Abstract
Chikungunya virus (CHIKV) is an alphavirus that infects millions of people every year, especially in the developing world. The selective expression of recombinant CHIKV capsid and envelope proteins results in the formation of self-assembled virus-like particles (VLPs) that have been shown to protect nonhuman primates against infection from multiple strains of CHIKV. This study describes the characterization, excipient screening, and optimization of CHIKV VLP solution conditions towards the development of a stable parenteral formulation. The CHIKV VLPs were found to be poorly soluble at pH 6 and below. Circular dichroism, intrinsic fluorescence, and static and dynamic light scattering measurements were therefore performed at neutral pH, and results consistent with the formation of molten globule structures were observed at elevated temperatures. A library of GRAS excipients was screened for their ability to physically stabilize CHIKV VLPs using a high-throughput turbidity based assay. Sugars, sugar alcohols, and polyanions were identified as potential stabilizers and the concentrations and combinations of select excipients were optimized. The effects of polyanions were further studied, and while all polyanions tested stabilized CHIKV VLPs against aggregation, the effects of polyanions on conformational stability varied.
Keywords: Stabilization, Thermal analysis, Vaccines, Particle size, Physical stability, Physicochemical properties, Preformulation, Spectroscopy, Protein formulation
Introduction
CHIKV is a positive, single-stranded RNA alphavirus that belongs to the Togaviridae family of viruses 1 and infects millions of individuals each year.2 Symptoms include rash, fever, and blisters; excruciating joint pain, however, is the hallmark of the disease and can last for months or years beyond other symptoms.3 The virus is rarely fatal (approximately 0.1% of all cases), although serious symptoms such as respiratory failure and brain infections have been reported. The more severe symptoms primarily effect the elderly and those with other medical conditions.4 The morbidity of this disease, as a result of the lingering joint pain and inflammation, results in significant economic losses and reductions in quality of life.5,6 Currently, no vaccine or anti-viral therapy exists, and CHIKV is treated with painkillers and general anti-inflammatories.4
CHIKV was first recognized in Tanzania in 1952,7 and historically outbreaks have been limited to developing countries.8 Recently, however, outbreaks have occurred in the developed world, and this has led to increased interest in understanding the disease for the purpose of establishing preventative and therapeutic measures. In 2005, 40% of the population Réunion, a French island off the coast of Madagascar, was infected with the disease,9 and in 2007, the first European outbreak occurred in Italy.10–12 CHIKV can be transmitted by the Asian tiger mosquito (Aedes albopictus),13 and the spread of this mosquito due to shipping and transport14 creates an environment in which CHIKV outbreaks are more likely to occur in places where the disease was uncommon in the past.15
In 2008 the Vaccine Research Center at the National Institutes of Health initiated development of a vaccine against CHIKV utilizing virus-like particles (VLPs). The genomic RNA of CHIKV encodes four nonstructural proteins (nsPs) required for replication and five polyproteins including a capsid protein (C) and envelope proteins (E3, E2, 6K, and E1).16 Expression of the capsid and envelope proteins (C-E3-E2-6K-E1) in HEK-293 (human embryonic kidney-293) cells leads to their assembly into VLPs with an external diameter of approximately 65 nm, as seen by electron microscopy, that resemble alphaviruses.17 Immunization of nonhuman primates with the VLPs produced high-titer neutralizing antibodies and protected against subsequent infection with two strains of the virus.17
In this work, we characterize the biophysical properties of purified, recombinant CHIKV VLPs, screen a library of GRAS (generally recognized as safe) excipients as potential stabilizers against physical degradation, and further optimize and evaluate selected polyanion excipients with the goal of providing preformulation characterization data for the development of a stable parenteral vaccine formulation.
Materials and Methods
Sample preparation, concentration determination, and osmolality measurements
Recombinant CHIKV VLPs were expressed and purified by the Vaccine Production Program at the NIH, using a scaled up process based on that described by Akahata et al.17 CHIKV VLPs were dialyzed from a stock solution of 218 mM sucrose, 7.2 mM K2HPO4, 3.8 mM KH2PO4, pH 7.0 into one of three different buffers: 20 mM citrate-phosphate buffer (pH 3–8) with an ionic strength of 0.15, 20 mM sodium phosphate buffer with an ionic strength of 0.15 (pH 7.0), or 10 mM sodium phosphate buffer (pH 7.0) without additional sodium chloride, as indicated in the text. CHIKV protein concentration was determined using a BCA Protein Assay Kit (Pierce, Rockford, IL). Excipients were prepared as concentrated solutions containing buffer to match the VLP. Excipient, VLP, and buffer were added together to achieve the concentrations indicated in the text. Osmolality measurements were made using an OSMETTE II (Precision Systems Inc., Natick, MA) osmometer.
Far-UV circular dichroism
CD measurements were performed using a Jasco J-815 Spectrophotometer (Great Dunmow, UK) equipped with a Peltier temperature controller. Spectra were collected with a scanning speed of 20 nm/min and a resolution of 1 nm from 195–260 nm using a 0.1 cm path length cuvette sealed with a Teflon stopper was used to measure 0.2 mg/mL CHIKV VLP solutions (on VLP protein basis). The CD signal at 210 nm was collected over a temperature range of 10 to 90 °C at 0.5 °C intervals and a heating rate of 15°C/hr.
Intrinsic fluorescence and static light scattering
A PTI QM-1 spectrofluorometer (Brunswick, NJ) equipped with a four-position Peltier temperature controlled cell-holder was used to obtain fluorescence emission spectra. A 1 cm pathlength cuvette was used to measure 0.2 mg/mL CHIKV VLP solutions. No inner filter corrections were applied. An excitation wavelength of 295 nm was used for simultaneously measuring light scattering and intrinsic tryptophan fluorescence. Emission spectra were collected over a range of 305 to 405 nm at a 1 nm/s collection rate. A second photomultiplier, placed at 180° to the emission detector was used to record light scattering. Spectra were collected at 2.5 °C intervals from 10 to 87.5°C with a 3 min equilibration at each temperature. Fluorescence intensity and peak positions were obtained by a mean spectrum center of mass method, subsequent to buffer subtraction. This provides wavelength maxima approximately 10°C higher than the actual maxima but with increased precision and signal to noise ratios compared to traditional peak maxima determinations. Data analysis was performed using Felix™ (PTI) and Origin (V 7.0) software.
Dynamic light scattering
Dynamic light scattering measurements were made using a Wyatt DynaPro Plate Reader dynamic light scattering instrument (Santa Barbara, CA). Five, 30 second acquisitions were collected for each sample, and data were collected continuously with a temperature ramp rate of 0.14 °C/min from 10–60 °C. Data were analyzed by percent mass using the Rayleigh spheres model and Dynapro version 7.2 software.
Turbidity assay
A high-throughput turbidity assay was developed to screen a library of GRAS excipients to identify stabilizers of CHIKV VLPs. An Agilent Cary 100 spectrophotometer was used to monitor the OD at 350 nm of 0.2 mg/mL CHIKV VLP solutions. Data were collected at 50 °C, at 2 minute intervals for a total time of 2 hours. Percent inhibition was determined by comparing the maximum observed turbidity of each excipient condition to a control without excipients.
Results and Discussion
Biophysical characterization of CHIKV VLPs
CHIKV VLPs were initially dialyzed into a 20 mM citrate/phosphate buffer at varying pH (pH 3–8) with an ionic strength of 0.15. Table 1 summarizes the visual inspection and UV absorbance analysis of these samples. After dialysis, significant precipitation was visually observed at pH 3–6; additionally, significant loss of material occurred in pH 3–5 samples following centrifugation as evidenced by a decrease in UV absorbance compared to control. Efforts to increase the solubility in this pH range, for example by removing sodium chloride or adding sucrose, were not successful. The pH 7 and 8 samples remained free of visible particles after dialysis; additional experiments were performed at pH 7.0. A potential reason for the observed low solubility below neutral pH is that Alphaviruses have been reported to undergo a change in conformation below neutral pH, and this change in conformation could have lower solubility.18,19
Table 1.
CHIKV VLP solution absorbance at 280 nm after dialysis into various buffers.
| Samplea | A280 nme | Visual Examination |
|---|---|---|
| Stockb | 0.91 | clear solution |
| pH 3 | 0.14 | precipitation |
| pH 4 | 0.06 | precipitation |
| pH 5 | 0.02 | precipitation |
| pH 5c | 0.02 | precipitation |
| pH 5d | 0.07 | precipitation |
| pH 6 | 0.42 | precipitation |
| pH 7 | 0.58 | clear solution |
| pH 8 | 0.56 | clear solution |
The samples were dialyzed from stock into a 20 mM citrate/phosphate buffer at the indicated pH with and ionic strength of 0.15 achieved by adding NaCl. After dialysis, the sample volume increased approximately 1.5 times. Results shown are from a single experiment.
218mM sucrose, 7.2 mM K2HPO4, 3.8 mM KH2PO4, pH 7.0
Dialyzed without additional NaCl.
Dialyzed without additional NaCl + 10% w/v sucrose.
The A280 nm of the supernatant was measured after centrifugation at 13,800 × g for 10 minutes in a ThermoScientific Sorvall microcentrifuge.
To obtain information about the secondary structure of the VLPs, circular dichroism experiments were performed. Figure 1a shows a CD spectrum of CHIKV VLP in 20 mM sodium phosphate buffer, pH 7.0 at 10°C. The spectrum indicates that the VLP proteins adopt a conformation that contains both α-helix and β-sheet secondary structure features. A more detailed analysis was not attempted due to the multiprotein nature of the VLPs. The spectrum displays a minimum at approximately 210 nm. The CD signal at 210 nm was monitored as a function of temperature from 10°C to 85°C (Figure 1b). A primary structural transition was observed with an onset of approximately 50.2°C; a second smaller, yet reproducible, transition with an onset of approximately 52.5°C was also observed.
Figure 1.
Circular dichroism analysis of CHIKV VLPs in 20 mM sodium phosphate buffer (I=0.15), pH 7.0, at 200 μg/mL protein. The far-UV CD spectrum (a) indicates mixed α-helix and β-sheet content. The CD signal at 210 nm was followed as a function of temperature from 10–87.5 °C (b). Error bars indicate standard deviation between two measurements.
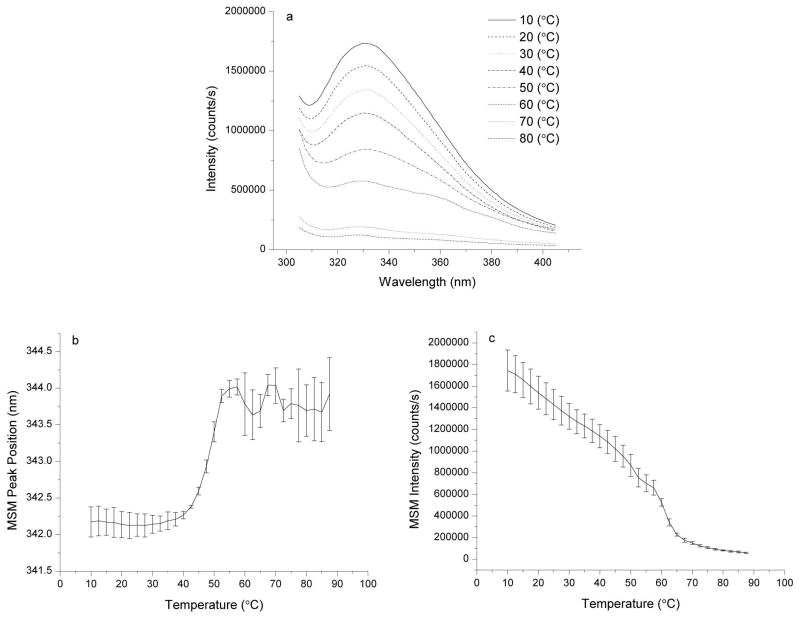
Intrinsic fluorescence experiments were performed by exciting the tryptophan residues in the VLPs at 295 nm. Changes in the polarity of the micro-environments of the tryptophan residues result in changes to both the intensity and peak position of the fluorescence emission spectra (Figures 2a–c). The mean spectral center of mass method was used to follow temperature-dependent changes in peak position (Figure 2b). The intensities at these wavelengths were also monitored (Figure 2c). Both methods show similar structural transition onsets at approximately 40°C–45°C. This result, in combination with the previously discussed CD data, suggests the VLPs may possess molten globule like behavior in the temperature range of 40°C–50°C, identified by a change or loss of tertiary structure without a loss of secondary structure. It must be noted, however, that the application of the molten globule state definition to a multi-protein containing virus-like particle has not been established.
Figure 2.
Intrinsic fluorescence data for CHIKV VLPs in 20 mM sodium phosphate buffer (I=0.15), pH 7.0, at 200 μg/mL protein. Tryptophan residues were excited at 295 nm, and the emission intensities from 305–405 nm were collected (a). The emission spectra were collected from 10–87.5°C and used to calculate the mean spectral center of mass peak positions (b) and intensities (c). Error bars indicate standard deviation between two measurements.
A combination of static (Figure 3a) and dynamic light scattering (Figure 3b) measurements were made to monitor the aggregation behavior of the VLPs as a function of temperature. Static light scattering measurements were made concurrently with the intrinsic fluorescence measurements described above, in which the scattered light was detected at a 90° angle from the excitation beam using a separate detector. DLS measurements indicate that the VLPs have a hydrodynamic radius of approximately 33 nm, which agrees well with previously published EM results in which the VLPs were found to have a diameter of approximately 65 nm.17 Both sets of light scattering results show an increase in scattering at approximately 45–50°C, suggesting that aggregation of the VLPs may be initiated or enhanced by the tertiary structural transition observed by fluorescence spectroscopy. These results indicate that the aggregating VLP species display characteristics consistent with a partially folded (molten globule-like) intermediate.
Figure 3.
Light Scattering data for CHIKV VLPs in 20 mM sodium phosphate buffer (I=0.15), pH 7.0 as a function of temperature. A VLP concentration of 200 μg/mL was used. Static light scattering (a) was collected concurrently with intrinsic fluorescence data by using a separate detector at a right-angle to the excitation. The hydrodynamic radius of the VLP particles was determined using dynamic light scattering (b). Error bars indicate standard deviation between two measurements.
Excipient screening, optimization, and conformational stability analysis
A high-throughput turbidity assay was used to screen a library of GRAS excipients for their ability to affect the colloidal stability the VLPs. This assay evaluated VLP aggregation kinetics by monitoring the optical density at 350 nm as a function of time at 50°C. Representative VLP aggregation profiles in the presence of several stabilizers, as well as a destabilizer, are shown in Figure 4, with the results summarized in Figure 5. Several different classes of excipients were examined.20 Some classes, such as amino acids and cyclodextrins, did not exhibit a significant effect on the aggregation of the VLPs, while detergents such as Tween 80 and Brij 35 increased the rate and extent of aggregation. Eight stabilizers that either significantly or completely inhibited VLP aggregation were identified from three classes of excipients including all sugars tested (dextrose, sucrose, trehalose, lactose), one sugar alcohol (mannitol), and various polyanions (sodium citrate, dextran sulfate, malic acid). Six of these candidate stabilizers, including at least one from each class, were selected for further study.
Figure 4.
Representative data from a high-throughput turbidity assay used to screen the ability of various excipients to inhibit CHIKV VLP aggregation. The OD at 350 nm of a 200 μg/mL solution of CHIKV VLP in 20 mM sodium phosphate buffer (I=0.15), pH 7.0 was measured at 50°C for 2 hours in the presence of 27 different excipients. Results are summarized in Figure 5.
Figure 5.
Summary results from a screen of 27 GRAS excipients to inhibit VLP aggregation. Turbidity inhibition is defined as the level of maximum turbidity observed for a sample in the presence of the indicated excipient relative to that observed in the control without excipient by monitoring the OD at 350 nm. Error bars indicate the standard deviation of at least two measurements. Turbidity inhibition by 20% (w/v) dextrose, 20% (w/v) sucrose, 0.2 M sodium citrate, 20% (w/v) mannitol, 20% (w/v) trehalose, 20% (w/v) lactose, and 0.15 M malic acid were found to be significantly different from the control by unpaired t-test (p<0.02). A 200 μg/mL solution of CHIKV VLPs in 20 mM sodium phosphate buffer (I=0.15), pH 7.0 was monitored at 50°C for 2 hours.
One important component of the CHIKV VLPs that has not been directly investigated in this study is the role that the integral lipid envelope plays in the spectral transitions and aggregation processes observed. The lipid layer surrounds the capsid protein core and serves as an anchor for the glycoprotein spikes of the VLP. It is conceivable that destabilizing effects of some of the excipients evaluated in this work could be result of partial destabilization of the lipid bilayer (e.g. surfactant class excipients). The role of the lipid bilayer in the VLP-excipient interaction is still relatively unknown, and will hopefully be elucidated in future works by this and other research groups.
The effect of various concentrations of the lead excipient candidates (dextrose, sucrose, trehalose, mannitol, sodium citrate, and malic acid) on VLP aggregation was evaluated. Figure 6 shows results from a concentration optimization of these six excipients. For all excipients tested here, inhibition of turbidity decreased with concentration. Of the three sugars tested, dextrose was the most effective; however, dextrose solutions of equal weight to volume ratio have approximately twice the contribution to osmolarity as disaccharides like sucrose and trehalose. For example, the osmolarity of a 5% w/v solution of dextrose is approximately 280 mOsm, whereas the osmolarity of a 10% w/v solution of sucrose or trehalose is approximately 290 mOsm. Therefore, it may be more appropriate to compare disaccharides and simple sugars at equimolar concentrations rather than equal weight volume concentrations. When compared on an equimolar basis, sucrose and trehalose are more effective at inhibiting the aggregation of CHIKV VLPs than dextrose. In addition, dextrose is a reducing sugar which may potentially lead to glycation of the proteins in the VLPs.21 Based on this information, dextrose was eliminated as a lead excipient candidate.
Figure 6.
Concentration optimization of excipient’s ability to inhibit CHIKV VLP aggregation as measured by turbidity assay. Excipients were selected based on the results of the initial turbidity screen (See Figure 5). Results shown are from a single experiment. Typical error is +/− 10%.
Based on the results of the excipient screening and optimization studies, promising excipient conditions are listed Table 2. These conditions were selected based on their ability to inhibit the aggregation of the VLPs, although some conditions possess a high solution osmolarity, discussed further below. The effects of these stabilizers on the conformational stability of the VLP were studied by far-UV CD and intrinsic fluorescence; however, no stabilizing effect on the conformational stability of the VLPs was seen (Figure S1).
Table 2.
Candidate excipient conditions for stabilizing CHIKV VLPs and corresponding osmolality values.a
| Excipient | Osmolality (mOsm/kg H2O)b |
|---|---|
| - | 252±0 |
| 10% (w/v) trehalose | 523±4 |
| 10% sucrose (w/v) | 571±27 |
| 10% dextrose (w/v) | 895±4 |
| 10% mannitol (w/v) | 898±4 |
| 0.1 M malic acid | 482±2 |
| 0.1 M sodium citrate | 506±5 |
20 mM sodium phosphate buffer with an ionic strength of 0.15 (NaCl) at pH 7 plus the indicated excipients.
Standard deviations of three measurements are reported. All osmolality values for the excipient containing samples were found to be significantly different from the sample without excipient by unpaired t-test (p<0.0001).
Effects of ionic strength on solution osmolality and inhibition of aggregation by select excipients
As shown in Table 2, many of the stabilizing conditions identified for CHIKV VLPs possess a high total osmolarity. In the absence of additional excipients, the osmolality of 20mM sodium phosphate buffer containing NaCl (I =0.15) approaches that of human plasma (Table 2). The addition of 10% w/v of trehalose or sucrose to this buffer, increases the osmolality values to 523 and 571 mOsm/kg H2O, respectively. Dextrose and mannitol 10% w/v solutions increase this value even further, to approximately 900 mOsm/kg H2O. This effect is not limited to the sugar and sugar alcohol classes, however, as the two polyanions, malic acid and sodium citrate, 0.1 M solutions have osmolality values of 482 and 506 mOsm/kg H2O, respectively. A lower solution osmolarity is optimally desired for clinical administration therefore the effect of lowering the solution osmolality on the ability of these excipients to inhibit CHIKV VLP aggregation was evaluated.
The ionic strength of the buffer was lowered from 0.15 to 0.035 by decreasing the sodium phosphate concentration from 20 to 10 mM and by eliminating the additional NaCl. The effects of sodium citrate and malic acid on the aggregation of CHIKV VLPs in both the high and low ionic strength buffers were then evaluated, as shown in Table 3. As expected, the osmolality of the lower ionic strength solutions decreases by approximately 220 mOsm/kg H2O compared to the high ionic strength buffer. Sodium citrate and malic acid both demonstrated increased inhibition of VLP aggregation in the lower ionic strength buffer compared to the higher ionic strength buffer. For example, in the presence of 50 mM sodium citrate, the aggregation of the VLPs is inhibited by 54% in the low ionic strength buffer but only by 35% at high ionic strength. At concentrations of citrate greater than 100 mM, the aggregation of VLPs is nearly completely inhibited in the low ionic strength buffer. For malic acid, at concentrations of 50 or 100 mM, the levels of inhibition of VLP aggregation are similar in both the low and high ionic strength buffers. At 150 mM and above, however, nearly complete inhibition of aggregation is observed in the low ionic strength buffers. In summary, by lowering the ionic strength of malic acid and sodium citrate solutions, similar or higher inhibition of VLP aggregation were achieved.
Table 3.
Percent inhibition of CHIKV VLP aggregation by addition of Na citrate and malic acid to high and low ionic strength buffers.
| Excipient | Concentration (M) | High Ionic strengtha | Low Ionic Strengthb | ||
|---|---|---|---|---|---|
|
| |||||
| Osmolality (mOsm/kg H2O) | % Inhibition of Aggregationc | Osmolality (mOsm/kg H2O) | % Inhibition of Aggregationc | ||
| Na Citrate | 0.05 | 383 | 35 | 166 | 54 |
| 0.1 | 506 | 71 | 297 | 100 | |
| 0.15 | 637 | - | 423 | 90 | |
| 0.2 | 758 | 88 | 524 | 96 | |
| 0.25 | 896 | - | 666 | 107 | |
|
| |||||
| Malic Acid | 0.05 | 367 | 37 | 151 | -1 |
| 0.1 | 482 | 71 | 277 | 79 | |
| 0.15 | 596 | 70 | 396 | 93 | |
| 0.2 | 762 | - | 488 | 90 | |
| 0.25 | 891 | - | 643 | 114 | |
Excipient added to 20 mM Na phosphate plus NaCl (I=0.15).
Excipient added to 10 mM Na phosphate.
Results shown are from a single experiment. Typical error is +/− 10%.
Effects of polyanions on the stability of CHIKV VLP
Several polyanions were found to stabilize CHIKV VLPs against aggregation, including sodium citrate, malic acid, and dextran sulfate. We have previously shown that polyanions have the ability to inhibit the aggregation of several growth hormones.22 In order to test the hypothesis that the protective effects observed are due to the polyanionic nature of these excipients, additional experiments were performed with these excipients as well as two other well-known polyanions, sodium sulfate and heparin. 22–24
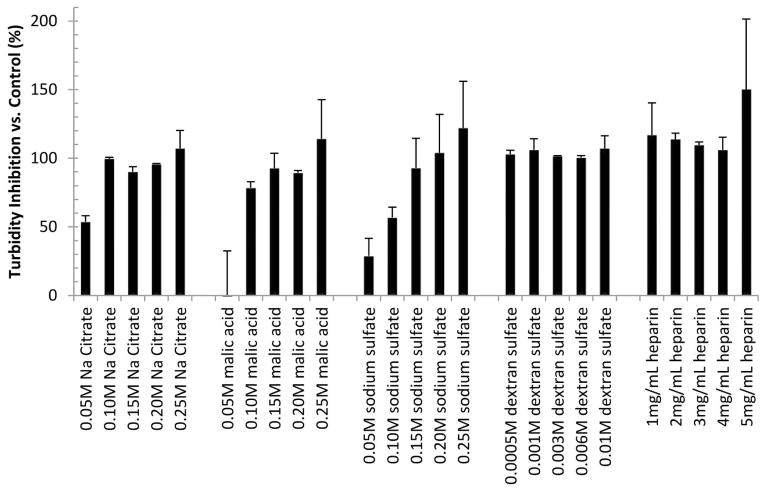
The effects of the five polyanions at various concentrations on the turbidity of CHIKV VLP solutions were evaluated (Figure 7). Sodium citrate and malic acid demonstrated similar behavior, with nearly complete inhibition of VLP aggregation at concentrations of 100 mM and above. At 50 mM, however, little protective effect was observed for malic acid, while sodium citrate induced approximately 50% turbidity inhibition. Higher concentrations (0.15 M) of sodium sulfate were required to achieve maximum stabilizing effects. For dextran sulfate and heparin, complete inhibition was seen at all concentrations tested here (0.5–10 mM and 1–5 mg/mL, respectively).
Figure 7.
Turbidity inhibition of CHIKV VLPs by addition of various polyanions. Turbidity inhibition is defined as the level of maximum turbidity observed of a sample in the presence of the indicated excipient relative to that observed in the control without excipient by monitoring the OD at 350 nm. Error bars indicated the standard deviation of at least two measurements. Turbidity inhibition by all tested concentrations of sodium citrate (p<0.01), and dextran sulfate (p<0.004) as well as 0.1–0.25 M malic acid (p<0.01), 0.1–0.25 M sodium sulfate (p<0.04), and 1–4 mg/mL heparin (p<0.02) were found to be significantly different from the control by unpaired t-test. A 200 μg/mL solution of CHIKV VLP in pH 7 sodium phosphate buffer (I=0.15) was monitored at 50°C for 2 hours.
The effects of heparin on the stability of CHIKV VLP solutions at lower concentrations were also studied. Figure 8 shows CD spectra for CHIKV VLP at 10 °C prior to and after heating to 90°C in the presence of various concentrations of heparin. In the absence of heparin and in the presence of 1 μg/mL of heparin, significant loss in CD signal is seen after heating to 90°C and cooling; however, in the presence of 50 or 100 μg/mL heparin, complete recovery of the CD signal is observed. This result suggests that heparin protects the secondary structure of CHIKV VLP from temperature induced denaturation at 50 μg/mL and above.
Figure 8.
CD spectra of CHIKV VLP in the absence and presence of heparin. Samples were measured at 10°C before (solid curve) and after heating to 87.5°C followed by subsequent cooling (broken curve). Refolding was performed without heparin (a) and in the presence of 1 μg/mL (b), 50 μg/mL, and 100 μg/mL (c).). Results shown are from a single experiment.
Figure 9a shows the OD 350 nm of CHIKV VLP solutions in the presence of 1–10 μg/mL heparin. Maximum protective effects are seen at concentrations of 6 μg/mL and above. Figure 9b shows the fluorescence peak position as a function of temperature for CHIKV VLP solutions in the presence of heparin. Even at the lowest concentration tested (1 μg/mL), significant destabilization of CHIKV VLP by heparin is seen as indicated by the lower temperature transition onsets.
Figure 9.
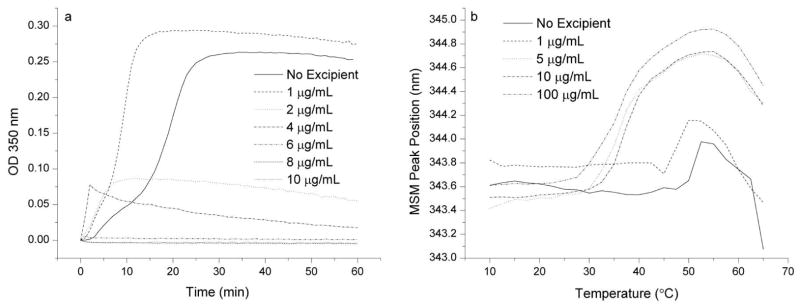
Turbidity (a) and mean spectral mass positions (b) of CHIKV VLP solutions containing heparin at concentrations indicated in figure legends. The OD at 350 nm was measured at 50°C in the presence of 1–10 μg/mL heparin for 2 hours. Emission spectra were collected from 305–405 nm with excitation at 295 nm as a function of temperature. 200 μg/mL solutions of CHIKV VLP in 10mM sodium phosphate buffer, pH 7.0 were measured in the presence of 1–100 μg/mL heparin. Results shown are from a single experiment.
The above results present an interesting scenario, showing that while heparin inhibits the aggregation of CHIKV VLP and protects refolding of the secondary structure, heparin also decreases the thermal stability of the VLP’s overall tertiary structure. Unfortunately, a concentration of heparin does not appear to exist that inhibits VLP aggregation without decreasing the VLP conformational stability. To this end, heparin in combination with other excipients was also examined; in each case, however, the VLP physical stability was not improved in combination with heparin over that observed for the other excipients alone (data not shown). Dextran sulfate was also observed to destabilize the structural stability of VLPs (data not shown).
One important component of the CHIKV VLPs that has not been directly investigated in this study is the role that the integral lipid envelope plays in the spectral transitions and aggregation processes observed. The lipid layer surrounds the capsid protein core and serves as an anchor for the glycoprotein spikes of the VLP. It is conceivable that destabilizing effects of the stress conditions and some of the excipients evaluated in this work could be result of partial destabilization of the lipid bilayer (e.g. surfactant class excipients). The role of the lipid bilayer in the VLP-excipient interaction is still relatively unknown, and will hopefully be elucidated in future works by this and other research groups.
Conclusions
Biophysical characterization of CHIKV VLPs was performed at neutral pH values, as poor solubility was observed at acidic pH (pH 6 and below). CHIKV VLPs with a radius of 33 nm were detected in solution by dynamic light scattering, which closely agrees with previously published EM results. Circular dichroism spectra indicate that CHIKV VLPs adopt a mixed α-helix and β-sheet conformation. Thermal unfolding studies suggest the presence of molten globule-like behavior between 45°C and 50 °C, a unique observation for VLPs. After unfolding of the tertiary structure, CHIKV VLP aggregates were observed visually as well as by static and dynamic light scattering.
A high-throughput turbidity-based assay was developed, and a screen of a GRAS excipient library using this method identified several stabilizers. Trehalose, sucrose, dextrose, mannitol, sodium citrate, and malic acid were identified as lead stabilizers and further tested for concentration optimization and conformational stability analysis. Studies of the effect of ionic strength on CHIKV VLP stability suggest that citrate and malic acid are more effective stabilizers in the absence of NaCl as an ionic strength modifier. Based on the efficacy of citrate and malic acid, additional polyanions were evaluated; all polyanions tested inhibited the aggregation of CHIKV VLP. Interestingly, malic acid and sodium citrate did not destabilize the conformational stability of CHIKV VLPs, while heparin and dextran sulfate significantly decreased the structural stability.
The data presented in this work suggest the following formulation may be acceptable for long-term stability of CHIKV VLP: 10 mM sodium phosphate, pH 7.0 along with(one or more of) 10% (w/v) trehalose, 10% w/v sucrose, 0.1 M sodium citrate, and/or 0.1M malic acid. Optimization studies evaluating excipient combinations, as well as long-term stability studies, are also recommended.
Supplementary Material
Conformational stability of CHIKV VLPs in the presence of excipients optimized based on their ability to inhibit aggregation of CHIKV VLP. The secondary structure of CHIKV VLPs was monitored at 210 nm by far-UV CD(a), and the tertiary structure was monitored by following the mean spectral mass position of the intrinsic fluorescence emission spectra. A 200 μg/mL solution of CHIKV VLP in 20 mM sodium phosphate buffer (I=0.15), pH 7.0 was used, with the excipients indicated in the figure legends. Results shown are from a single experiment.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. Journal of General Virology. 2007;88(9):2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 2.Staples JE, Breiman RF, Powers AM. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clinical Infectious Diseases. 2009;49(6):942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 3.Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Ali Mohamed A, de Lamballerie X, Charrel R, Tolou H. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine. 2007;86(3):123–137. doi: 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 4.Enserink M. Chikungunya: No Longer a Third World Disease. Science. 2007;318(5858):1860–1861. doi: 10.1126/science.318.5858.1860. [DOI] [PubMed] [Google Scholar]
- 5.Seyler T, Hutin Y, Ramanchandran V, Ramakrishnan R, Manickam P, Murhekar M. Estimating the burden of disease and the economic cost attributable to chikungunya, Andhra Pradesh, India, 2005–2006. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(2):133–138. doi: 10.1016/j.trstmh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Gerardin P, Fianu A, Malvy D, Mussard C, Boussaid K, Rollot O, Michault A, Gauzere BA, Breart G, Favier F. Perceived morbidity and community burden after a Chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC medicine. 2011;9:5. doi: 10.1186/1741-7015-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. The Journal of hygiene. 1956;54(2):177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariuki Njenga M, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CHL, Sang R, Sergon K, Breiman R, Powers AM. Tracking epidemic Chikungunya virus into the Indian Ocean from East Africa. Journal of General Virology. 2008;89(11):2754–2760. doi: 10.1099/vir.0.2008/005413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taubitz W, Cramer JP, Kapaun A, Pfeffer M, Drosten C, Dobler G, Burchard GD, Löscher T. Chikungunya Fever in Travelers: Clinical Presentation and Course. Clinical Infectious Diseases. 2007;45(1):e1–e4. doi: 10.1086/518701. [DOI] [PubMed] [Google Scholar]
- 10.Moro ML, Grilli E, Corvetta A, Silvi G, Angelini R, Mascella F, Miserocchi F, Sambo P, Finarelli AC, Sambri V, Gagliotti C, Massimiliani E, Mattivi A, Pierro AM, Macini P. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: A prognostic cohort study. Journal of Infection. 2012;65(2):165–172. doi: 10.1016/j.jinf.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A. Infection with chikungunya virus in Italy: an outbreak in a temperate region. The Lancet. 370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 12.Moro ML, Gagliotti C, Silvi G, Angelini R, Sambri V, Rezza G, Massimiliani E, Mattivi A, Grilli E, Finarelli AC, Spataro N, Pierro AM, Seyler T, Macini P. Chikungunya Virus in North-Eastern Italy: A Seroprevalence Survey. The American Journal of Tropical Medicine and Hygiene. 2010;82(3):508–511. doi: 10.4269/ajtmh.2010.09-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enserink M. A Mosquito Goes Global. Science. 2008;320(5878):864–866. doi: 10.1126/science.320.5878.864. [DOI] [PubMed] [Google Scholar]
- 15.Simon F, Savini H, Parola P. Chikungunya: A Paradigm of Emergence and Globalization of Vector-Borne Diseases. Medical Clinics of North America. 2008;92(6):1323–1343. doi: 10.1016/j.mcna.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiological reviews. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akahata W, Yang Z-Y, Andersen H, Sun S, Holdaway HA, Kong W-P, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16(3):334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dollery SJ, Delboy MG, Nicola AV. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol. 2010;84(8):3759–3766. doi: 10.1128/JVI.02573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Piper A, Meilleur F, Hernandez R, Heller WT, Brown DT. Conformational changes in Sindbis virus induced by decreased pH are revealed by small-angle neutron scattering. J Virol. 2012;86(4):1982–1987. doi: 10.1128/JVI.06569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein excipient interactions: Mechanisms and biophysical characterization applied to protein formulation development. Advanced Drug Delivery Reviews. 2011;63(13):1118–1159. doi: 10.1016/j.addr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Fischer S, Hoernschemeyer J, Mahler H-C. Glycation during storage and administration of monoclonal antibody formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70(1):42–50. doi: 10.1016/j.ejpb.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Joshi SB, Kamerzell TJ, McNown C, Middaugh CR. The interaction of heparin/polyanions with bovine, porcine, and human growth hormone. J Pharm Sci. 2008;97(4):1368–1385. doi: 10.1002/jps.21056. [DOI] [PubMed] [Google Scholar]
- 23.Vardhanabhuti B, Allen Foegeding E. Effects of dextran sulfate, NaCl, and initial protein concentration on thermal stability of β-lactoglobulin and α-lactalbumin at neutral pH. Food Hydrocolloids. 2008;22(5):752–762. [Google Scholar]
- 24.Volkin DB, Middaugh CR. The characterization, stabilization, and formulation of acidic fibroblast growth factor. Pharm Biotechnol. 1996;9:181–217. doi: 10.1007/0-306-47452-2_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conformational stability of CHIKV VLPs in the presence of excipients optimized based on their ability to inhibit aggregation of CHIKV VLP. The secondary structure of CHIKV VLPs was monitored at 210 nm by far-UV CD(a), and the tertiary structure was monitored by following the mean spectral mass position of the intrinsic fluorescence emission spectra. A 200 μg/mL solution of CHIKV VLP in 20 mM sodium phosphate buffer (I=0.15), pH 7.0 was used, with the excipients indicated in the figure legends. Results shown are from a single experiment.