which natural selection operates [64].
This epigenetic facilitation of mirror neuron development does not consider the role of experience marginal; instead, it is often fundamental in triggering and guiding developmental trajectories. In this regard, several examples illustrate how experience might interact and shape the raw materials provided by genes. For example, the case of the callosity of skin cells in birds: even if many cells have the potential to develop callosity as consequence of pressure and friction during movement of the hind leg, only some cells present callosity at birth or soon after birth, likely the result of genetic assimilation and epigenetic mechanisms [65] [66].
Similar epigenetic principles may be responsible for mirror neuron development, and such development appears to strictly depend on early postnatal sensorimotor experiences and social interactions. Epigenetic mechanisms underlying mirror neuron formation are yet unknown. There are certainly areas of investigation that are worth considering in future research, involving these mechanisms and their stabilization and assimilation into genetic sequences (Box 3). In molecular biology, some of these mechanisms are currently under investigation and scientists are making progress in understanding them [67-69].
Highlights.
We propose a model that could explain how mirror neurons can emerge during development
We examine mirror neurons variations and their plasticity
Environmental factors, by acting on brain plasticity, produce stable functional brain circuits
MNs are subjected to functional modification through their interaction with social environment
Another important consequence of this perspective is that it may provide insights for explaining some key visual features of mirror neuron activity, such as their modulation according to the space where the action is performed [70] or the type of object that is grasped ([71]; see also [72] for a review). This variation in mirror neuron activity may be a consequence of the fact that, in adulthood, the environment can still exert important influences on how these neurons, and the networks in which they are connected, adjust and adapt their neuronal and functional properties to the contextual features of the environment and the individual's social experiences (see Figure 2).
Figure 2.
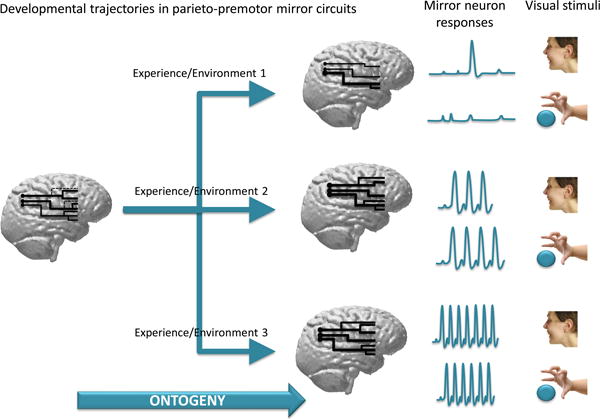
The Figure shows how different experiences might produce a variety of patterns of parietal-premotor circuits, which retain some of the basic configured features present at birth. A consequence of these changes is that mirror neurons will be produced that, in different individuals, might result in differential responses (here represented in terms of frequency of neuronal discharge) to the same set of visual stimuli (right side of the figure).
Conclusion
The epigenetic account predicts that there will be variation in mirror neuron developmental paths, and for ultimately acquired properties. Though at first it may seem that the properties of mirror neurons are homogeneous, this might be due to similarities in early environments (e.g., monkeys' rearing conditions). Furthermore, this account predicts that environmental differences early in development, or under intensive sensorimotor training, should produce variations mirror neuron properties (neural response patterns) that tune them to specific stimuli related to others' actions (e.g., space or the use of tools).
Examining developmental characteristics of mirror neurons within a comparative perspective, we may consider mirror neurons to be a product of an evolved learning process, and at the same time, as a form of adaptation, having been adapted to a given set of environmental conditions [73] for which plasticity (at brain and behavioral levels) plays an important role. In this way, plasticity may be a potential adaption to unforeseen changes in environmental conditions [73].
Established after maturation, mirror neurons are not simply making a gross match of the execution and observation of self/other actions, but they are often modulated by various detailed aspects of actions [3] [7] [8] [9], suggesting that they could be characterized not simply as fixed machinery to establish conceptual association, but as “real” variable neural circuitry subject to functional modification through the individual's history of interactions with environmental conditions. Indeed, neurophysiological research on the posterior parietal cortex (PPT) has demonstrated that areas with mirror neurons contain several other types of neurons that appear to code grasping and space in relation to actions [74-77]. Thus, it is likely that this area, and the different neurons present within it, contribute to different aspects of action-perception that could be related not only to sensorimotor transformation, but also to other cognitive processes, such as space coding, and object and biological motion processing. In an evolutionary perspective, the neuronal properties of these neurons have been suitable for sensorimotor transformations, but they have been probably exapted to perform functions in other domains. The variety of neuronal properties described in the PPT highlights that sensorimotor integration is probably exploited to accomplish several functions within the physical domain (to interact with objects), as well as the social domain (to interact with other individuals).
In conclusion, an epigenetic account offers a powerful hypothesis to allow developmental changes in sensorimotor systems, including latent variation in mirror neuron activities, which may have contributed to niche-construction of highly sophisticated human social environments during the course of past primate evolutionary history. While genetic and associative accounts fail to consider the complexity of the interaction between genetic and non-genetic contributions, we think this new account may be utilized to solve many challenges of understanding the functional significance of mirror neurons and mirror neuron systems to subserve social interactions and, thereby provide novel insights in understanding the meanings of their disorders within an evolutionary context.
Box 2. Questions for future directions of Evo-Devo hypothesis.
The Evo-Devo hypothesis of mirror neurons is a useful approach for understanding fundamental phenotypic traits of organisms, in contrast to dichotomous views of the relationship between innate/acquired, adaptation/plasticity and genes/environment. This Evo-Devo view raises new questions and future directions for research to determine the mechanisms for mirror neuron evolution, such as:
What molecular differences, at birth and during development, exist between standard visuomotor neurons and mirror neurons?
What molecular differences exist, if any, between postnatal and adult development of mirror neurons? How do such differences affect patterns of mirror neuron discharge?
What specific socio-environmental stimuli are able to trigger specific patterns of molecular changes underlying mirror neurons?
When in development, if any, is there an adaptive sensitive period for mirror neurons formation? If there is a sensitive period, is it more sensitive to social-environmental, compared to non-social, stimuli? Do face mirror neurons have a developmental trajectory different from hand mirror neurons?
What is the cognitive function of mirror neurons, beyond that operated by the visuomotor mapping?
What are the cognitive and behavioural deficits following mirror neurons knocking out?
What can comparisons among primates, including humans, tell us about the phylogenetic history of mirror neurons?
What conditions have lead to the stabilization of the generalization process for creating mirror neurons, and how do these conditions vary depending on the phylogenetic history of the species presenting them?
Acknowledgments
This research was supported by the Division of Intramural Research, NICHD, and NICHD P01HD064653, and by the Funding Program for World-leading Innovative R&D on Science and Technology. We thank Valentina Sclafani and Sebo Uithol for their comments on an early version of this paper.
Glossary
- Adaptation
is a trait that contributes to the fitness of the organism. In the traditional evolutionary perspective, the source of variation of a trait is mainly genetic and this variation is involved in the trait's expression. According to a more recent evolutionary theoretical view, (i.e., the Extended Synthesis), the stabilization of a trait within a population could occur through different processes [36]: e.g., hard inheritance, namely selection on genetic variations; soft inheritance, selection on non-genetic variations; [39] and evolution of plasticity [36]. Adaptation, therefore, includes but does not refer exclusively to the result of a selective process acting on genes and ultimately favoring the emergence and fixation of a novel trait, which contributes to a specific function. Further, adaptations can be inherently plastic.
- Adaptiveness
refers to phenotypic plasticity during development; namely, the ability of a single genotype to produce more than one alternative form of morphology, physiological states, or behaviour, in response to environmental conditions [40]. The environment (and especially the prenatal environment) is the primary source of variation in phenotypic plasticity [40]. New variants of a trait can emerge as the result of developmental plasticity in which individuals differ in their response to the cellular, chemical, or social/parental environment at different stages of development. In other words, adaptiveness results from the plastic ability to overcome unforeseen environmental events [40] [36].
- Canalization
is a mechanism that narrows the range of developmental possibilities. It represents the bias that an organism has toward acquiring some forms of a trait, with a corresponding decrease in plasticity during ontogenesis (i.e., there are a greater number of developmental possibilities earlier, compared to later, in development). Canalization buffers traits against perturbation due to non-specific experiential influence and non-standard genetic variations.
- Epigenetics
is the study of genetic and non-genetic factors acting upon cells to selectively control gene expression. Epigenetics results in increasing phenotypic complexity during development. Epigenetic mechanisms are generally understood as chromatin modifications of genes, which allow differential access of complex of transcription factor to DNA sequences. Epigenetics also includes that study of heritable patterns of gene expression between generations, which result from methylation of DNA, chromatin structure, and genomic imprinting.
- Evo – devo
stands for “evolutionary developmental biology” and refers to a field of biology addressing the origin and evolution of development. It investigates the modifications of development and developmental processes that lead to the production of novel features. Three elements – epigenetics, genomic control, and environmental control – and their integration underlie and unify evolution. Epigenetic mechanisms are essential because there is no one-to-one relationship between genotype and phenotype. The genotype is the starting point and the phenotype is the endpoint of epigenetic control, while ecology is a vehicle for key innovation and integrated change during development that affects evolutionary change.
- Exaptation
refers to the change in function of preexisting structure during phylogenesis under appropriate condition of selection. A trait, previously shaped from natural selection for a function or alternatively resulted from a learning process, may be reused for a new function with evolutionary value.
- Mirror neurons
are neurons with visuomotor properties originally found in two anatomically-connected cortical areas of the macaque monkeys: the ventral premotor cortex and the inferior parietal lobule. They discharge both during the execution and observation of hand/mouth goal-directed motor act and facial gesture. These neurons are not activeated by the observation of objects, or of biological movements mimicking the action but lacking the target object. Similar neurons have been recently found also in the primary motor cortex (citation). Some of these neurons are part of the cortico-spinal tract and may also have inhibitory discharge. The most important property of mirror neurons is the congruence they show, both in terms of goals and means to achieve the goal, between the effective observed and the effective executed action.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Di Pellegrino G, et al. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, et al. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 3.Fogassi L, et al. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 4.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 5.Gallese V. Before and below ‘theory of mind’: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):659–69. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 7.Caggiano V, et al. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Current Biology. 2011;21(2):144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Caggiano V, et al. Mirror neurons encode the subjective value of an observed action. Proceedings of the National Academy of Sciences. 2012;109(29):11848–11853. doi: 10.1073/pnas.1205553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari PF, et al. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. Journal of cognitive neuroscience. 2005;17(2):212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y, et al. Potential role of monkey inferior parietal neurons coding action semantic equivalences as precursors of parts of speech. Soc Neurosci. 2010;5:105–117. doi: 10.1080/17470910802625306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochat MJ, et al. Responses of mirror neurons in area F5 to hand and tool grasping observation. Exp Brain Res. 2010;204(4):605–16. doi: 10.1007/s00221-010-2329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyes C. Where do mirror neurons come from? Neuroscience & Biobehavioral Reviews. 2010;34(4):575–583. doi: 10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Cook R, et al. Mirror neurons: From origin to function. Behavioral and Brain Sciences. 2013 doi: 10.1017/S0140525X13000903. In press. [DOI] [PubMed] [Google Scholar]
- 14.Giudice MD, et al. Programmed to learn? The ontogeny of mirror neurons. Developmental science. 2009;12(2):350–363. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Bonini L, Ferrari PF. Evolution of mirror systems: a simple mechanism for complex cognitive functions. Annals of the New York Academy of Sciences. 2011;1225(1):166–175. doi: 10.1111/j.1749-6632.2011.06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraga MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rideout WM, et al. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001;293(5532):1093–1098. doi: 10.1126/science.1063206. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J. Epigenetics: unfinished symphony. Nature. 2006;441(7090):143–145. doi: 10.1038/441143a. [DOI] [PubMed] [Google Scholar]
- 19.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in neuroendocrinology. 2008;29(3):386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24(1):1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 21.Fagiolini M, et al. Epigenetic influences on brain development and plasticity. Current opinion in neurobiology. 2009;19(2):207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakyan VK, Beck S. Epigenetic variation and inheritance in mammals. Current opinion in genetics & development. 2006;16(6):573–577. doi: 10.1016/j.gde.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Bird A. DNA methylation patterns and epigenetic memory. Genes & Development. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 24.Fischer A, et al. Recovery of learning and memory is associated with chromatin remodeling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 25.Cooper R, et al. Associative (not Hebbian) learning and the mirror neuron system. Neuroscience Letters. 2012;540:28–36. doi: 10.1016/j.neulet.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari PF, et al. Neonatal imitation in rhesus macaques. PLoS biology. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catmur C, et al. Sensorimotor learning configures the human mirror system. Current Biology. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Catmur C. Sensorimotor learning and the ontogeny of the mirror neuron system. Neuroscience letters. 2013;540:21–27. doi: 10.1016/j.neulet.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Cavallo A, et al. Timecourse of mirror and counter-mirror effects measured with transcranial magnetic stimulation. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barchiesi G, Cattaneo L. Early and late motor responses to action observation. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss049. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catmur C, et al. Associative sequence learning: the role of experience in the development of imitation and the mirror system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1528):2369–2380. doi: 10.1098/rstb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiesel TN. The postnatal development of the visual cortex and the influence of environment. Bioscience reports. 1982;2(6):351–377. doi: 10.1007/BF01119299. [DOI] [PubMed] [Google Scholar]
- 34.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26(1003):17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 35.Zeigler HP, Bischof HJ. Vision, brain, and behavior in birds. The MIT Press; 1993. [Google Scholar]
- 36.Dor D, Jablonka E. From cultural selection to genetic selection: a framework for the evolution of language. Selection. 2001;1(1):33–56. [Google Scholar]
- 37.Clayton DF. Role of gene regulation in song circuit development and song learning. Journal of neurobiology. 1997;33(5):549–571. [PubMed] [Google Scholar]
- 38.Griffiths PE, Machery E. Innateness, canalization, and ‘biologicizing the mind’. Philosophical Psychology. 2008;21(3):397–414. [Google Scholar]
- 39.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 40.Leppänen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nat Rev Neurosci. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 42.Holtmaat A, Sovoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 43.Van Sluyters RC, Levitt FB. Experimental strabismus in the kitten. Journal of Neurophysiology. 1980;43(3):686–699. doi: 10.1152/jn.1980.43.3.686. [DOI] [PubMed] [Google Scholar]
- 44.Gallese V, et al. Motor Cognition and its role in the phylogeny and ontogeny of intentional understanding. Dev Psychol. 2009;45(1):103–13. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- 45.Shaw DJ, Czekóová K. Exploring the Development of the Mirror Neuron System: Finding the Right Paradigm. Developmental Neuropsychology. 2013;38(4):256–271. doi: 10.1080/87565641.2013.783832. [DOI] [PubMed] [Google Scholar]
- 46.Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neurosciences. 1998;21(5):188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 47.Anderson ML. Neural reuse: A fundamental organizational principle of the brain. Behavioral and brain sciences. 2010;33(4):245. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- 48.Pievani T, Serrelli E. Exaptation in human evolution: how to test adaptive vs exaptive evolutionary hypotheses. Journal of Anthropological Sciences. 2011;89:9–23. doi: 10.4436/jass.89015. [DOI] [PubMed] [Google Scholar]
- 49.Fitch WT. Evolutionary Developmental Biology and Human Language Evolution: Constraints on Adaptation. Evolutionary biology. 2012;39(4):613–637. doi: 10.1007/s11692-012-9162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heyes C. Mesmerizing mirror neurons. Neuroimage. 2010;51(2):789–791. doi: 10.1016/j.neuroimage.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 51.Fantz RL. Pattern vision in newborn infants. Science. 1963;140:296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MH, Morton J. Biology and cognitive development: The case of face recognition. Oxford: Blackwell Publishing; 1991. [Google Scholar]
- 53.Macchi Cassia V, et al. Can a nonspecific bias toward top-heavy patterns explain newborns' face preference? Psychological Science. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 54.Mondloch CJ, et al. Face perception during early infancy. Psychological Science. 1999;10:419–422. [Google Scholar]
- 55.Turati C, et al. Newborns' face recognition: role of inner and outer facial features. Child Development. 2006;77:297–311. doi: 10.1111/j.1467-8624.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 56.Valenza E, et al. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- 57.White B, et al. Observations on the development of visually-directed reaching. Child Development. 1964;35:349–364. doi: 10.1111/j.1467-8624.1964.tb05944.x. [DOI] [PubMed] [Google Scholar]
- 58.Van Der Meer A. Keeping the arm in the limelight: Advanced visual control of arm movements in neonates. European Journal of Pediatric Neurology. 1997;4:103–108. doi: 10.1016/s1090-3798(97)80040-2. [DOI] [PubMed] [Google Scholar]
- 59.Van Der Meer A, et al. The functional significance of arm movements in neonates. Science. 1995;267:693–695. doi: 10.1126/science.7839147. [DOI] [PubMed] [Google Scholar]
- 60.Von Hofsten C. An action perspective on motor development. Trends in Cognitive Sciences. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Paukner A, et al. Delayed imitation of lipsmacking gestures by infant rhesus macaques (Macaca mulatta) PloS one. 2011;6(12):e28848. doi: 10.1371/journal.pone.0028848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrari PF, et al. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. Journal of Cognitive Neuroscience. 2012;24(5):1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner SL, et al. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science Signaling. 2005;309(5742):1857. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 64.Gilad Y, et al. Natural selection on gene expression. TRENDS in Genetics. 2006;22(8):456. doi: 10.1016/j.tig.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Waddington CH. The evolution of an evolutionist. Columbia University Press; New York: 1975. [Google Scholar]
- 66.Speybroeck L. From epigenesis to epigenetics. Annals of the New York Academy of Sciences. 2002;981(1):61–81. [PubMed] [Google Scholar]
- 67.Dulac C. Brain function and chromatin plasticity. Nature. 2010;465(7299):728–35. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. The Quarterly Review of Biology. 2009;84(2):131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 69.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 70.Caggiano V, et al. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Current Biology. 2011;21(2):144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Caggiano V, et al. Mirror neurons encode the subjective value of an observed action. Proceedings of the National Academy of Sciences. 2012;109(29):11848–11853. doi: 10.1073/pnas.1205553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casile A, et al. The mirror neuron system: A fresh view. The Neuroscientist. 2011;17(5):524–38. doi: 10.1177/1073858410392239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bateson P, Gluckman P. Plasticity, robustness, development and evolution. Cambridge University Press; 2011. [DOI] [PubMed] [Google Scholar]
- 74.Rozzi S, et al. Functional organization of inferior parietal lobule convexity in the macaque monkey: Electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. European Journal of Neuroscience. 2008;28(8):1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- 75.Hyvärinen J. Posterior parietal lobe of the primate brain. Physiological Reviews. 1982;62(3):1060. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- 76.Yokochi H, et al. Inferior parietal somatosensory neurons coding face-hand coordination in Japanese macaques. Somatosensory & Motor Research. 2003;20(2):115–125. doi: 10.1080/0899022031000105145. [DOI] [PubMed] [Google Scholar]
- 77.Fujii N, et al. Dynamic social adaptation of motion-related neurons in primate parietal cortex. PLoS One. 2007;2(4):e397. doi: 10.1371/journal.pone.0000397. [DOI] [PMC free article] [PubMed] [Google Scholar]


