Abstract
Background
Lowering dietary sodium and adhering to medication regimens are difficult for persons with heart failure (HF). Because these behaviors often occur within the family context, this study evaluated the effects of family education and partnership interventions on dietary sodium (NA) intake and medication adherence (MA).
Methods
HF patients and family member (FM) dyads (N = 117) were randomized to: usual care (UC), Patient-FM education (PFE), or a family partnership intervention (FPI). Dietary NA (3-day food record), Urine NA (24-hour urine) and MA (MEMS®) were measured at baseline (BL) prior to randomization, and at 4 and 8 months (M).
Results
FPI and PFE reduced Urine NA at 4 M, and FPI differed from UC at 8 M (p=.016). Dietary NA decreased from BL to 4M with both PFE (p=.04) and FPI (p=.018) lower than UC. The proportion of subjects adherent to NA intake (≤ 2500 mg/day) was higher at 8 M in PFE and FPI vs UC (χ2(2)=7.076, p=.029). MA did not differ among groups across time. Both FPI and PFE groups increased HF knowledge immediately after intervention.
Conclusions
Dietary NA intake, but not MA, was improved by the PFE and FPI interventions compared with UC. UC was less likely to be adherent with dietary NA. Greater efforts to study and incorporate family-focused education and support interventions into HF care are warranted.
Keywords: self management, adherence, dietary sodium, medication adherence, autonomy support
Introduction
Self-management of heart failure (HF), particularly reduction of dietary sodium and medication adherence, is challenging for patients and their families. One recommendation for management of HF is a low sodium diet (2 to 3 grams/day), and 2000 mg per day is recommended for those considered Stage C.1 Yet this lifestyle change is fraught with difficulties of adherence and recidivism. HF patient and family education guidelines1 include strategies to follow a low sodium diet, yet efforts to consume lower sodium diets are thwarted by the pervasive amounts of hidden sodium in foods, cultural preferences, and the excess levels of the taste of “saltiness” to which many have become accustomed.2, 3 Persons with HF attempting to lower their dietary sodium rarely receive objective feedback on their intake and may erroneously believe they are adherent.
Medication adherence in HF is also challenging and is notably low, ranging from 31% to 79% depending on the medication of interest.4–6 Not following a low sodium diet and lack of medication adherence have both been associated with adverse outcomes such as fluid retention,7 and hospitalizations for heart failure. 8–11 Following a therapeutic diet and taking medications require certain knowledge and skills, yet education alone may not improve self-care or outcomes.12, 13
HF is a chronic illness in which family members (FMs) provide tangible and social support, motivation, and communication vital to effective self-care. 14–20 FMs may share or take full responsibility for preparing low sodium meals, and/or they may set up medication schedules and refills. Thus provision of family education seems a logical component of HF care, yet little evidence on family care in HF is available to guide practice.
The structure of the family and components of family functioning (i.e. the way the family communicates, adapts to problems and new situations, and solves problems) may also affect HF patients’ psychological and behavioral responses including their self-care.21–23 Lack of support is associated with poor self- management behaviors in chronic illness and HF, and perceived family criticism has been associated with adverse health behavior as well as relapse in diet and health behaviors in patients with diabetes, asthma, and those attempting weight loss.24
Self-determination theory, a motivation theory,25, 26 posits that performance of a desired behavior can be influenced through autonomy support, which is characterized by empathy, choice, problem solving, and avoidance of controlling language (e.g “you must” or “you should”). Autonomy support from health care providers has resulted in improved adherence to behavioral changes such as better glucose control in diabetics,27 weight loss,28 smoking cessation,29 and medication adherence.30, 31 In a pilot study, a brief autonomy support intervention with family members demonstrated greater reduction of dietary sodium intake by persons with HF compared to education alone; however, only short term effects after 3 months without sustained change were observed.32, 33 A more intense and/or longer family intervention to maintain the behavior change was suggested.
Dietary sodium and medication self- management are influenced not only by the family factors but also by individual patient characteristics. These include age, gender, and ethnicity, clinical characteristics such as severity of illness, and comorbidities with high self-care regimens. Behavioral characteristics such as motivation, self efficacy, mood states and depression, and the prerequisite knowledge and skills to perform the behaviors (such as understanding role of dietary sodium in HF, recognition of high sodium foods and alternatives, and skills such as label reading and alternative food preparation) are also influencing factors.33 These factors are important to consider in the design of an education and family focused intervention to improve self- care in HF.
The purpose of this clinical trial (EducatioN and SuPport Interventions to Improve Self CaRE: ENSPIRE), was to test a HF patient-family partnership intervention (FPI) designed to reduce dietary NA and improve medication adherence in comparison to a patient-family education intervention (PFE) and usual care (UC). The hypotheses were: (1) HF patients receiving the FPI will have a greater decrease in dietary NA intake (24-hour urine NA; 3-day food record) and a greater proportion considered adherent (≤2500mg sodium/day) than those receiving PFE or UC; and (2) HF patients receiving the FPI will have better adherence to HF medications than those receiving PFE or UC. We also examined whether the intervention improved the patient’s HF knowledge and perceived autonomy support and reduced family criticism.
METHODS
Design
A three group, randomized design with data collected at baseline (BL), and after 4 and 8 months (M) was used. HF patients and one family member were randomized as dyads. The three randomization groups were: 1) usual care (UC); 2) patient and family education (PFE) and 3) family partnership intervention (FPI) The intervention was provided between 1–2 months after BL measures were obtained. The time frame between BL and 4M was considered the initiation phase of behavior change with the time frame between 4–8 M considered the maintenance phase of behavior change. We expected to see the greatest change between the 0–4 M with little additional change during the 4–8 M phase. The protocol and informed consent documents were approved by the Emory University Institutional Review Board and all participating institutions.
Sample
The sample was recruited from three large university affiliated outpatient HF clinics, selected for their provision of care for a large number of HF patients through multidisciplinary teams of HF physicians, cardiology fellows, clinical and advanced practice nurses, pharmacists and social workers. Inclusion criteria for HF patients were: diagnosis of HF confirmed in the medical record, age 30–79 years, NYHA Class II–III, English fluency, telephone access, on optimal HF medication regimen unless documented contraindication including angiotension-converting enzyme inhibitors (ACEI) or angiotension II receptor blockers (ARB), beta adrenergic blocking agent, and diuretics, eligible for a low NA diet, ambulatory, adequate renal function as evidenced by glomerular filtration rate>30, and a participating family member (FM) who was designated as the primary person helping with HF self-care and interacting with the HF patient at least 2–3 times/week. HF patient exclusion criteria were: acute myocardial infarction in the past 6 months, significant angina, HF secondary to untreated condition, planned cardiac surgery, impaired cognition, psychiatric diagnosis, and uncorrected visual/hearing problem. FMs had to be >19 years of age, willing to participate and without conditions that would impair their ability to participate in the intervention sessions such as impaired cognition or psychiatric diagnosis. Study enrollment took place from March 2005 to July 2008, and both the HF patient and FM gave written informed consent.
Overview of the Interventions
Usual Care (UC) Group
Participants in the UC group received an informational brochure Taking Control of Your Heart Failure (Heart Failure Society of America; St Paul Minn) and usual care from their health care providers. UC related to patient education in the recruitment settings was assessed by comparing HF education standards, materials, and observed practices, and were comparable among the three sites. All provided patient teaching regarding general overview of HF, HF medications, and dietary NA, and family members tended to be included if present. To maintain interest in the project, a study newsletter was mailed once to the UC group at 4–5 M and contained an update on the number of study participants and reminder of remaining study activities.
Patient-Family Education (PFE) Group
After BL data collection, dyads participated in an educational session (approximately 1 hour) delivered by a trained master’s prepared research nurse. Content included: 1) general HF overview, symptoms of fluid overload, rationale for and ways to modify dietary NA intake, cues to take medications regularly and maintain refills, and other self- management activities such as weighing daily and physical activity. Time was allowed for individual questions. By 2M, dyads in the PFE group attended a second, 2-hour, group session focused on reinforcing education about dietary NA and medication-taking behaviors. This group was conducted by a trained master’s prepared nurse and registered dietitian. This session included active learning activities such as selection of low NA foods, meal planning, and adapting recipes. Coordinated written and media resources were provided including materials developed for the study, brochures (Taking Control of Your Heart Failure, How to Follow a Low Sodium Diet, Heart Failure Medicines; HFSA, St. Paul, MN), and a DVD (Heart Failure Basics to Better Care; Milner- Fenwick; Hunt Valley, MD).
Self-monitoring and specific feedback on the target behavior are important components of successful behavior change interventions.34 Examples of successful behavior change interventions in which self-monitoring or feedback on the target behavior include self -monitoring of blood glucose to achieve optimal glucose levels and control in persons with diabetes,35, self- monitoring of weight to achieve weight loss in overweight individuals,36 and audit/feedback to health providers to improve health provider clinical performance.37 Therefore, in our study, participants also received individual feedback in the form of a written report regarding their dietary NA intake and MA. (See variables and measurement section) The feedback included their dietary NA intake derived from analysis of a three-day food (3DFR) record that listed the participant’s specific high and low sodium foods and the results of their baseline 24-hour urine sodium (Urine NA) compared with the goal of 2000 mg/day. Medication adherence data obtained from the MEMS® (% of prescribed doses taken correctly) were also included in the report. This information was presented in text and graph formats, and at each of the study time points, the report was updated with their dietary NA intake, high NA foods and alternatives, 24-hour Urine NA results, and medication adherence so participants could track their progress. For the maintenance phase, the PFE dyads received a telephone booster educational session after the 4M data collection point during which the research nurse reviewed their 4M NA intake, medication data, and reinforced the education. Between 4–5M, participants also were mailed study newsletters containing strategies to lower dietary NA, new seasonal low NA recipes, and reinforcement of medication-taking behaviors.
Family Partnership (FPI) Group
After BL data collection and by 2M, the FPI group initially received the same protocol for dyadic teaching, and the individualized feedback on dietary NA and medications as described for the PFE group. In addition they attended two, 2-hour small group FPI sessions led by a trained master’s prepared research nurse who began the session with a brief discussion of reinforcing dietary and medication education with patient and family members together. Then break-out patient and family member education and training sessions were held. The content and discussions included: 1) perceptions of living with HF or a family member with HF, 2) principles of autonomy supportive communication, 3) HF self- care scenarios with role playing of responses based on autonomy supportive approaches which have been previously validated.32, 33 Coordinated written materials about family partnership and autonomy supportive communication, a family focused brochure (Tips for Family and Friends, HFSA, St. Paul, MN), as well as all the written and DVD materials given to the PFE group were provided. For the maintenance phase, dyads received a scripted booster telephone call during which information about the patient’s 4M dietary NA results were reviewed with reinforcement of efforts to reduce dietary NA and take medications. In addition the research nurse used a script tailored on the dyad’s BL and 4M autonomy support and family criticism scores (see measures) to reinforce strategies for working together through autonomy supportive communication. The FPI group was mailed study newsletters with similar information as sent to PFE, with the addition of tips for implementing an autonomy supportive family partnership.
All PFE and FPI intervention protocol activities (dyadic and group sessions; telephone calls) were scripted but allowed time for participants to raise concerns or questions for discussion. Fidelity of the intervention was monitored by random investigator attendance at group sessions, and review of interventionist completed check sheets for coverage of specific content and activities at each session. Interventionists were retrained periodically. Overall participant adherence to the intervention, defined as the percent of the dyad who received greater than 50% of the intervention was 81.8% for PFE and 87.9% for FPI groups.
Measurement of Variables
Participant Characteristics
Demographic and clinical characteristics for the HF patients were collected from self-report and the medical record. The Charlson Comorbidity index (CMI) was used to quantify other chronic conditions.38 Measures of height and weight were obtained in the Clinical Research Center, and body mass index (BMI) was calculated using a standard formula. Type and dose of diuretics were collected, and furosemide equivalents were calculated39 to account for residual effects of loop diuretics on sodium excretion. FM characteristics of age, gender, relationship to HF participant, and education were obtained from self report.
Dietary NA
Dietary NA, the primary outcome, was measured in two ways. At each time point, HF participants completed a 3DFR which was reviewed for accuracy, completeness, and portions, then analyzed by the research registered dietician blinded to group assignment using Food Processor SQL, a well validated software program (version 10.2, ESHA Research) to obtain a mean daily sodium intake. The ESHA master database contains over 30,000 food items with data sources obtained from the latest USDA Standard Reference database, items from the U. S. Continuing Survey of Food Intake by Individuals database, food manufacturers fast food companies, and from literature sources, and has strong concordance with other established nutritional databases.40
Because 95% of ingested NA is excreted in urine in steady state if renal function is adequate,41 participants collected a 24-hour urine on the third day of maintaining their 3DFR which was analyzed for NA, creatinine, and urea. Written and verbal instructions on collecting a 24-hour urine and collection supplies were provided, and the research nurse called the day before the scheduled collection to review procedures. Creatinine and urea were also assayed and incorporated into standard formulas to determine renal function and completeness of the urine collections. Record reviews and patient interviews were employed to verify that no urine collections were obtained within 2 weeks of a medication or dose change.
Medication Adherence
Medication adherence was also measured two ways. The objective measure was the Medication Events Monitoring System (MEMS®) which is a microelectronic monitoring device applied as a cap to a medication bottle. The monitor records each time a cap is removed from a medication bottle; data are downloaded to a computer database for analysis. For this study, two HF medications were monitored which included a primary daily heart failure medication (ACEI, ARB or Beta Blocker), and a diuretic. Monitoring occurred for a minimum of 2 weeks at baseline, and again at 4 and 8 Ms. Participants kept a written calendar to record times/reasons the MEMS® was not used (i.e. travel, hospitalization, etc). MEMS® data were calculated for the monitored days including percent of the prescribed doses that were taken and taken on schedule, and percent of days the dose was taken correctly. The primary variable for calculating individual adherence was the number of actual dosages/number of desired dosages X 100 to achieve % adherence rate for each monitored HF drug. MEMS® caps have been found to be a reliable and valid measure of medication adherence, 42–45 and they have been used successfully with HF patients.46–49
Participants also completed the Morisky Medication Adherence Scale (MMAS).50 The 8-item instrument assesses medication-taking behaviors using a yes/no response for 7 items and a 5 point Likert response for one item asking ”How often do you have difficulty remembering to take medications?” Slight modifications in wording were made to the 8-item version with the author’s permission to refer specifically to taking HF medications. A panel of nursing experts confirmed appropriateness of the changes. Scores range from 1–12 with higher scores reflecting better adherence. Scores were trichotomized with a score of <6 representing low adherence, 6–8 representing medium adherence, and >8 representing high adherence.51 The original MMAS has reported reliability, sensitivity and specificity,51 and the internal consistency reliability of the revised instrument was acceptable in this study with Cronbach’s alpha of .72.
Heart Failure Knowledge
HF knowledge was assessed by the Atlanta Heart Failure Knowledge Test (AHFKT.v1),52 a 27-item questionnaire that includes items on HF pathophysiology, diet, symptom assessment, and medication taking behaviors. The sum of correct scores was converted to a 0–100% scale. The AKHKT has a 5th grade reading level, and both content and construct validity were established through correlations with selfcare measures.52 Internal consistency reliability was adequate with Cronbach’s alpha of .84.52
Patient Perceived Autonomy Support
Autonomy support was measured by the 15-item Family Care Climate Questionnaire –Patient version (FCCQ-P).53 Items address the degree of perception of the participating FMs’ support and communication regarding lifestyle changes associated with HF. Higher scores on the FCCQ-P reflect higher perceived autonomy support for HF self management. Cronbach’s alpha in this study was .80.
Perceived Family Criticism
Perceived Family Criticism (PFC) was measured by the updated 7-item PFC scale of the Family Emotional Involvement and Criticism Scale (FEICS-PC).54 This scale measures general criticism from family which is viewed as antithetical to autonomy support. Responses from items are summed for a total score ranging from 0–28 with higher scores representing higher perceived criticism from family. The PFC has reported reliability coefficients of .82,54 .73 in a pilot study with HF patients,53 and .82 in the current study. Both the autonomy support and PFC scores were used for providing tailored information and counseling to the FPI dyads during intervention components.
Depressive Symptoms
Because depressive symptoms have been associated with reduced self management behaviors and adherence, depressive symptoms were measured as a control variable using the Beck Depression Inventory-II (BDI-II), 55, 56 a well-established measure with 21 items rated on a 0–3 scale. Total scores ≥ 14 indicate presence of depressive symptoms. The Cronbach’s alpha for this sample was .90.
Data Analysis
Descriptive statistics were used to describe the sample and evaluate underlying distribution assumptions. Analysis of variance (ANOVA), chi-square tests and Pearson correlations were run to test for associations between categorical and continuous variables and for group differences at baseline. Intent to treat procedures were followed for hypothesis testing. Missing data were evaluated to determine the extent due to attrition and other reasons as well as to evaluate if any measures were predictive of missing values. These evaluations were performed to determine if the assumptions were met for MCAR (missing completely at random), CD-MCAR (covariate dependent MCAR) or MAR (missing at random).57 Because of some incomplete urine collections, urine NA data were imputed for 8.1% of the possible data points for subjects still enrolled at each time point who had otherwise full data used in the analysis, using unbiased multivariate regression imputation that incorporated related variables of BMI and urinary volume. Urine NA values and MA measures were analyzed for group, time, and group-by-time interaction effects using multi-level modeling (MLM) treating group and time as factors. Post hoc pairwise comparisons were conducted (Sidak multiple comparisons error rate adjustment), which included contrasts for the hypothesized initiation phase (BL to 4M) and maintenance phase (4 to 8M), with p values of ≤ .05 considered significant. In the contrast analysis for urine NA, covariates were variables related to the outcome (p < .10) and/or predictive of missing over time. To further examine adherence and clinical relevance of changes in dietary NA as reflected by 24-hour Urine NA, participants were categorized as adherent (Urine NA levels ≤ 2500 mg/day) or non-adherent ( > 2500 mg/day) to compare proportions at each time point using Chi square.
RESULTS
Sample Characteristics
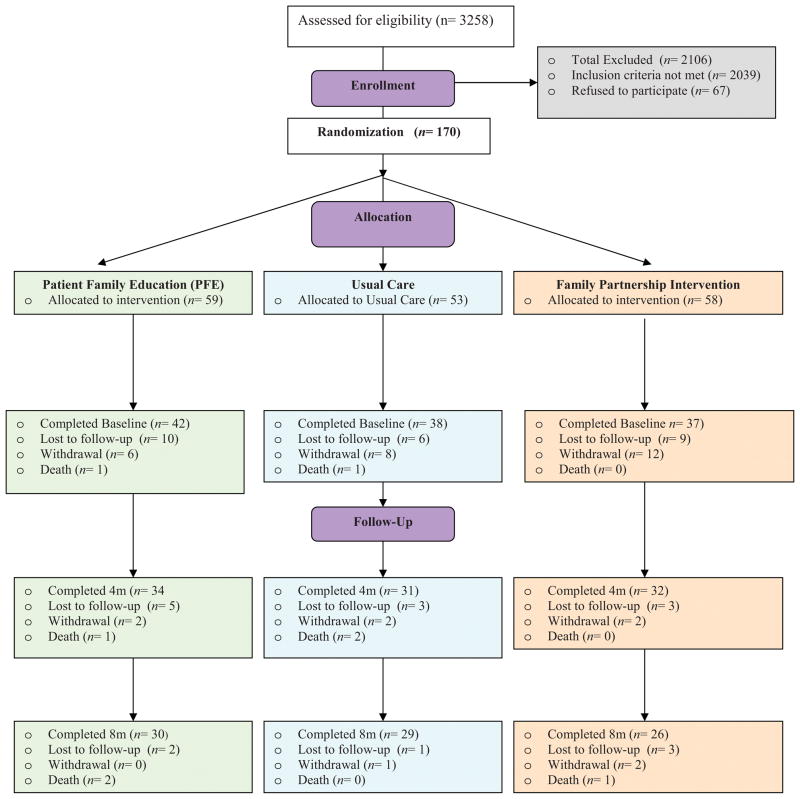
The recruitment procedures resulted in 170 consented dyads, and the CONSORT chart (Figure 1) reflects randomization/attrition from groups. There was an overall attrition rate of 31% between consent and BL data collection because of 2 deaths, and 51 withdrawals/loss to follow-up due to the HF patients’ progressive illness, family member illness or drop, loss of interest in the study, travel or other activities that interfered with ability to participate. Thus, 117 dyads completed the BL data collection, and the randomization procedures resulted in no significant differences among groups in demographic or clinical characteristics (Table 1). Of the participants enrolled at BL, 21(17.9%) left the study before 4M (3 deaths, 6 withdrawn, and 12 loss to follow-up) and an additional 11 participants left the study between 4M and 8M (3 deaths, 2 withdrawn, 6 loss to follow-up) for an overall attrition rate of 27.4% (Figure 1). Attrition was even across the 3 groups with no significant differences between groups on amount of missing data at 4M (χ2(2)=0.845, p=.655) and at 8M (χ2(2)=0.394, p=.821).
Figure 1.
Consort Chart
Table 1.
Demographic and Clinical Characteristics of Persons with HF
| Variable | Total N = 117 |
Usual Care n =38 |
PFE n =42 |
FPI n=37 |
p-values ANOVA or χ2 tests |
|---|---|---|---|---|---|
| Age Range years | 28 – 78 | 31 – 74 | 28 – 78 | 38 – 78 | p=.793 |
| M±SD | 55.9 ± 10.5 | 55.8 ± 10.3 | 56.7 ± 11.1 | 55.1 ± 10.2 | |
| Gender M/W% | 63/37 | 68/32 | 55/45 | 68/32 | p=.361 |
| Education | |||||
| % ≥ College | 47.9 | 55.3 | 52.4 | 35.1 | p=.167 |
| Ethnicity | |||||
| % Caucasian | 42 | 37 | 43 | 46 | p=.717 |
| % AA | 58 | 63 | 57 | 54 | |
| Family Member | |||||
| % spouse/partner | 52.6 | 56.8 | 40.5 | 62.2 | p=.203 |
| % adult child/sibling^ | 22.4 | 24.3 | 23.8 | 18.9 | |
| % other^ | 25.0 | 18.9 | 35.7 | 18.9 | |
| NYHA Class | |||||
| % Level II | 72.6 | 76.3 | 69.0 | 73.0 | p=.766 |
| % Level III | 27.4 | 23.7 | 31.0 | 27.0 | |
| LVEF M±SD | 26.9 ± 13.7 | 24.4 ± 11.2 | 25.8 ± 12.8 | 30.7 ± 16.5 | p=.178 |
| N=91 | n=30 | n=32 | n=29 | ||
| Charlson | |||||
| Comorbidity Index M±SD | 3.1 ± 2.2 | 2.9 ± 1.8 | 3.0 ± 2.1 | 3.2 ± 2.7 | p=.846 |
| BMI M±SD | 33.7± 8.4 | 33.1± 7.9 | 33.7± 8.5 | 34.4± 9.0 | p=.799 |
| Serum Creatinine (mg/dl) M±SD | 1.30± .41 | 1.37± .53 | 1.26± .36 | 1.29± .32 | p=.496 |
| Diuretics | |||||
| Thiazides (%) | 7.8 | 7.9 | 9.8 | 5.6 | p=.658 |
| Potassium sparing | |||||
| Spironolactone (%) | 40.9 | 34.2 | 43.9 | 44.4 | p=.593 |
| Furosemide | 33.0 | 31.6 | 34.1 | 33.3 | |
| equivalents (%)* | 42.6 | 39.5 | 43.9 | 44.4 | |
| 0 (0 mg/day) | 17.4 | 18.4 | 17.1 | 16.7 | |
| 1 (≤40 mg/day) | 2.6 | 5.3 | 0.0 | 2.8 | |
| 2 (41–80 mg/day)^ | 4.4 | 5.3 | 4.9 | 2.8 | p=.734 |
| 3 (81–160 mg/day)^ | |||||
| 4 (>160 mg/day)^ | |||||
Furosemide equivalents: Furosemide 80 mg = torsemide 40; bumetanide 3 mg; ethacrynic acid 50 mg.39
These categories were combined for the purpose of comparing the groups via chi-square analysis.
Age ranged up to 78 years, the mean age was in the mid fifties; women comprised over 30% of the sample, and more than 50% were African American. (Table 1) This was a fairly well educated group in that 47% had college education. HF participants were mainly NYHA Class II, tended to be slightly overweight with 36% having BDI-II scores indicating mild to moderate depressive symptoms. The participating FMs were predominately spouses, and the next largest category was adult child. Family members ranged in age from 19 to 78 years with an average of (mean 52.3±13.3) and were characterized as 83% women, 59% African American, and 48% had college degrees.
Detailed information on patients’ BL medications and doses revealed a varied diuretic pattern in terms of regimens and combinations. Furosemide equivalents as well as the percent on hydrochlorothiazide (HCTZ) and spironolactone did not differ by group. Approximately one-third (33 %) were not on daily loop diuretics although prn diuretic regimens were present.
Dietary Sodium
Table 2 presents the unadjusted means and standard deviations of the dietary NA from the 3DFR and 24-hr urine. Dietary NA intake values showed great variation at all time points, and are described here to emphasize patterns of intake and the high range of dietary NA ingestion.(Table 2) At BL, there were no group differences (F(2,112)=1.402, p=.250). The unadjusted mean decrease in the Urine NA in the FPI group from BL to 4M was 677 mg/day, and this decrease was maintained with a slight further decrease of a mean of 216 mg/day from 4M to 8M; FPI had significantly lower Urine NA than UC at 8M. The PFE group decreased by an average of 556 mg/day between BL and 4M and leveled off between 4–8M. UC decreased by a mean of 524 mg/day by 4M, but began to slightly increase between 4–8Ms. Dietary NA values obtained from the 3DFR were equivalent by group at BL (F(2,112)=0.405, p=.668). The (unadjusted) mean decrease in the 3DFR NA in the FPI group from BL to 4M was 706 mg/day with a small increase at 8 M. The PFE group decreased 724 mg/day between BL and 4M with negligible decrease between 4–8M. UC actually increased their 3DFR NA by 402 mg/day from BL to 4M, but decreased by 425 mg/day from 4M to 8M. Correlations between the self-report 3 DFR and Urine NA were significant (p < .05) at .34 at baseline, .41 at 4 M and .47 at 8 M. These relationships are consistent with prior studies.33, 41
Table 2.
Unadjusted Means for Daily Dietary Sodium from 3-day Food Record and 24-hr Urine (mg)
| Group | Baseline N M ±SD (mg) (Min-Max) |
4 M N M ±SD (mg) (Min-Max) |
8-M N M ±SD (mg) (Min-Max) |
|---|---|---|---|
|
| |||
| Subjects Enrolled [UC, PFE, FPI] | 117 [38, 42, 37] | 96 [31, 33, 32] | 85 [29, 30, 26] |
|
| |||
| UC | 38 | 30 | 29 |
| 3-day food record | 2483 ± 999 (768–4819) | 2885 ± 1571 (149–7548) | 2460 ± 1153 (783–5001) |
|
| |||
| 24-hour urine | 37 | 30 | 29 |
| 4238 ± 2044 (1656–9757) | 3714 ± 1912 (1196–8947) | 3879 ± 2138 (870–9591) | |
|
| |||
| PFE | 40 | 33 | 29 |
| 3-day food record | 2727 ± 1792 (522–9251) | 2003 ± 1066 (709–4846) | 1938 ± 986 (308–4981) |
|
| |||
| 24-hour urine | 41 | 33 | 30 |
| 3657 ± 1604 (1219–7498) | 3101 ± 1536 (851–6371) | 3224 ± 2349 (506–8694) | |
|
| |||
| FPI | 37 | 32 | 25 |
| 3-day food record | 2753 ± 1411 (705–7327) | 2047 ± 1123 (394–4870) | 2245 ± 1363 (679–5428) |
|
| |||
| 24-hour urine | 37 | 32 | 26 |
| 3610 ± 1765 (736–9269) | 2933 ± 1904 (322–7061) | 2717 ± 1621 (1035–6578) | |
Numbers of subjects enrolled at each time are provided overall and by group to present missing values due to attrition, or incomplete urine collections. Note: Groups did not differ on dietary sodium intake at baseline. Correlations of self-report from the 3-day food record and 24-hr sodium were: .31 at baseline, .41 at 4 M, and .47 at 8 M.
Comparison of Groups at Initiation and Maintenance Phases
Participants who were missing at 8M either due to overall attrition and/or inadequate urinary NA tended to have higher depressive symptoms (BDI-II scores) at BL (t(112)=1.897, p=.06). Thus, these data meet the criteria for covariate-dependent MCAR assumptions, and BDI-II scores were included in the models along with covariates of gender and furosemide equivalents. Multi-level models (MLM) were used to maximize all available data to test for group, time and group-by-time effects for urine NA and 3DFR NA levels adjusting for gender and depression in both models, and adding furosemide equivalents to the urine NA model. While not statistically significant, participants with missing data at 4M and 8M did have slightly higher urine NA levels at BL, indicating a trend towards MAR assumptions, which are further accounted for in the MLM procedures.
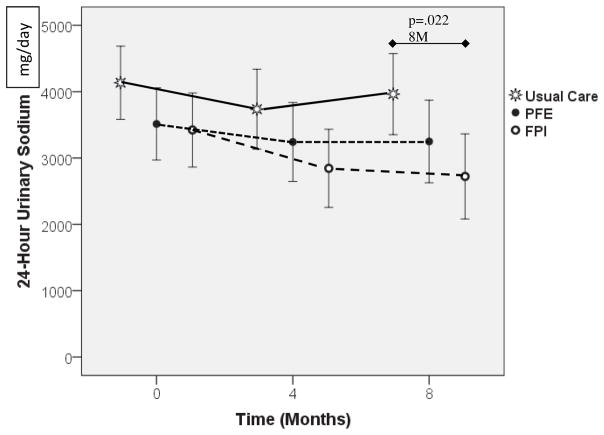
Figure 2 shows the adjusted mean 24-hour Urine NA across time by study groups (with 95% confidence intervals) from the MLM ANCOVA adjusting for the covariates of gender, BDI-II scores, and furosemide equivalents. Overall there was a significant group difference (F(2,102.508)=4.736, p=.01) and close to significant time effect (F(2,182.262)=2.762, p=.06). There was no group difference at BL (F(2,231.970)=1.915, p=.15), nor at 4M (F(2,250.521)=2.181, p=.115). However, at 8M there was a group difference (F(2,262.677)=3.863, p=.022), where post hoc pairwise comparison using SIDAK adjustment revealed that the FPI group had significantly lower Urine NA than UC (mean difference 1241.038, SE=449.427, p=.018) which is a moderate-to-large effect size of 0.54 using Cohen’s d58 with an observed power of 75.6%. While not statistically significant, it is worth noting that the FPI group reduced their urinary NA(covariate adjusted) by an average of 578 mg/d (SE=336, p=.239) from 0 to 4M (based on 32 FPI participant at 4M, the estimated effect size was 0.30 which is small) and by a mean of 700 mg/d (SE=360, p=.152) from BL-8M ((based on 26 FPI participants at 4M the effect size is estimated at 0.38 which is small-to-moderate). These are clinically significant reductions. Neither UC, nor PFE achieved adjusted mean reductions more than 500 mg/day from BL.
Figure 2.
24-hour Urine Sodium Over Time. Markers indicate means adjusted for covariates (gender, BDI, Furosemide Equivalents) for each group. Error bars indicate 95% confidence intervals. At 8mo there was a significant group difference (p=.022): post hoc tests indicated FPI group was significantly lower than UC at 8 M (p=.018). Covariates: Gender (p=.001), BDI-II (p<.001), Furosemide Equivalents (p=.012).
Gender was significant in the model with women having lower 24-hour urine NA compared to males (p=.001). Higher 24-hour urine NA were found in those with higher BDI-II scores (p<.001), and higher BL Furosemide equivalents (p=.01).
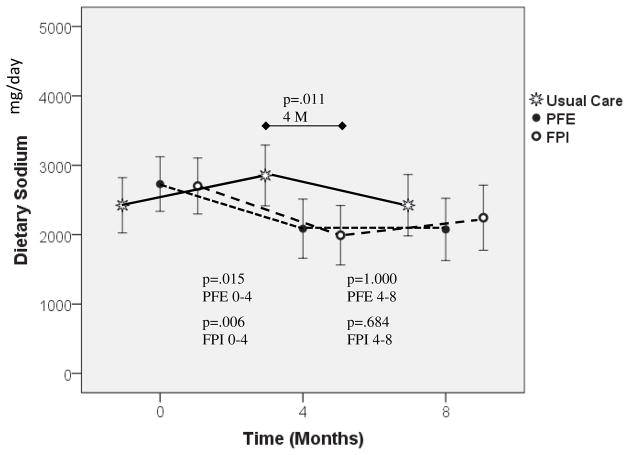
Figure 3 shows the mean dietary sodium from the 3DFR across time by study groups (with 95% confidence intervals) from the MLM ANCOVA adjusting for the covariates of gender and BDI-II. There was no group difference (F(2,113.094)=0.817, p=.444) but time (F(2,189.252)=4.344, p=.014) and group-by-time interaction (F(4,189.090)=3.970, p=.004) effects were observed. Gender was significant in the model with women having lower 3DFR dietary sodium values compared to men (p=.001). Those with higher BDI-II scores did not have significantly higher 3DFR dietary NA(p=.152), however the BDI-II scores were kept in the model given the association between depression and attrition at 8M. There was no group difference at BL or at 8M, but there was at 4M (F(2,240.955)=4.606, p=.011), where post hoc pairwise comparison using SIDAK adjustment revealed that both PFE (p=.042; moderate effect size 0.45) and FPI (p=.018; moderate effect size 0.51) had lower self-report dietary NA than UC. Significant time effects were found for PFE (F(2,189.360)=5.417, p=.005) and FPI (F(2,189.004)=4.972, p=.008). Post hoc tests for PFE revealed significant reductions from BL-4M (p=.015 moderate effect size 0.49, 79% power) and BL-8M (p=.019, moderate effect size 0.51, 76% power) with no change from 4–8M. Post hoc tests for FPI revealed significant reductions from 0–4M (p=.006, moderate effect size 0.55, 85% power), with no change from 4–8M.
Figure 3.
Dietary (3DFR) Sodium Over Time. Markers indicate means adjusted for covariates (gender and BDI) for each group. Error bars indicate 95% confidence intervals. At 4mo there was a significant group difference (p=.011): post hoc tests indicated PFE was significantly lower than UC (p=.042) and FPI group was significantly lower than UC (p=.018). No significant time effect was found for UC. However there were significant time effects for PFE (p=.005) [post hoc tests indicated significant reduction between BL-4mo (p=.015) and no significant change from 4–8Mo (p=1.000)] and for FPI (p=.008) [post hoc tests indicated significant reduction between 0–4mo (p=.006) and no significant change from 4–8M (p=.684)]. Covariates: Gender (p=.001), BDI (p=.152).
Analysis of Proportions Adherent to Dietary NA Recommendations
Adherence to dietary NA intake was defined as Urine NA ≤ 2500 mg/day based on the guidelines of 2–3 g/day and the patient education and intervention goals emphasizing this range. Groups were compared on the proportion considered adherent at each time point. By 8M, both the PFE and the FPI groups doubled their percent adherent from baseline, and both had >50% participants who were adherent compared to the usual care group (Figure 4). The proportion of participants considered adherent to dietary sodium intake was higher in FPI (43.8% 4M; 61.5% 8M) and PFE (36.4% 4M, and 53.3% 8M) groups compared to UC (30.0% 4M; 27.6% 8M). At 8M, the proportions significantly differed with those in the UC group being less likely to be in the ≤2500 group (χ2(2)=7.07, p=.029) (Figure 4).
Figure 4.
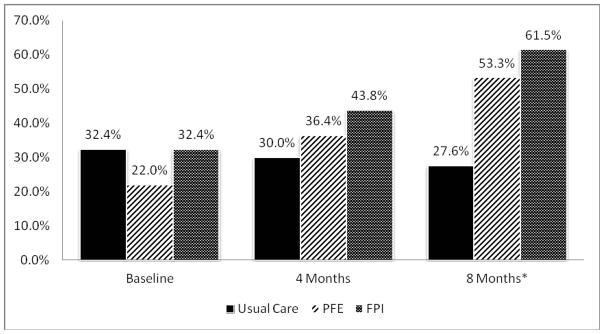
Proportion in Each Group Adherent to Low Sodium Diet (≤ 2,500 mg/day) Across Time. UC is less likely to be in the adherent (≤2500mg/day) category than intervention groups at 8 M, Chi Square= 7.076, p=0.029.
Medication Adherence
In general, adherence to HF medications was high (>80%) at BL and across time in this sample, however missing MEMS® data were high due to poor participant compliance with the system. The MA data are presented in Table 3 and include the MEMS® monitoring for the three groups at all time-points for both the monitored primary HF medication and diuretic. The data reflect the mean % of prescribed doses taken at each time point. More men were missing the monitored HF medicine doses at 4M (χ2(1)=3.650, p=.056) and younger participants had more missing diuretic doses at all 3 time points.
For the HF medication, The MLM ANOVA revealed that there was no group, time, or group by time interaction for the MA variable when controlling for age, gender, NYHA class, and CMI. For diuretic adherence, there were group differences (F(2,97.219)=3.353, p=.039), and differences across time (F(2,150.474)=3.192, p=.044) when controlling for age (p=.011), gender (p=.526), NYHA (p=.097), and CMI (p=.026). A difference between BL and 8M (mean difference 6.525, SE=2.583, p=.037) was found for diuretic adherence, and post hoc tests revealed this was between UC and FPI (mean difference=14.582, SE=5.670, p=.032) with FPI demonstrating lower diuretic adherence than UC at 8M. Older HF participants and those with more comorbidities had greater MA adherence.
The MMAS was analyzed by percentages within each group who were considered at the level of high adherence (score>8).51 There were no significant group differences among groups for the proportions of high adherence at BL, 4M and 8M respectively: UC 81.6%, 87.1%, 86.2%; PFE 84.6%, 80.6%, 76.7%, and FPI 88.9%, 71.9%, 80.8%.
HF Knowledge, Autonomy Support, and Perceived Criticism
The AHFKT, FCCQ-P and Perceived Family Criticism (PC) scores were examined to determine if the interventions had the intended effect of increased HF knowledge in both intervention groups, and increased autonomy support and lower perceived criticism in the FPI group (See Table 4). No differences by group were found on any score at BL. Immediately after the BL dyadic educational intervention session, HF patient knowledge scores in the PFE and FPI groups increased from means in the 70% correct range to means of 83.4% (± 8.3) and 84.0% (± 11.0) respectively. Because UC did not have a BL dyadic education session, they did not have an immediate post intervention measure. Analysis of covariance of AHFKT scores by group using the BL knowledge score and education level as covariates revealed group differences at 4M (F 2,88 =3.169, p =.047, moderate effect size d=0.54),58 and post hoc testing revealed that FPI was slightly higher than UC (p=0.02). However, all groups showed significant declines in knowledge across time.
Table 4.
HF Knowledge (AHFKT), Autonomy Support (FCCQ-P), and Perceived Family Criticism (PFC) Scores in HF patients by group across time.
| Group | Baseline N M ±SD |
Immediately post BL dyad teaching intervention | 4- Ma N M ±SD |
8-M N M ±SD |
|---|---|---|---|---|
|
| ||||
| Subjects Enrolled [UC, PFE, FPI] | 117 [38, 42, 37] | 96 [31, 33, 32] | 85 [29, 30, 26] | |
|
| ||||
| UC | 38 | 31 | 29 | |
| AHFKTb | 71.8 ± 14.4 | 53.5 ± 10.7 | 56.8 ± 11.1 | |
| FCCQ-P | 5.7 ± 0.9 | 5.8 ± 1.0 | 5.8 ± 0.8 | |
| PFC | 1.9 ± 0.9 | 1.9 ± 1.0 | 1.8 ± 0.9 | |
|
| ||||
| PFE | 39 | 32 | 30 | |
| AHFKTb | 73.7 ± 11.3 | 83.3 ± 8.2 | 58.8 ± 8.2 | 62.2 ± 6.8 |
| FCCQ-P | 6.0 ± 0.8 | 6.1 ± 0.6 | 6.1 ± 0.9 | |
| PFC | 2.0 ± 0.9 | 1.8 ± 0.9 | 1.7 ± 0.9 | |
|
| ||||
| FPI | 36 | 32 | 26 | |
| AHFKTb | 72.5 ± 12.4 | 84.0 + 11 | 58.3 ± 11.5 | 61.5 ± 10.3 |
| FCCQ-P | 5.8 ± 1.0 | 6.0 ± 1.3 | 6.2 ± 0.7 | |
| PFC | 1.7 ± 0.7 | 1.5 ± 0.7 | 1.6 ± 0.6 | |
AHFKT – Atlanta Heart Failure Knowledge Test; FCCQ-P – Family Care Climate Questionnaire – patient version; PFC = perceived family criticism. Using ANCOVA with BL knowledge and education as covariates and SIDAK post hoc comparisons:
At 4 M, there were significant differences between groups: F(2,88)=3.169, p= 0.047, post hoc pairwise comparisons show that there was significant differences between UC and FPI (mean difference 4.898, SE=2.109, p=0.02);
For all 3 groups, 4M and 8M scores are lower than BL (p<.001).
Overall, FCCQ-P scores were high for all groups. Participants in the FPI group demonstrated a slight increase in FCCQ-P scores, from BL to 4M and continued to increase at 8M; however this was not a significant change when analyzed with analysis of covariance using the BL value as a covariate. The PFE group did not differ significantly on FCCQ-P scores from UC or FPI groups at any time point. PFC scores also did not show a group difference using analysis of covariance adjusting for the BL value.
DISCUSSION
Based on the objective 24-hour urine NA measures, the theory based FPI intervention reduced dietary NA intake compared with UC at 8M. The FPI, in addition to the education and feedback, was effective in fostering the initiation and maintenance of lifestyle changes needed to reduce dietary NA. The data reflect a sustained effect of lowering dietary NA intake in the FPI group even though we expected to see little change between 4 and 8 M (maintenance).Telephone booster sessions which included education and autonomy support counseling maintained the initial reduction in dietary sodium in the FPI group, demonstrating the importance in reinforcing both dietary education and supportive family communication. Although the PFE group exhibited clinically meaningful decreases in dietary sodium intake by 8M, the decline did not differ from UC and was more gradual in the initiation phase in comparison to FPI. This suggests that the PFE group may have required the second individualized dietary sodium intake feedback component as well as the telephone booster education session to support the changes. Both FPI and PFE groups increased the proportion of participants whose urinary sodium values reflected dietary sodium intake closer to goal of < 2500 mg/day by 8 M. Both FPI and PFE reduced their self-reported dietary sodium intake by 4M in comparison with UC, and sustained this effect. Although routine usual care in the recruitment settings was strong, the data suggest UC group exhibited little change and remained far above recommended dietary NA intake for HF patients. Structured family education and support approaches, repetitive and reinforced patient and family education, and methods to provide patient feedback on dietary sodium intake were essential to affect clinical change in dietary sodium intake.
Covariates (gender, depressive symptoms, furosemide equivalents) were important in the statistical model. Similar to other studies, women tended to have greater dietary adherence than men.59 Those with greater depressive symptoms were less adherent and were more likely to drop from the study or have incomplete urine collections reflecting greater difficulty attending to both their diet self-care and study requirements. The significance of furosemide equivalents in the model may have been a reflection of illness severity with those on higher diuretic doses having greater difficulty with dietary adherence.
The interventions did not affect medication adherence. In general, medication adherence was high at baseline with all groups close to or greater than 80%. Wu and colleagues have reported acceptable adherence as ≥ 88% in predicting event free survival,60 as well as the benefit of feedback on medication-taking behavior. The lack of intervention effect may have been from a ceiling effect at BL limiting the opportunity for improvement. Another possible explanation may be that medication-taking behaviors may be less interdependent than managing diet in this sample. Alternatively, a stronger dose of the intervention may have been required. Other factors that may influence medication taking behavior besides knowledge and family support, such as access to medicines or socioeconomic factors, were beyond the scope of the intervention. Of interest is the decrease in the FPI diuretic adherence at 8 M which coincided with lower dietary sodium intake, perhaps reflecting greater self adjustment of their diuretics; however this requires further evaluation.
In a survey of 439 functionally independent persons with HF, 78% of respondents reported that involved family members criticized or badgered them about care related to their HF, and family barriers were negatively associated with patient’s self-management adherence.18 Our intervention was designed to facilitate a family partnership by improving autonomy support and reducing family criticism perceived by the HF patient, reflecting improved support in the family context. The use of scenarios and discussion was an effective way to teach autonomy support strategies to the patients and family members, which resulted in slightly greater (although not statistically significant) perceived autonomy support by the patient. A higher dose of intervention may be needed to observe a reported change in these family patterns and perceptions which may have been well established. Alternatively, the perceived autonomy support scores were high and there may have been a ceiling effect with the instrument, thus more sensitive measures of autonomy support in HF may be needed. The two intervention groups (FPI and PFE) did not differ in dietary sodium intake at the end of the 8 M. Thus, having both patient and family member together in the PFE sessions may have contributed to a feeling of partnership/support. However, as demonstrated in the mean Urine NA values, the FPI group had more rapid decline in dietary sodium intake indicating that an intervention focused on teaching families supportive communication did result in the FPI participants having greater expected initiation as well as maintenance of dietary behavior change.
Participant knowledge about HF increased significantly in both intervention groups immediately after the baseline dyadic education sessions. However, retention of knowledge across time in all groups was remarkably low. Educational strategies in clinical and research settings are needed to enhance knowledge retention. Self-management of HF is complex and ongoing often for years, thus HF patients and family members need ongoing reinforcement and repetition of education to fully understand how to manage the illness as it progresses. Although having knowledge is important, having the resources about HF and diet available to help with daily self-management activities may have been more important than the ability to recall information about HF and sodium content in foods in a testing format.
The study supports aspects of the conceptual model21 by the positive relationships observed between gender, depressive symptoms (BDI-II scores), furosemide equivalents and self-management behavior of dietary NA reduction. Additionally and more importantly, strategies to improve knowledge and autonomy support in the family context were effective in improving the self -management behavior of reducing dietary NA intake. These supported theoretical relationships add to the knowledge base of how patients may be motivated to adhere to HF self-management behaviors and provide additional insight for the usefulness of the model and self-determination theory in understanding HF self-care behaviors.
There are limitations to this study. First, participant attrition was high. The HF population is one that experiences exacerbations of symptoms, frequent hospitalizations, and death, making outpatient behavioral intervention participation at prescribed times challenging, and resulting in the need for correction of missing data. Attrition is compounded by family member loss to the study as well, which is not uncommon in family focused studies. Second, regarding intervention effects and design, pragmatically, the patient-family dyad could not be blinded to the intervention, and the effects of the individual intervention components (education, feedback on dietary sodium and medication-taking, family support) cannot be identified. Both the PFE and FPI groups had the opportunity to learn about their actual dietary sodium from the feedback reports and viewed this as a highly valuable aspect of the intervention, yet a possible limitation is any bias introduced from the education intervention which included feedback on an outcome of the study. While the 24 hour urine collection occurred on the 3rd day of the 3DFR, it is unlikely that participants were able to manipulate the measurements without having learned from the intervention education/counseling. Feedback on NA measures were based on 24h urine and 3DFR from the earlier time point which was 4 months prior. Third, measures of dietary sodium intake are imprecise and may be affected by inaccurate or incomplete self-report, medications and loop diuretics, and other physiological mechanisms. 61, 62 Although furosemide equivalents were used to correct for the effect of loop diuretics on Urine NA, this may be inexact. The one 24-hour urine and one 3DFR at each time point may not reflect true day to day behavior. However, few studies have reported an objective measure of dietary sodium intake, and given the overall high urine NA values and wide ranges, particularly for the UC group, we suggest that participants did not alter their diets only because they were being tested. Fourth, the use of the electronic medication monitoring system may not be the best approach for measuring medication adherence in this group as many were adverse to using the system, used alternative methods such as pill boxes which may have limited cap openings, and challenged the reported medication adherence data received during feedback sessions as being too low. Even though detailed instructions on how to use the MEMS® was provided by experienced research nurses, the system was problematic, especially for measuring two medications. The congruence between the self-report MMAS and MEMS® system helped verify and interpret the data trends.
This study has several strengths. The eligibility criteria standardized the effect of varying medication regimens and enrolled HF participants receiving optimal medical treatment consistent with guidelines but earlier in the HF trajectory (Class II and III). In addition, this sample had moderately high representation of African Americans, an underrepresented minority group who are disproportionately affected by HF and not often well represented in studies.63,14, 64 The use of objective and self-report measures for both dietary sodium intake and medication adherence was useful in that the patterns of data over time were congruent for both types of measures and both variables increasing confidence in the findings. Participants especially liked the feedback reporting system providing them with an accurate picture of their dietary sodium intake. If feedback had relied on using only the self-reported dietary sodium from the 3 DFR, on average, persons with HF would have received information indicating they were close to recommended sodium intake and would not have recognized the need to increase efforts to reduce their sodium. This accurate knowledge is important for self-monitoring for behavior change 34 and in current clinical practice, persons with HF receive little tailored objective feedback on their self-care behaviors. Although strong trends in reduced dietary sodium were noted at 4M, this is a short time frame for a behavior change, and the second feedback report at 4 M and measure at 8M were important. The overall participant adherence to the intervention was high, and the booster telephone intervention for the FPI group dyads to promote autonomy support was also tailored which was a strength of the intervention. Attrition and missing outcome measures due to lack of complete urinary collections were challenging in the data analysis. An indepth analysis of missing data was conducted to examine variables to include to correct for this in the analysis. The important relationship between depressive symptoms and attrition and inclusion of depressive symptoms in the model increases confidence in the findings. The analysis of the change in urinary sodium and the clinically meaningful approach of the proportion of achieving the recommend sodium intake goal coincided with the trends for the other analyses.
The study adds evidence for the benefits of incorporating family members into the education and counseling of HF patients. Agren and colleagues16 tested a dyad intervention and found greater HF patient perceived control, but acknowledged the need for more intense and frequent family involvement. Lofvenmark et al65, 66 tested a family member only education group and found no effect on the HF patients’ anxiety, depression, or quality of life or rehospitalization, but identified social support as an area requiring greater provider attention. More research is needed to identify the salient factors and efficient ways to provide family care.
In practice, family involvement may be inadequate due to lack of provider attention to families in education or unavailability of the appropriate HF family member at the time patient education is provided. Additionally, the benefits of supporting family members in a strategy of motivating communication based on autonomy support approaches are evident in the outcomes of this study reflecting desired behavior change related to dietary sodium, increased HF knowledge and perceived support from family members. Although this was a complex study and intervention, the outcomes provide directions for future work focused on an important resource in HF care, the family. These approaches will require further study and simplification to refine the approach and develop clinically feasible, family education and support interventions. For example, the educational component of the PFE and FPI interventions could be adapted to a video or internet format and tested to increase access and reach larger numbers of dyads. In practice, family focused care should be incorporated to a greater degree by increased attention to incorporating a designated family caregiver consistently into education and counseling sessions, and by teaching family members greater support strategies. Family focused care to achieve desired patient behavior change deserves additional development, study and testing for its effects on other important outcomes such as HF hospitalizations and family caregiver outcomes.
Table 3.
HF Medication Adherence based on the MEMS (number of actual dosages/number of prescribed dosages X 100 = % adherence)
| Group | Baseline N M ±SD (%)(Min-Max) |
4- M N M ±SD (%)(Min-Max) |
8-M* N M ±SD (%)(Min-Max) |
|---|---|---|---|
|
| |||
| Subjects Enrolled [UC, PFE, FPI] | 117 [38, 42, 37] | 96 [31, 33, 32] | 85 [29, 30, 26] |
|
| |||
| UC | 32 | 25 | 23 |
| HF Medication | 79.5 ± 26.2 (15 – 100) | 87.1 ± 14.3 (52 – 100) | 87.9 ± 14.6 (48 – 100) |
|
| |||
| Diuretic | 26 | 22 | 25 |
| 89.2 ± 15.4 (48 – 100) | 89.7 ± 12.7 (62 – 100) | 88.0 ± 14.4 (49 – 100) | |
|
| |||
| PFE | 35 | 27 | 26 |
| HF Medication | 91.2 ± 12.5 (43 – 100) | 89.2 ± 16.4 (33 – 100) | 89.5 ± 14.1 (52 – 100) |
|
| |||
| Diuretic | 34 | 27 | 26 |
| 91.9 ± 13.6 (53 – 100) | 85.6 ± 17.8 (43 – 100) | 83.8 ± 19.9 (33 – 100) | |
|
| |||
| FPI | 34 | 22 | 20 |
| HF Medication | 87.3 ± 19.7 (38 – 100) | 85.5 ± 19.6 (29 – 100) | 82.9 ± 22.6 (29 – 100) |
|
| |||
| Diuretic * | 29 | 18 | 16 |
| 83.8 ± 26.1 (10 – 100) | 84.9 ± 23.0 (33 – 100) | 73.2 ± 30.1 (19 – 100) | |
No significant overall differences by group or time.
For diuretic doses, there was a significant decline in FPI between BL and 8M (p=.037), and there was a significant difference between usual care and FPI at 8 M (p=.032).
Acknowledgments
This study was supported by (1) A Family Partnership Intervention for Heart Failure”, (RO1 R08800) National Institute of Nursing Research, NIH 07/01/04-11/30/09, PI: S. Dunbar and in part by PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources and PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources and Nitromed for unrestricted educational grant.
We acknowledge Judy Robinson, RN, MSN, Kendaly Meadows, RN, MA, Bridget Fielder, RN, MSN and Christina Quinn, RN, PhD for their outstanding contributions to this project.
Footnotes
Disclosures
There are no disclosures or conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WHW, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of Cardiac Failure. 16(6):e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Bentley B, De Jong MJ, Moser DK, Peden AR. Factors related to nonadherence to low sodium diet recommendations in heart failure patients. European Journal Of Cardiovascular Nursing. 2005;4(4):331–336. doi: 10.1016/j.ejcnurse.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SJ, Lane KA, Welch J, Perkins SM, Brater DC, Murray MD. Medication and dietary compliance beliefs in heart failure. Western Journal Of Nursing Research. 2005;27(8):977–993. doi: 10.1177/0193945905280253. [DOI] [PubMed] [Google Scholar]
- 4.Wu JR, Moser DK, Lennie TA, Peden AR, Chen YC, Heo S. Factors influencing medication adherence in patients with heart failure. Heart & Lung. 2008;37(1):8–16. doi: 10.1016/j.hrtlng.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clinic Proceedings. 2011;86(4):273–281. doi: 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setoguchi S, Choudhry NK, Levin R, Shrank WH, Winkelmayer WC. Temporal trends in adherence to cardiovascular medications in elderly patients after hospitalization for heart failure. Clinical Pharmacology and Therapeutics. 2010;88(4):548–554. doi: 10.1038/clpt.2010.139. [DOI] [PubMed] [Google Scholar]
- 7.Gudmundsson K, Lynga P, Karlsson H, Rosenqvist M, Braunschweig F. Midsummer Eve in Sweden: a natural fluid challenge in patients with heart failure. European Journal of Heart Failure. 2011;13(11):1172–1177. doi: 10.1093/eurjhf/hfr124. [DOI] [PubMed] [Google Scholar]
- 8.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clinical Pharmacology and Therapeutics. 2009;85(6):651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart & Lung: Journal of Acute & Critical Care. 2001;30(4):294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 10.Bennett SJ, Huster GA, Baker SL, Milgrom LB, Kirchgassner A, Birt J, Pressler ML. Characterization of the precipitants of hospitalization for heart failure decompensation. American Journal of Critical Care. 1998;7(3):168–174. [PubMed] [Google Scholar]
- 11.Arcand J, Ivanov J, Sasson A, Floras V, Al-Hesayen A, Azevedo ER, Mak S, Allard JP, Newton GE. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. American Journal of Clinical Nutrition. 2011;93(2):332–337. doi: 10.3945/ajcn.110.000174. [DOI] [PubMed] [Google Scholar]
- 12.Clark AM, Freydberg CN, McAlister FA, Tsuyuki RT, Armstrong PW, Strain LA. Patient and informal caregivers’ knowledge of heart failure: necessary but insufficient for effective self-care. European Journal of Heart Failure. 2009;11(6):617–621. doi: 10.1093/eurjhf/hfp058. [DOI] [PubMed] [Google Scholar]
- 13.Saarmann L, Daugherty J, Riegel B. Patient teaching to promote behavioral change. Nursing Outlook. 2000;48(6):281–287. doi: 10.1067/mno.2000.107277. [DOI] [PubMed] [Google Scholar]
- 14.Flynn KJ, Powell LH, Mendes de Leon CF, Muñoz R, Eaton CB, Downs DL, Silver MA, Calvin JE. Increasing self-management skills in heart failure patients: a pilot study. Congestive Heart Failure. 2005;11(6):297–302. doi: 10.1111/j.1527-5299.2005.04361.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith B, Forkner E, Krasuski RA, Galbreath AD, Freeman GL. Educational attainment has a limited impact on disease management outcomes in heart failure. Disease Management. 2006;9(3):157–166. doi: 10.1089/dis.2006.9.157. [DOI] [PubMed] [Google Scholar]
- 16.Agren S, Evangelista LS, Hjelm C, Stromberg A. Dyads affected by chronic heart failure: a randomized study evaluating effects of education and psychosocial support to patients with heart failure and their partners. Journal of Cardiac Failure. 2012;18(5):359–366. doi: 10.1016/j.cardfail.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang B, Luttik ML, Dracup K, Jaarsma T. Family caregiving for patients with heart failure: types of care provided and gender differences. Journal of Cardiac Failure. 2010;16(5):398–403. doi: 10.1016/j.cardfail.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illness. 2010;6(1):22–33. doi: 10.1177/1742395309354608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molloy GJ, Johnston DW, Witham MD. Family caregiving and congestive heart failure. Review and analysis. Eur J Heart Fail. 2005 Jun;7(4):592–603. doi: 10.1016/j.ejheart.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Saunders MM. Factors associated with caregiver burden in heart failure family caregivers. Western Journal of Nursing Research. 2008;30(8):943–959. doi: 10.1177/0193945908319990. [DOI] [PubMed] [Google Scholar]
- 21.Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. Journal of Cardiovascular Nursing. 2008;23(3):258–265. doi: 10.1097/01.JCN.0000305093.20012.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher R, Luttik M, Jaarsma T. Social Support and Self Care in Heart Failure. The Journal of cardiovascular nursing. 2011;26(6):439–445. doi: 10.1097/JCN.0b013e31820984e1. [DOI] [PubMed] [Google Scholar]
- 23.Riegel B, Vaughan Dickson V, Goldberg L, Deatrick J. Factors associated with the development of expertise in heart failure self care. Nursing Research. 2007;56(4):235–243. doi: 10.1097/01.NNR.0000280615.75447.f7. [DOI] [PubMed] [Google Scholar]
- 24.Fiscella K, Campbell TL. Association of perceived family criticism with health behaviors. Journal of Family Practice. 1999;48(2):128–134. [PubMed] [Google Scholar]
- 25.Ryan RM, Deci EL. A self-determination theory approach to psychotherapy: The motivational basis for effective change. Canadian Psychology/Psychologie canadienne. 2008;49(3):186–193. [Google Scholar]
- 26.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. The American Psychologist. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 27.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychology. 2004;23(1):58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- 28.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal Of Personality And Social Psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, Deci EL. Testing a self-determination theory intervention for motivating tobacco cessation: Supporting autonomy and competence in a clinical trial. Health Psychology. 2006;25(1):91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 30.Williams GC, Patrick H, Niemiec CP, Williams LK, Divine G, Lafata JE, Heisler M, Tunceli K, Pladevall M. Reducing the health risks of diabetes: how self-determination theory may help improve medication adherence and quality of life. The Diabetes Educator. 2009;35(3):484–492. doi: 10.1177/0145721709333856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychology. 1998;17(3):269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- 32.Clark PC, Dunbar SB. Family Partnership Intervention: A guide for a family approach to care of patients with heart failure. AACN Clinical Issues. 2003;14(4):467–476. doi: 10.1097/00044067-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Dunbar SB, Clark PC, Deaton C, Smith AL, De AK, O’Brien MC. Family education and support interventions in heart failure: a pilot study. Nursing Research. 2005 May-Jun;54(3):158–166. doi: 10.1097/00006199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Artinian NT, Magnan M, Sloan M, Lange MP. Self-care behaviors among patients with heart failure. Heart & Lung: The Journal Of Critical Care. 2002;31(3):161–172. doi: 10.1067/mhl.2002.123672. [DOI] [PubMed] [Google Scholar]
- 35.Virdi NDM, Nigam S, Kozma C, Raja P. The association of self-monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non-insulin treatment. Diabetes Technol Ther. 2012;14(9):790–798. doi: 10.1089/dia.2012.0047. [DOI] [PubMed] [Google Scholar]
- 36.Akers JD, Cornett RA, Savla JS, Davy KP, Davy BM. Daily self-monitoring of body weight, step count, fruit/vegetable intake, and water consumption: a feasible and effective long-term weight loss maintenance approach. Journal of the Academy of Nutrition & Dietetics. 2012;112(5):685–692. e682. doi: 10.1016/j.jand.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig TJ, Perlin JB, Fleming BB. Self-reported performance improvement strategies of highly successful Veterans Health Administration facilities. American Journal of Medical Quality. 2007;22(6):438–444. doi: 10.1177/1062860607304928. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of Classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. The American journal of cardiology. 2006;97(12):1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 40.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Archives of Internal Medicine. 2002;162(14):1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 41.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, Robertson J, Brown WM, McFarlane M, Group TCR. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. American Journal of Epidemiology. 2001;153(10):996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 42.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S57–60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 43.De Geest S, Abraham I, Dunbar-Jacob J. Measuring transplant patients’ compliance with immunosuppressive therapy. Western Journal of Nursing Research. 1996;18(5):595–605. doi: 10.1177/019394599601800509. [DOI] [PubMed] [Google Scholar]
- 44.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA: The Journal Of The American Medical Association. 1989;261(22):3273–3277. [PubMed] [Google Scholar]
- 45.Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia. 1995;36(11):1111–1117. doi: 10.1111/j.1528-1157.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 46.Bohachick P, Burke LE, Sereika S, Murali S, Dunbar-Jacob J. Adherence to angiotensin-converting enzyme inhibitor therapy for heart failure. Progress in Cardiovascular Nursing. 2002;17(4):160–166. doi: 10.1111/j.0889-7204.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 47.Fulmer TT, Feldman PH, Kim TS, Carty B, Beers M, Molina M, Putnam M. An intervention study to enhance medication compliance in community-dwelling elderly individuals. Journal of Geontological Nursing. 1999;25(8):6–14. doi: 10.3928/0098-9134-19990801-04. [DOI] [PubMed] [Google Scholar]
- 48.Wu J-R, Corley DJ, Lennie TA, Moser DK. Effect of a medication-taking behavior feedback theory-based intervention on outcomes in patients with heart failure. Journal of Cardiac Failure. 2012;18(1):1–9. doi: 10.1016/j.cardfail.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J-R, Chung M, Lennie TA, Hall LA, Moser DK. Testing the psychometric properties of the Medication Adherence Scale in patients with heart failure. Heart & Lung. 2008;37(5):334–343. doi: 10.1016/j.hrtlng.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of adherence behavior. Medical Care. 1986;24:67–76. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of Clinical Hypertension. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Reilly CM, Higgins M, Smith A, Gary RA, Robinson J, Clark PC, McCarty F, Dunbar SB. Development, psychometric testing, and revision of the Atlanta Heart Failure Knowledge Test. Journal of Cardiovascular Nursing. 2009;24(6):500–509. doi: 10.1097/JCN.0b013e3181aff0b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark PC, Dunbar SB. Preliminary reliability and validity of a family care climate questionnaire for heart failure. Family, Systems & Health. 2003;21(3):281–291. [Google Scholar]
- 54.Shields CG, Franks P, Harp JJ, Campbell TL, McDaniel SH. Family Emotional Involvement and Criticism Scale (FEICS): II. Reliability and validity studies. Family Systems Medicine. 1994;12(4):361–377. [Google Scholar]
- 55.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 56.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal Of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 57.Hedeker D, Giggons RD. Longitudinal Data Analysis. Hoboken, NJ: Wiley-Interscience – A John Wiley & Sons, Inc publication; 2006. [Google Scholar]
- 58.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrnece Erlbaum Associates; 1988. [Google Scholar]
- 59.Chung ML, Moser DK, Lennie TA, Worrall-Carter L, Bentley B, Trupp R, Armentano DS. Gender differences in adherence to the sodium-restricted diet in patients with heart failure. Journal of Cardiac Failure. 2006;12(8):628–634. doi: 10.1016/j.cardfail.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J-R, Moser DK, De Jong MJ, Rayens MK, Chung ML, Riegel B, Lennie TA. Defining an evidence-based cutpoint for medication adherence in heart failure. American heart journal. 2009;157(2):285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arcand J, Floras JS, Azevedo E, Mak S, Newton GE, Allard JP. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: the confounding effect of loop diuretics. American Journal of Clinical Nutrition. 2011;93(3):535–541. doi: 10.3945/ajcn.110.004457. [DOI] [PubMed] [Google Scholar]
- 62.Gerber LM, Mann SJ. Inaccuracy of self-reported low sodium diet. American Journal of Human Biology. 2012;24(2):189–191. doi: 10.1002/ajhb.22213. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian U, Hopp F, Mitchinson A, Lowery J. Impact of provider self-management education, patient self-efficacy, and health status on patient adherence in heart failure in a Veterans Administration population. Congestive Heart Failure. 2008;14(1):6–11. doi: 10.1111/j.1751-7133.2008.07174.x. [DOI] [PubMed] [Google Scholar]
- 64.Okin PM, Kjeldsen SE, Dahlof B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circulation Cardiovascular Quality & Outcomes. 2011;4(2):157–164. doi: 10.1161/CIRCOUTCOMES.110.960112. [DOI] [PubMed] [Google Scholar]
- 65.Lofvenmark C, Saboonchi F, Edner M, Billing E, Mattiasson A-C. Evaluation of an educational programme for family members of patients living with heart failure: a randomised controlled trial. Journal of Clinical Nursing. 2013;22(1–2):115–126. doi: 10.1111/j.1365-2702.2012.04201.x. [DOI] [PubMed] [Google Scholar]
- 66.Lofvenmark C, Karlsson MR, Edner M, Billing E, Mattiasson A-C. A group-based multi-professional education programme for family members of patients with chronic heart failure: effects on knowledge and patients’ health care utilization. Patient Education & Counseling. 2011;85(2):e162–168. doi: 10.1016/j.pec.2010.09.026. [DOI] [PubMed] [Google Scholar]