Abstract
Rapid eye movement (REM) sleep disturbances predict poor clinical outcomes in posttraumatic stress disorder (PTSD) and major depressive disorder (MDD). In MDD, REM sleep is characterized by activation of limbic and paralimbic brain regions compared to wakefulness. The neural correlates of PTSD during REM sleep remain scarcely explored, and comparisons of PTSD and MDD have not been conducted. The present study sought to compare brain activity patterns during wakefulness and REM sleep in 13 adults with PTSD and 12 adults with MDD using [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (PET). PTSD was associated with greater increases in relative regional cerebral metabolic rate of glucose (rCMRglc) in limbic and paralimbic structures in REM sleep compared to wakefulness. Post-hoc comparisons indicated that MDD was associated with greater limbic and paralimbic rCMRglc during wakefulness but not REM sleep compared to PTSD. Our findings suggest that PTSD is associated with increased REM sleep limbic and paralimbic metabolism, whereas MDD is associated with wake and REM hypermetabolism in these areas. These observations suggest that PTSD and MDD disrupt REM sleep through different neurobiological processes. Optimal sleep treatments between the two disorders may differ: REM-specific therapy may be more effective in PTSD.
1. Introduction
Posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) are two stress-related disorders that are associated with the disruption of rapid-eye movement (REM) sleep (Benca et al., 1992; Ross et al., 1989; Lustberg and Reynolds, 2000; Adrien, 2002). In turn, subjective sleep complaints and objective indices of sleep disruption are associated with increased daytime symptom severity in PTSD and MDD, alcohol or substance misuse, suicidality (e.g., Saladin et al., 1995; Agargun et al., 1997; Krakow et al., 2000; Krakow et al., 2002; Agargun and Cartwright, 2003), and poor treatment outcomes (Buysse et al., 1997; Buysse et al., 1999). Whether REM sleep disturbances arise from similar or distinct changes in brain activity patterns in REM sleep relative to wakefulness in PTSD and MDD is unknown. Identifying the neural underpinnings of REM sleep in PTSD and MDD may provide novel insights into the common or distinctive pathophysiology of these disorders and help guide development of new treatments.
Few studies have compared the neural correlates of the two disorders. Lanius and colleagues (2007) found that the presence of comorbid MDD along with PTSD was associated with greater bilateral activation of the anterior and posterior cingulate cortex and lesser activation of the left insula in response to imagery evoking a stress response when compared to PTSD uncomplicated with MDD during wakefulness. The pattern of limbic and paralimbic hyperactivity may thus be more diffuse in MDD than in PTSD.
Only one preliminary study has investigated the neurobiological correlates of PTSD during wakefulness and sleep (Germain et al., in press). Consistent with the hypothesis that REM sleep naturally activates the threat response network (Germain et al., 2008), veterans with PTSD showed increased relative regional metabolic rate of glucose (rCMRglc) in limbic and paralimbic systems during wakefulness and REM sleep compared to veterans without PTSD. Aversive stimuli, in the form of flashbacks during wakefulness and nightmares during REM sleep, account for these differences between veterans with and without PTSD through activation of the threat response network. Those with PTSD perceive threat and experience limbic and paralimbic hyperactivity, while those without PTSD suffer no such dysfunction.
Prior neuroimaging studies have shown that, compared to healthy participants, adults with MDD have greater rCMRglc in REM sleep compared to wakefulness in the amygdala, paralimbic system, midbrain reticular formation, and executive frontal cortex (Nofzinger et al., 2004). These findings of paralimbic and paralimbic hyperactivity in REM sleep may underlie a neurotransmitter unbalance that results in the increased sleep latency and multiple awakenings associated with depression (Smiley et al., 1999).
While previous studies have compared the REM sleep correlates of PTSD and MDD to healthy participants separately, no study as of yet has directly compared the mechanisms of REM sleep disruption between the two disorders. The apparent partial overlap in brain regions that show increased rCMRglc from wakefulness to REM sleep in both PTSD and MDD samples suggest that REM sleep anomalies observed in both disorders may arise from similar underlying neurobiological processes. To explore this hypothesis, we compared changes rCMRglc during wakefulness and REM sleep in participants with PTSD and MDD. We hypothesized that veterans with PTSD would show greater increases in rCMRglc in REM sleep versus wakefulness sleep compared to participants with MDD in limbic and paralimbic brain regions due to activation of the neural threat network by aversive REM stimuli such as nightmares, which are symptomatic of PTSD.
2. Methods
2.1 Participants
A sample of convenience was assembled for the purposes of this study by drawing from prior and ongoing studies (MH083055; PI: Germain; PT073961#; Germain; MH6627, PI: Nofzinger; MH061566, PI: Nofzinger). The sample included 13 combat-exposed military veterans with a current diagnosis of PTSD (mean 29.5 ± SD 6.4, range 22–45, 3 female) and 12 civilian adults who met diagnostic criteria for MDD (mean age 34.0 years ± standard deviation 7.2 years, range 25–46, 9 female). All participants provided written informed consent. Studies were reviewed and approved by the University of Pittsburgh Institutional Review Board.
Participants in the PTSD group were veterans of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), recruited through public advertisement in Western Pennsylvania. The presence of PTSD was determined by the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1990). Exclusion criteria were left-handedness, use of medications known to affect sleep or wake function in the past 3 months up to the first day of the study, current comorbid sleep disorder or another Axis I psychiatric disorder, unstable acute or chronic general medical condition, or a current or recent history (past 3 months) of alcohol or substance abuse or dependence. Comorbid MDD was excluded in PTSD veterans using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (First et al., 1996). The Beck Depression Inventory (Beck, 1961) was used to assess the severity of depressive symptoms in this group.
Participants in the MDD group were non-veterans recruited through similar advertisement. Participants with depression met criteria for MDD as assessed by the SCID, and Hamilton Depression Rating Scale (Hamilton, 1960). Exclusion criteria for the depressed group included left handedness, recent usage of antidepressant, anxiolytic, or antipsychotic medications; presence of suicidal ideation; and diagnosis of bipolar disorder or comorbid Axis I disorders.
2.2 Procedures
After providing informed consent and completing the screening procedures, participants in both studies completed the Pittsburgh Quality Sleep Index (PSQI) (Buysse et al., 1989) to assess overall sleep quality. Participants underwent a magnetic resonance imaging (MRI) scan to detect any preexisting brain abnormalities and to co-register with the positron emission tomography (PET) scans. Following participant positioning in a standard head coil, the MRI scan included a brief scout T1-weighted image, a fast spin-echo T2-weighted image, and proton-density weighted images, using high-resolution MPRAGE for regional placement. Using Statistical Parametric Mapping (SPM) software, version 8 (SPM8 software package; Wellcome Department of Cognitive Neurology; London, England), the MR images were normalized to the ICBM 152 template (Montreal Neurological Institute) with the unified segmentation technique (Ashburner and Friston, 2005).
Eligible participants slept for five nights at the Neuroscience and Clinical Translational Research Center (N-CTRC). Polysomnography (PSG) recordings were conducted on all nights and included bilateral and central electroencephalograph (EEG) channels (C3, C4, O1, O2), electrooculogram (EOG) channels, and an electrocardiogram (EKG) channel.
The participants’ first night in the N-CTRC was used to rule out sleep disorders, and the second night was used as a baseline night. PSG data collected during the baseline night were used to determine sleep parameters such as sleep latency, wake time after sleep onset, sleep duration, sleep efficiency, total time spent in REM sleep, REM fragmentations, and average REM count, an automated measure of REM density (Doman et al., 1995).
On the morning following the baseline night, participants underwent a waking [18F] FDG PET scan using the established [18F]-fluoro-2-deoxy-D-glucose (FDG) method (Nofzinger et al., 1998). Participants were injected with 5 mCi of [18F] FDG at 2–4 hours after wake-up time. During the 20 minute uptake period, participants were monitored by EEG to verify continuous wakefulness. Participants were then transported to the PET center for their waking PET scan. The REM sleep PET scan occurred on either the third or the fifth night. The fourth night allowed for recovery, where participants were left undisturbed during sleep. For the REM scans, injection of 5 mCi of [18F] FDG occurred at the start of the participant's second period of REM sleep as determined by a sleep technologist observing the PSG recordings. After the 20-minute uptake period, participants were awakened and transported to the PET center for scanning procedures.
A 15-minute transmission scan and a 30-minute emission scan—consisting of six sequential 5 minute segments to allow for exclusion of segments disrupted by subject movement—were obtained for both waking and REM states at 60 minutes following the [18F] FDG injection. The FDG images were then aligned and averaged over 60–90 minutes post-injection via established methods (Woods et. al, 1993), co-registered to the corresponding structural MR scans, and smoothed with a 10mm FWHM Gaussian filter. All FDG PET scans were performed with an ECAT HR+ scanner in 3D (volume) mode with individually molded thermoplastic head holders to minimize participant head movement and allow for proper head positioning.
2.3 Statistical Analysis
Image analysis was conducted using SPM8 (Wellcome Department of Cognitive Neurology; London, England) for voxel-by-voxel analysis of the differences in regional rCMRglc between the PTSD and MDD groups. Image alignment and MR-PET co-registration were performed using previously established methods (Woods et al., 1992; Minoshima et al., 1993; Wiseman et al., 1995). A full factorial design was used to study the interaction between group (PTSD vs. MDD) and state (Wakefulness vs. REM sleep). Two separate interaction analyses were conducted to 1) to identify brain regions that show greater increases in rCMRglc from wakefulness to REM sleep in participants with PTSD compared to those with MDD; and 2) to identify brain regions that show greater increases in rCMRglc from wakefulness to REM sleep in participants with MDD compared to PTSD veterans. Mean rCMRglc values for specific regions of interest within significant clusters were extracted using the MATLAB toolbox rex (web.mit.edu/swg/rex/rex.pdf). Significant interactions were then decomposed using two-sample t-tests to comparing the two groups during wakefulness and during REM sleep. Because of our a priori approach, a statistical threshold of alpha = 0.05 was used for uncorrected cluster-level p-value.
3. Results
3.1 Sample Characteristics
Demographic and clinical information are summarized in Table 1. The PTSD and MDD groups did not differ significantly in terms of age (t = 1.91, p = 0.08), though the MDD group had a greater proportion of female participants. The two groups did not differ significantly in terms of PSQI scores or PSG sleep or REM measures. The mean BDI score for the PTSD group was 8.33 + 5.53, indicating symptoms below clinical thresholds. In the MDD group, the mean HRSD scores was 21.00 + 2.23, indicate moderate to severe depressive symptoms.
Table 1.
Clinical and polysomnographic measures for MDD and PTSD participants.
| Measure | PTSD | MDD | t (df=17) | p |
|---|---|---|---|---|
| PSQI (Mean ± SD) | 8.0 ± 3.7 | 9.1 ± 2.9 | 0.81 | 0.43 |
| CAPS - Total* | 52.8 ± 18.3 | - | - | - |
| Sleep latency (minutes) | 17.5 ± 17.5 | 36.9 ± 38.0 | 1.62 | 0.13 |
| Time spent asleep (minutes) | 416.7 ± 39.9 | 381.5 ± 59.3 | −1.73 | 0.1 |
| Sleep efficiency (%) | 91.2 ± 4.9 | 85.6 ± 9.6 | −1.82 | 0.09 |
| Wake time after sleep onset (minutes) | 23.0 ± 17.7 | 28.1 ± 32.0 | 0.49 | 0.63 |
| Percent REM sleep | 28.3 ± 4.4 | 27.2 ± 5.5 | −0.58 | 0.57 |
| Percent NREM sleep | 71.7 ± 4.4 | 72.8 ± 5.5 | −0.58 | 0.57 |
| Percent Delta sleep | 9.5 ± 6.7 | 11.1 ± 7.8 | 0.53 | 0.61 |
| REM fragmentations | 6.9 ± 3.9 | 6.5 ± 4.1 | 0.29 | 0.78 |
| Total REM count | 1122.2 ± 604.5 | 888.6 ± 424.9 | −1.13 | 0.27 |
| Total NREM time (minutes) | 271.4 ± 84.6 | 276.6 ± 39.3 | 0.2 | 0.85 |
| Average REM count | 9.6 ± 5.0 | 8.6 ± 3.5 | −0.58 | 0.57 |
The Clinician Administered PTSD Scale (CAPS) was only administered to PTSD participants.
3.2 Group by State Interaction
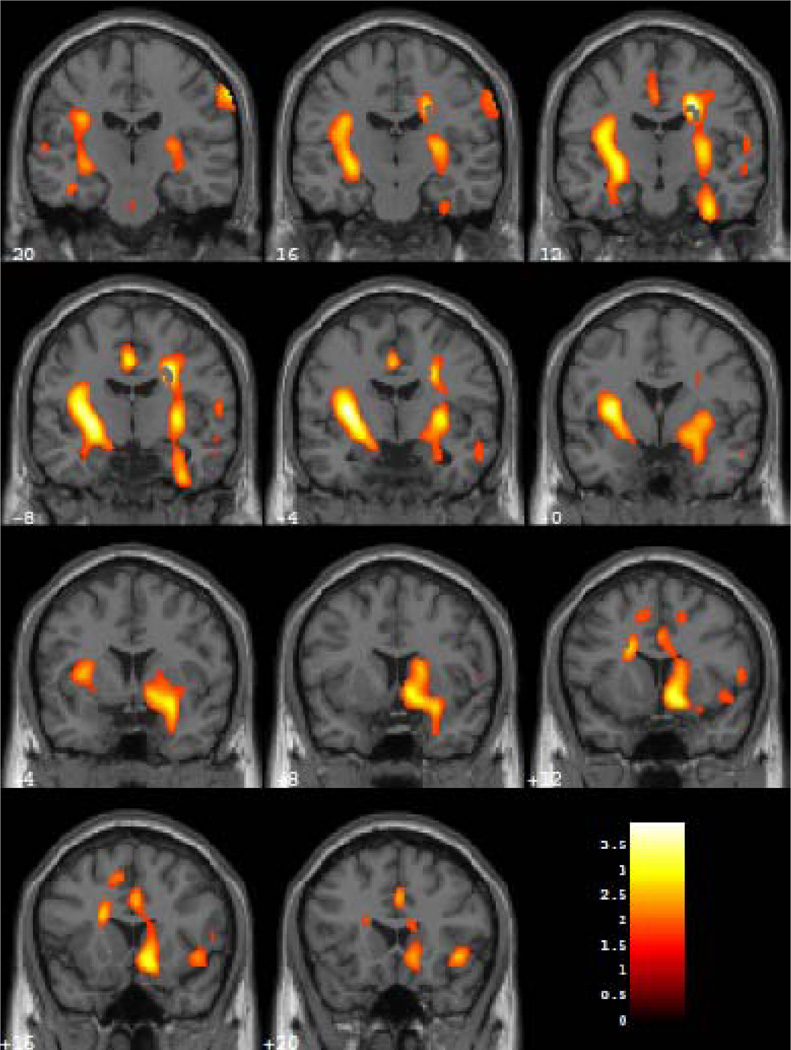
Veterans with PTSD showed greater increases in relative rCMRglc in REM sleep compared to wakefulness than did MDD participants in two clusters (Figure 1). The first cluster of 3,758 voxels contained left basal ganglia, including the claustrum, putamen, and globus pallidus; left limbic and paralimbic structures, including the amygdala, hippocampus, uncus, insula, and parahippocampal gyrus; and left fusiform gyrus, superior temporal gyrus, and pontine reticular formation (Z = 3.64, cluster level puncorrected = 0.013, MNI x, y, and z coordinates of voxel of maximum significance: −32, −4, 6).
Figure 1.
Coronal sections from MNI Y coordinates −20 to 20 in 4 mm slices where veterans with PTSD showed greater increases in rCMRglc during REM sleep relative to wakefulness compared to participants with MDD. Colors correspond to degree of PET scan activation, as measured by relative regional cerebral metabolic rate of glucose metabolism (rCMRglc), with yellow colors indicating higher activation than red colors.
A similar cluster of 3,654 voxels in the right hemisphere also showed significantly greater increases in relative rCMRglc from wakefulness to REM sleep in veterans with PTSD compared to MDD participants (Z = 3.47, cluster level puncorrected = 0.015, MNI x, y, and z coordinates of voxel of maximum significance: 26, −10, 36). This region included the claustrum, putamen, head and body of the caudate nucleus, amygdala, hippocampus, uncus, subcallosal gyrus, insula, anterior cingulate cortex, parahippocampal gyrus, inferior temporal gyrus, ventrolateral and orbital prefrontal cortical areas, and pontine reticular formation.
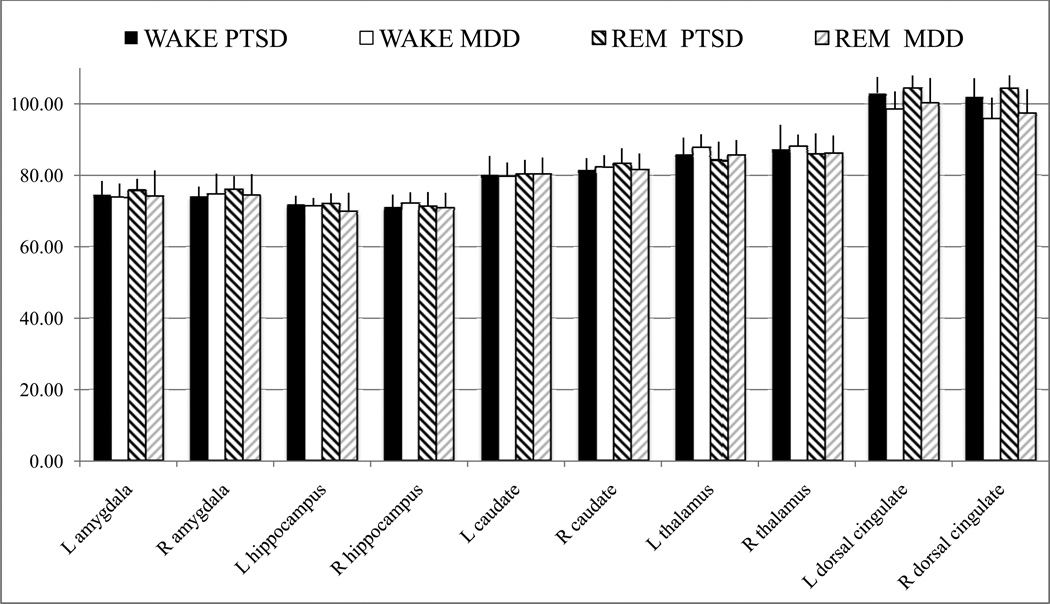
Mean and standard deviations for rCMRglc values in some of these brain regions are presented in Table 2 and Figure 2.
Table 2.
Means (standard deviations) for regional cerebral metabolic rate of glucose in selected regions of interest for participants with MDD and PTSD during wakefulness and REM sleep.
| Hippocamp | Dorsal Cingulate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | us | Caudate | Thalamus | Cortex | |||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||
| MDD | WAKE | 73.86 | 74.68 | 71.39 | 72.32 | 79.72 | 82.25 | 87.71 | 88.10 | 98.68 | 95.77 |
| (3.86) | (5.77) | (2.28) | (2.94) | (3.87) | (3.36) | (3.78) | (3.30) | (4.80) | (5.98) | ||
| REM | 74.25 | 74.41 | 69.92 | 70.98 | 80.29 | 81.74 | 85.85 | 86.30 | 100.29 | 97.33 | |
| (7.14) | (5.95) | (5.21) | (4.12) | (4.67) | (4.39) | (4.03) | (4.86) | (6.94) | (6.78) | ||
| PTSD | WAKE | 74.36 | 74.12 | 71.82 | 71.13 | 80.02 | 81.35 | 85.88 | 87.20 | 103.06 | 101.97 |
| (4.08) | (2.73) | (2.46) | (3.49) | (5.41) | (3.46) | (4.66) | (6.94) | (4.48) | (5.22) | ||
| REM | 75.87 | 75.96 | 72.00 | 71.36 | 80.26 | 83.30 | 84.15 | 86.01 | 104.58 | 104.24 | |
| (3.14) | (3.83) | (2.95) | (3.97) | (4.06) | (4.28) | (5.25) | (5.73) | (3.36) | (3.75) | ||
Figure 2.
Means and standard deviations for regional cerebral metabolic rate of glucose in selected regions of interest for participants with MDD (light bars) and PTSD (darker bars) during wakefulness (full bars) and REM sleep (hashed bars). R = right, L = left.
Participants with MDD did not show any clusters of significantly greater increases in relative rCMRglc from wakefulness to REM sleep compared to veterans with PTSD.
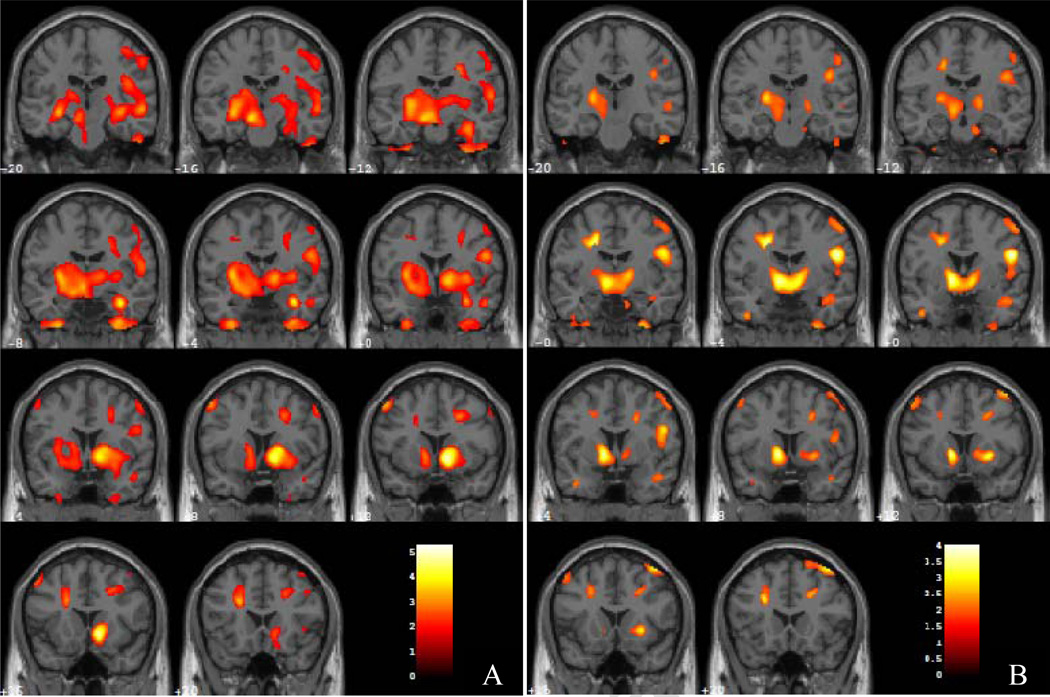
3.3 Post-hoc analyses: Group comparisons during wakefulness
During wakefulness, no regions showed cluster level significance for greater relative rCMRglc in PTSD veterans than in participants with MDD. Participants with MDD showed greater relative rCMRglc than veterans with PTSD in one large bihemispheric cluster of 31,985 contiguous voxels (Z = 4.22 puncorrected < 0.001, MNI x, y, and z coordinates: 12, 14, −6) (Figure 2A). This cluster included, bilaterally, structures of the basal ganglia, such as the head, body, and tail of the caudate nucleus, the claustrum, the putamen, and the globus pallidus; and limbic and paralimbic structures such as the amygdala, hippocampus, subcallosal gyrus, insula, dorsal cingulate cortex, and parahippocampal gyrus. This cluster also included the right medial prefrontal cortex, precentral and postcentral gyri, and bilateral cuneus and precuneus as well as the right mammillary body, bilateral ventral posterolateral thalamic nucleus, and anterior pons. Finally, this cluster of significance extended bilaterally to the anterior and posterior cerebellar lobes.
3.4 Post-hoc analyses: Group comparisons during REM sleep
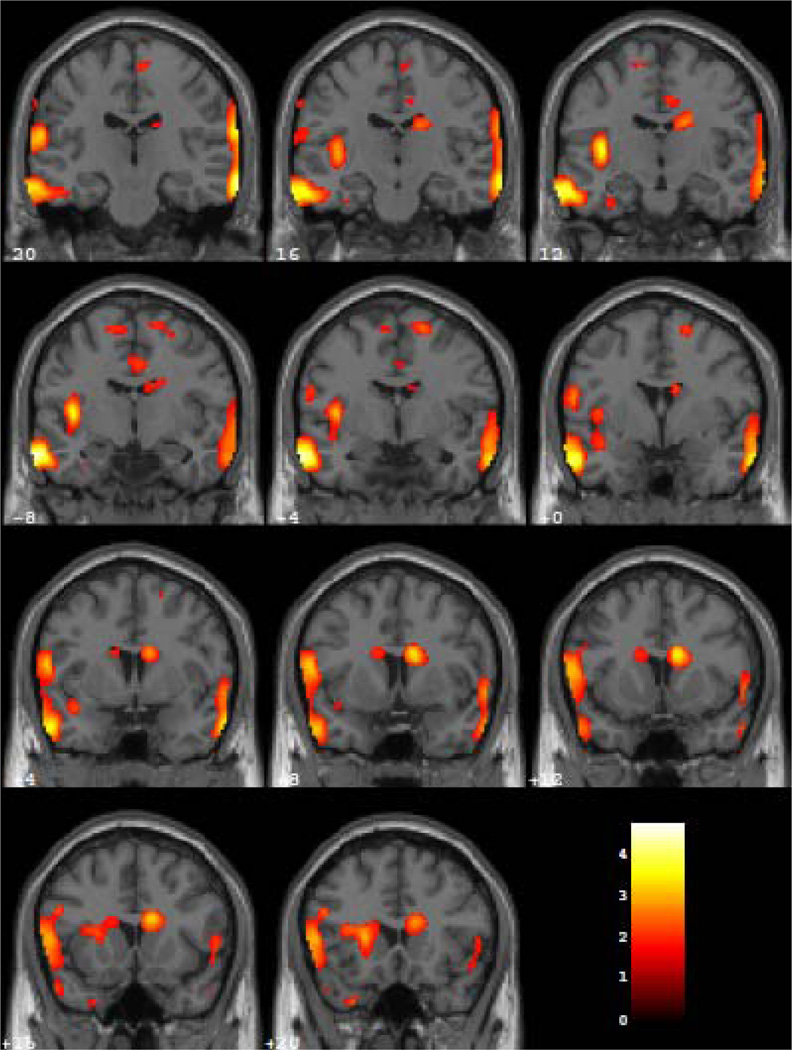
During REM sleep, veterans with PTSD showed significantly greater relative rCMRglc than participants with MDD in three clusters (Figure 3). The first cluster (Z = 3.93, puncorrected = 0.011, K = 3763 voxels, MNI x, y, and z coordinates: 72, −26, −16) encompassed the right ventrolateral prefrontal, lateral temporal, and inferior parietal cortical areas and the temporo-parieto-occipital junction. The other two clusters corresponded to similar regions in the left hemisphere. Specifically, veterans with PTSD showed greater relative rCMRglc in the left ventrolateral prefrontal cortex, temporal and parietal cortex, and temporo-parieto-occipital junction (Z = 3.68; puncorrected = 0.045, K = 2162 voxels, MNI x, y, and z coordinates: −64, −26, 12; and Z = 3.86; puncorrected = 0.017, K = 3249 voxels, MNI x, y, and z coordinates: −66, −6, −24).
Figure 3.
Coronal sections from MNI Y coordinates −20 to 20 in 4 mm slices where participants with MDD showed greater relative rCMRglc that veterans with PTSD during wakefulness (A) and REM sleep (B). Colors correspond to degree of PET scan activation, as measured by relative regional cerebral metabolic rate of glucose metabolism (rCMRglc), with yellow colors indicating higher activation than red colors.
Participants with MDD showed significantly greater relative rCMRglc than veterans with PTSD in two clusters (Figure 2B). The first cluster of 2560 contiguous voxels included the basal ganglia and the paralimbic system bilaterally (Z = 3.30; puncorrected = 0.031; MNI x, y, and z coordinates: −10, 10, −2). This cluster included bilaterally the claustrum, putamen, globus pallidus, caudate nucleus, and parahippocampal gyrus, as well as the left anterior cingulate cortex, and substantia nigra. Other regions in this cluster included the left red nucleus, left ventral posterolateral and ventrolateral thalamic nuclei, and left hypothalamus. The second cluster contained the posterior lobe of the left cerebellum (Z = 3.02, puncorrected = 0.047, K = 2121 voxels, MNI x, y, and z coordinates: −48, −56, −50).
4. Discussion
This study shows different patterns of cerebral brain glucose metabolism during wakefulness and REM sleep in veterans with PTSD and participants with MDD. As hypothesized, PTSD veterans showed greater relative increases in limbic and paralimbic regions in REM sleep versus wakefulness compared to participants with MDD. Consistent with past research (Nofzinger et al., 2004), MDD was associated with state-independent hypermetabolism in these regions.
The greater increase in relative rCMRglc in limbic and paralimbic regions during REM sleep relative to wakefulness in veterans with PTSD may underlie or result from nightmares and other dysphoric dreams that characterize PTSD but not MDD (American Psychiatric Association, 1994), or the two could represent separate manifestations of a single process of PTSD REM sleep disturbance. The observed increase in rCMRglc in these brain regions during REM sleep in veterans with PTSD parallels changes observed in response to aversive stimuli in PTSD during wakefulness using other neuroimaging methods (e.g., Shin et al., 2001; Gilboa et al., 2004; Shin et al., 2004). Activation paradigms using scripts of non-distressing dreams and nightmares during wakefulness may provide an experimental method to assess whether mental imagery is the source or result of heightened limbic and paralimbic activity, or whether the two are different results of a separate process, in patients with PTSD compared to patients with MDD.
Post-hoc analyses revealed that MDD patients showed greater activation than veterans with PTSD veterans in limbic and paralimbic structures (amygdala and medial prefrontal cortex) and basal ganglia structures (caudate nucleus, putamen, and globus pallidus) during wakefulness. These regions are associated with affective and cognitive processes (e.g., Mayberg, 1997; Botvinick et al., 1999; Phillips et al., 2003a; Phillips et al., 2003b) and reward processing (Pizzagalli et al., 2001; Phillips et al., 2003a). The present findings support the notion that MDD is characterized by abnormal activity in limbic and paralimbic regions, as well as in the basal ganglia, and demonstrates underlying differences in basal neural activity between MDD and PTSD during wakefulness. A weaker pattern of differences was observed during REM sleep, whereby patients with MDD continued to show greater relative rCMRglc compared to veterans with PTSD in basal ganglia structures. More specifically, limbic and paralimbic regions, including the amygdala, hippocampus, and medial prefrontal cortex, showed greater rCMRglc in those with MDD than PTSD veterans during wakefulness but similar rCMRglc between the two groups during REM sleep. Thus, according to our findings and past findings of MDD hyperactivity relative to healthy adults in REM sleep (Nofzinger et al., 2004), MDD may be associated with state-independent hypermetabolism in these brain regions.
Finally, PTSD veterans showed a greater activation in temporo-parieto-occipital regions and inferior ventrolateral prefrontal cortex during REM sleep compared to participants with MDD. Hypermetabolism in PTSD during REM sleep in these regions may disrupt dreaming. Lesions in these areas have been related to cessation of dreaming, thus suggesting a relationship between these brain regions and mental imagery (Solms, 2000). Thus, increased metabolism in these brain regions in combination with a hyperactivation of the limbic system during REM sleep relative to wakefulness in PTSD may contribute to nightmares.
Limitations of this preliminary study relate to the absence of a healthy comparison group to more precisely quantify the observed group differences relative to healthy awake and sleeping brain activity. A second limitation relates to the absence of a detailed trauma history for participants with MDD, which make it impossible to disentangle the potential contribution of past exposure to trauma to the present findings, even though MDD participants not meeting diagnostic criteria for past or current PTSD. Third, the sample assembled from archival data for this preliminary study also limited the ability to balance sex distribution in the two study groups. Also, our attempt to isolate the effects of PTSD from those of other psychiatric conditions resulted in an atypical sample of PTSD veterans without an Axis I diagnosis that may differ from the larger population of veterans with PTSD. Larger samples and better characterization of trauma history will be necessary in future studies to fully evaluate the potential contribution of prior adverse experiences and sex on brain activity patterns across states in these two stress-related disorders.
Nevertheless, this is the first study to explore and characterize differences in relative rCMRglc during wakefulness and REM sleep in MDD and PTSD. These preliminary findings suggest that sleep disturbances associated with MDD may arise from waking and REM sleep hypermetabolism in limbic and paralimbic regions as well as in the basal ganglia, whereas REM-related hypermetabolism in limbic areas in combination with heightened activity of brain areas involved in mental imagery may subserve disturbed dreaming in PTSD.
Figure 4.
Coronal sections from MNI Y coordinates −20 to 20 in 4 mm slices where veterans with PTSD showed greater relative rCMRglc than participants with MDD during REM sleep. Colors correspond to degree of PET scan activation, as measured by relative regional cerebral metabolic rate of glucose metabolism (rCMRglc), with yellow colors indicating higher activation than red colors.
Acknowledgements
We thank the research participants, and the staff at the University of Pittsburgh Neuroscience and Clinical Translational Research Center for their role in obtaining and processing PET scans and EEG data and Olga Milgrom for instruction with usage of SPM. This study was supported by funding from the National Institutes of Health (MH083035, PI: Germain, HL082610, PI: Buysse; UL1RR024153 and UL1TR000005; PI: Reiss; MH061566 and MH66227, PI: Nofzinger) and the Department of Defense (PT073961, PI: Germain).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Med Rev. 2002;6:341–351. [PubMed] [Google Scholar]
- Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119:33–39. doi: 10.1016/s0165-1781(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Agargun MY, Kara H, Solmaz M. Subjective sleep quality and suicidality in patients with major depression. J Psychiatr Res. 1997;31:377–381. doi: 10.1016/s0022-3956(96)00037-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, Keane TM, Klauminzer G. A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behav Ther. 1990;13:187–188. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Frank E, Lowe KK, Cherry CR, Kupfer DJ. Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry. 1997;41:406–418. doi: 10.1016/S0006-3223(96)00041-8. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Tu XM, Cherry CR, Begley AE, Kowalski J, Kupfer DJ, Frank E. Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry. 1999;45:205–213. doi: 10.1016/s0006-3223(98)00198-x. [DOI] [PubMed] [Google Scholar]
- Doman J, Detka C, Hoffman T, Kesicki D, Monahan JP, Buysse DJ, Reynolds CF, Coble PA, Matzzie J, Kupfer DJ. Automating the sleep laboratory: Implementation and validation of digital recording and analysis. Int J Biomed Comput. 1995;38:277–290. doi: 10.1016/s0020-7101(05)80010-8. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinical Version: Adminstration Booklet. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, James J, Insana SP, Herringa R, Mammen O, Price J, Nofzinger E. A window into the invisible wound of war: Functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2012.05.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurgery Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow B, Artar A, Warner TD, Melendrez D, Johnston L, Hollifield M, Germain A, Koss M. Sleep disorder, depression, and suicidality in female sexual assault survivors. Crisis. 2000;21:163–170. doi: 10.1027//0227-5910.21.4.163. [DOI] [PubMed] [Google Scholar]
- Krakow B, Melendrez D, Johnston L, Warner TD, Clark JO, Pacheco M, Pedersen B, Koss M, Hollifield M, Schrader R. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190:442–452. doi: 10.1097/00005053-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lustberg L, Reynolds CF. Depression and insomnia: Questions of cause and effect. Sleep Med Rev. 2000;4:253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA. Automated detection of the intercommissural line for stereotactic localization of functional brain. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Carter C, Luna B, Price JC, Meltzer CC, Miewald JM, Reynolds CF, Kupfer DJ. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry. 2004;61:695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Price J, Meltzer CC, Townsend D, Buysse DJ, Reynolds CF, Dachille M, Matzzie J, Kupfer DJ, Moore RY. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Research Protocols. 1998;2:191–198. doi: 10.1016/s1385-299x(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Dansky BS, Kilpatrick DG. Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav. 1995;20:643–655. doi: 10.1016/0306-4603(95)00024-7. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic-cholinergic interac-tions in the primate basal forebrain. Neuroscience. 1999;93:817–829. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23:843–850. doi: 10.1017/s0140525x00003988. [DOI] [PubMed] [Google Scholar]
- Wiseman M, Nichols T, Woods R, Sweeney J, Mintun M. Stereotaxic techniques comparing foci intensity and location of activation areas in the brain as obtained using positron emission tomography (PET) J Nucl Med. 1995;36 [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]