Abstract
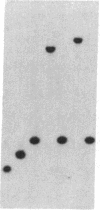
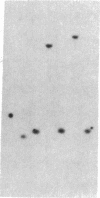
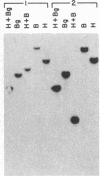
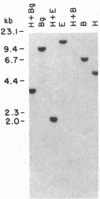
Integrated hepatitis B virus (HBV) DNA is almost invariably found in hepatocellular carcinomas (HCC) which develop in HBV carriers. Integrated HBV DNAs from two single-integration HCCs (C3 and C4) have been cloned, and the cellular integration sites have been analyzed. Integrated HBV DNA of C3 is present in chromosome 6 and contains a nearly complete linear HBV genome. The HBV DNA integration in tumor C3 was not associated with major rearrangements of cellular DNA. In contrast, the integrated HBV DNA in C4 contains a large inverted repeat of HBV DNA, in which each repeat consists of a linear HBV DNA segment similar to that present in C3. The C4 integration was also accompanied by a cellular DNA translocation at the HBV integration site. The translocation occurred between chromosomes 17 and 18, along with a deletion of at least 1.3 kilobases of chromosome 18 DNA at the translocation site. Our data support a model in which postintegration rearrangement of integrated HBV and cellular DNA results in the generation of chromosomal aberrations. These chromosomal aberrations may function in a multistage mechanism leading to fully malignant HCC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Dejean A., Brechot C., Tiollais P., Wain-Hobson S. Characterization of integrated hepatitis B viral DNA cloned from a human hepatoma and the hepatoma-derived cell line PLC/PRF/5. Proc Natl Acad Sci U S A. 1983 May;80(9):2505–2509. doi: 10.1073/pnas.80.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., Solt D., Cameron R., Laishes B., Ogawa K., Medline A. Newer insights into the pathogenesis of liver cancer. Am J Pathol. 1977 Nov;89(2):477–482. [PMC free article] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Nelson J. A., McDougall J. K. Small fragments of herpesvirus DNA with transforming activity contain insertion sequence-like structures. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4736–4740. doi: 10.1073/pnas.81.15.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E., Theile M. Virus-induced gene mutations of eukaryotic cells. Hum Genet. 1983;63(1):1–12. doi: 10.1007/BF00285389. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Hino O., Kitagawa T., Sugano H. Relationship between serum and histochemical markers for hepatitis B virus and rate of viral integration in hepatocellular carcinomas in Japan. Int J Cancer. 1985 Jan 15;35(1):5–10. doi: 10.1002/ijc.2910350103. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Hino O., Nomura K., Sugano H. Dose-response studies on promoting and anticarcinogenic effects of phenobarbital and DDT in the rat hepatocarcinogenesis. Carcinogenesis. 1984 Dec;5(12):1653–1656. doi: 10.1093/carcin/5.12.1653. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Koch S., von Loringhoven A. F., Hofschneider P. H., Koshy R. Amplification and rearrangement in hepatoma cell DNA associated with integrated hepatitis B virus DNA. EMBO J. 1984 Sep;3(9):2185–2189. doi: 10.1002/j.1460-2075.1984.tb02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy R., Koch S., von Loringhoven A. F., Kahmann R., Murray K., Hofschneider P. H. Integration of hepatitis B virus DNA: evidence for integration in the single-stranded gap. Cell. 1983 Aug;34(1):215–223. doi: 10.1016/0092-8674(83)90152-6. [DOI] [PubMed] [Google Scholar]
- Kuhlmann I., Achten S., Rudolph R., Doerfler W. Tumor induction by human adenovirus type 12 in hamsters: loss of the viral genome from adenovirus type 12-induced tumor cells is compatible with tumor formation. EMBO J. 1982;1(1):79–86. doi: 10.1002/j.1460-2075.1982.tb01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Taira M., Yaginuma K., Kobayashi M., Yoshida E., Koike K. Inversely repeating integrated hepatitis B virus DNA and cellular flanking sequences in the human hepatoma-derived cell line huSP. Proc Natl Acad Sci U S A. 1985 Jan;82(1):208–212. doi: 10.1073/pnas.82.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston C. W., Jonak G. J., Rogler C. E., Astrin S. M., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982 Jun;29(2):385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Sherman M., Su C. Y., Shafritz D. A., Summers J., Shows T. B., Henderson A., Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. doi: 10.1126/science.2996131. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from a chronically infected liver. J Virol. 1984 Jun;50(3):832–837. doi: 10.1128/jvi.50.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler C. E., Summers J. Novel forms of woodchuck hepatitis virus DNA isolated from chronically infected woodchuck liver nuclei. J Virol. 1982 Dec;44(3):852–863. doi: 10.1128/jvi.44.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Shouval D., Sherman H. I., Hadziyannis S. J., Kew M. C. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981 Oct 29;305(18):1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Ziemer M., Garcia P. D., Crawford R., Hsu H., Valenzuela P., Rutter W. J. Cloning and analysis of integrated hepatitis virus sequences from a human hepatoma cell line. J Virol. 1984 Sep;51(3):776–787. doi: 10.1128/jvi.51.3.776-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Yang J. Q., Eckhardt L. A., Harris L. J., Birshtein B. K., Marcu K. B. Products of a reciprocal chromosome translocation involving the c-myc gene in a murine plasmacytoma. Proc Natl Acad Sci U S A. 1984 Feb;81(3):829–833. doi: 10.1073/pnas.81.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich H. F. Oncogenic and nononcogenic mutants of adenovirus 12: induction of chromosome aberrations and cell divisions. Prog Exp Tumor Res. 1973;18:260–272. doi: 10.1159/000393170. [DOI] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi M., Yoshida E., Koike K. Hepatitis B virus integration in hepatocellular carcinoma DNA: duplication of cellular flanking sequences at the integration site. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4458–4462. doi: 10.1073/pnas.82.13.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]