Abstract
Most transcription factors specify the subset of genes that will be actively transcribed in the cell by stimulating transcription initiation at these genes, but MYC has a fundamentally different role. MYC binds E-box sites in the promoters of active genes and stimulates recruitment of the elongation factor P-TEFb and thus transcription elongation. Consequently, rather than specifying the set of genes that will be transcribed in any particular cell, MYC’s predominant role is to increase the production of transcripts from active genes. This increase in the transcriptional output of the cell’s existing gene expression program, called transcriptional amplification, has a profound effect on proliferation and other behaviors of a broad range of cells. Transcriptional amplification may reduce rate-limiting constraints for tumor cell proliferation and explain MYC’s broad oncogenic activity among diverse tissues.
The cancer-associated protein MYC is a transcription factor that increases the production of transcripts from active genes. It appears to play an important role at the pause-release and/or elongation stages of transcription.
TRANSCRIPTIONAL REGULATION
Transcription factors bind specific DNA sequences and regulate the recruitment and activity of the transcription apparatus at genes (Ptashne and Gann 1997; Lee and Young 2013). The process of transcription consists of at least three steps: initiation, elongation, and termination (Fuda et al. 2009; Malik and Roeder 2010; Zhou et al. 2012). During initiation, the transcription apparatus, which consists of RNA polymerase II (Pol II) and various cofactors, is recruited to genes by transcription factors. A short transcript is produced by Pol II and pause factors typically induce pausing 20–50 bp downstream of the transcriptional start site. Elongation proceeds after the elongation factor P-TEFb, which consists of Cdk9 and cyclin T, is recruited, and phosphorylates the pause factors and Pol II. Transcription termination is stimulated by recognition of polyadenylation site sequences by factors associated with Pol II during elongation.
It has long been clear that specific transcription factors are responsible for recruiting Pol II to selected genes during transcription initiation, but evidence emerged in the last decade that argues for an additional level of control at the pause-release and/or elongation stage of transcription for a large number of genes (Fuda et al. 2009; Nechaev and Adelman 2011; Zhou et al. 2012; Conaway and Conaway 2013). For example, in various human cells, Pol II was found to occupy the promoters of the majority (∼70%) of protein-coding genes, but full-length transcripts were detected at only a subset of these genes (Guenther et al. 2007). Similarly, a large fraction of Drosophila genes with roles in development were found to show evidence of transcription initiation, but not elongation (Muse et al. 2007; Zeitlinger et al. 2007). These results indicated that Pol II pausing occurs at many genes and suggested that pause control is an important step in global gene regulation.
Further investigation in mammalian cells revealed that Pol II initiates transcription bidirectionally and this divergent transcription produces short RNA species at active promoters, with full-length transcripts occurring predominantly across protein-coding genes following pause release (Core et al. 2008; Seila et al. 2008). Recent studies indicate that the RNAs produced by antisense transcription from promoters of protein-coding genes account for a large fraction of long noncoding RNA (lncRNA) species in mammalian cells (Sigova et al. 2013). Thus, Pol II molecules initiate divergent transcription at a large fraction of the genes in the genome, are subjected to pausing in both directions, and only a portion of the initiated Pol II molecules are released to produce the longer transcripts recognized as messenger RNAs (mRNAs) and lncRNAs. This further supports the idea that promoter-proximal pausing is a general feature of Pol II transcription and suggests that regulation of pause release influences both mRNA and lncRNA levels. Genome-wide studies show that the negative elongation factors NELF, DSIF, and Gdown1 co-occupy most promoters with paused Pol II, and that the positive elongation factors P-TEFb and TFIIS, generally, control pause release at actively transcribed genes (Chao and Price 2001; Core et al. 2008; Gilchrist et al. 2010; Nechaev et al. 2010; Rahl et al. 2010; Cheng et al. 2012; Jishage et al. 2012). Thus, the control of promoter-proximal pausing and transcription elongation by these and other factors is important to global gene regulation.
MYC and MAX
MYC is a master regulator of cellular proliferation. Under normal physiologic conditions it connects growth-factor stimulation to cellular proliferation and cell-cycle progression. MYC coordinates these cellular events by forming a heterodimer with MAX and binding E-box sequences (Blackwood and Eisenman 1991). The MYC basic helix-loop-helix and leucine zipper (bHLH-LZ) domains, which are located at its carboxyl terminus, are responsible for dimerization with MAX and for DNA binding. MYC has multiple transcription activation domains (TADs) in its amino terminus that recruit transcription cofactors and chromatin regulators (McMahon et al. 1998, 2000; Park et al. 2001; Knoepfler et al. 2006). MAX also contains a bHLH-LZ domain, but lacks TADs. Similarly, other MAX dimerization partners such as Mnt and Mad contain bHLH-LZ domains to facilitate dimerization with MAX, but lack TADs (Ayer et al. 1993; Hurlin et al. 1997). MYC protein levels increase following growth-factor stimulation resulting in MYC binding to increasing amounts of the constitutively expressed MAX. MAX/MAX, Mad/MAX, and Mnt/MAX dimers can also bind E-box sequences, and because these proteins lack transcriptional activation domains, these are thought to have a different transcriptional impact than MYC/MAX heterodimers. MAX/MAX and Mad/MAX binding to these sites may maintain an open chromatin structure at MYC/MAX binding sites that would allow for rapid activation of MYC-regulated genes following MYC protein induction (Ayer and Eisenman 1993; Baudino and Cleveland 2001). Consistent with this idea, MYC appears to require active chromatin modifications to bind the genome (Guccione et al. 2006; Nie et al. 2012; Soufi et al. 2012). There is evidence that RNA Pol II and other components of the transcription machinery can be loaded at promoters before MYC binding (Guccione et al. 2006; Lin et al. 2012; Nie et al. 2012), suggesting that MYC is not required to recruit the transcription apparatus to these promoters (see Sabò and Amati 2013).
MYC Regulates Transcriptional Elongation
Eberhardy and Farnham first reported that MYC regulated transcription of the human CAD gene through a P-TEFb-dependent regulatory mechanism (Eberhardy and Farnham 2001; Eberhardy and Farnham 2002). RNA Pol II was found to be constitutively bound to the CAD promoter, whereas full-length mRNA and RNA Pol II at the 3′ end of genes was detected only in S phase coincident with MYC occupancy. Furthermore, the E-box sites at the CAD promoter were dispensable for RNA Pol II recruitment. Thus, for the CAD gene, MYC binding was apparently required for transcription elongation, but not for RNA Pol II initiation.
MYC has been shown to interact with P-TEFb subunits CycT1 and CDK9 in vitro and in vivo via Myc’s TAD (Eberhardy and Farnham 2002; Kanazawa et al. 2003; Gargano et al. 2007; Rahl et al. 2010). The MYC and CycT1 interaction requires MYC Box I and MYC Box II in the TAD—the ability of these MYC domains to activate expression of a Gal4 transactivation assay correlated with their CycT1 binding (Eberhardy and Farnham 2002). Cyclin T1 interacts with MYC through its cyclin boxes, which is similar to this cyclin’s binding mode to the acidic activation domains of other transcription factors such as CIITA and RelA (Kanazawa et al. 2000; Barboric et al. 2001). The MYC, CycT1, and Cdk9 complex isolated by Peterlin and colleagues can phosphorylate the RNA Pol II carboxy-terminal domain in vitro (Kanazawa et al. 2003). Furthermore, direct recruitment of P-TEFb can substitute for Myc binding in CAD transcriptional activation (Eberhardy and Farnham 2002).
Studies of the control of the CCND2 gene also suggested a role for MYC in transcriptional steps subsequent to initiation. Eilers and colleagues found that MYC and FoxO regulate distinct steps in the transcription cycle at the CCND2 gene (Bouchard et al. 2004). Here the PI3K pathway, by regulating FoxO function, is responsible for preinitiation complex formation. Pol II and other components of the transcription initiation apparatus were found to be loaded at CCND2 in the absence of MYC activity.
Rahl et al. (2010) described multiple lines of evidence that MYC’s dominant transcriptional role at most genes in embryonic stem cells is to regulate transcriptional pause release. For example, they found that reducing the levels of MYC caused a reduction in the levels of elongating Pol II, but had little effect on the levels of promoter-proximal Pol II in genome-wide chromatin immunoprecipitation-sequencing (ChIP-seq) assays. This is in contrast to the effect of reducing the levels of the pluripotency transcription factor Oct4, which reduced the levels of both promoter-proximal Pol II and elongating Pol II at its target genes. As described below, further studies revealed the significance of this mode of transcriptional regulation in cancer cells, in which elevated levels of MYC cause transcriptional amplification by increasing transcriptional pause release (Lin et al. 2012).
The control of transcriptional pause release by MYC plays a key role in control of the pluripotent ground state in murine embryonic stem cells (mESCs) (Marks et al. 2012). mESCs can be grown in two different conditions, referred to here as 2i and serum conditions, which produce two distinguishable cell states. When grown under 2i conditions, mESCs express low levels of c-Myc and show relatively low RNA Pol II pause release across the genome (a high ratio of initiating vs. elongating RNA Pol II). When grown in serum conditions, mESCs express relatively high levels of c-Myc and show higher levels of pause release across the genome. Thus, MYC’s role in regulating transcriptional pause release appears to be key to the control of embryonic stem cell pluripotency.
GENERAL TRANSCRIPTION ELONGATION CONTROL FACTORS
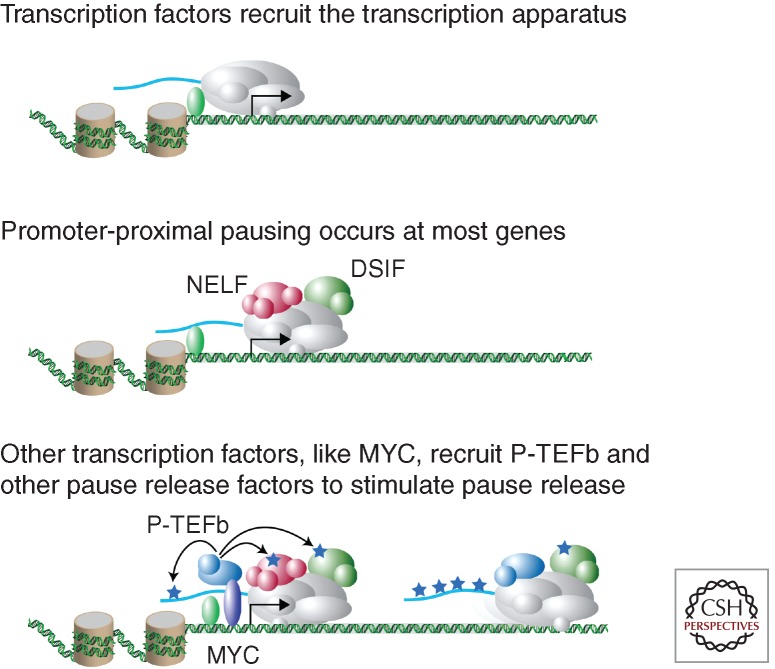
The promoters of many genes in mammalian cells can be found occupied by Pol II together with the negative elongation factors DSIF, NELF, and Gdown1 at positions located approximately 30–50 bp downstream of the transcription start site (Fig. 1), also known as promoter-proximal pause sites (Fuda et al. 2009; Nechaev and Adelman 2011; Zhou et al. 2012). DSIF, which consists of Spt4 and Spt5 subunits, was isolated as a factor that is essential for 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole-induced transcriptional inhibition (Wada et al. 1998). NELF is a multisubunit complex that functions with DSIF to repress transcriptional elongation through binding Pol II in the promoter-proximal region (Yamaguchi et al. 1999). Gdown1 adds an additional layer of promoter-proximal negative regulation that plays an important role in Mediator-dependent gene activation (Hu et al. 2006; Cheng et al. 2012; Jishage et al. 2012). Thus, Pol II occupies pause sites at a large population of genes together with this set of negative elongation factors, and the functions of these negative factors must be overcome to allow full-length transcript production.
Figure 1.
Key regulatory steps leading to transcriptional pause release. (Top) Transcription factors bind to specific DNA elements and recruit the transcription apparatus and Pol II to the transcription start site. (Middle) DSIF, NELF, and other pausing factors co-occupy regions near transcription start sites with Pol II. Pol II begins transcription from the initiation site, but pausing factors cause it to stall approximately 50 bp downstream from the start site. (Bottom) Transcription factors, including MYC, and cofactors recruit pause-release factors such as P-TEFb, which phosphorylates the pausing factors, DSIF and NELF, and Pol II, leading to elongation. Additional pause-release factors, including TFIIS and FACT, facilitate pause release and elongation. Adapted from Rahl et al. (2010) with permission from the author.
Pause release and processive transcript elongation requires the recruitment of pause-release factors, which include P-TEFb, TFIIS, and FACT (Fuda et al. 2009; Nechaev and Adelman 2011; Zhou et al. 2012). P-TEFb phosphorylates multiple key substrates important for stimulating pause release including DSIF, NELF, and the carboxy-terminal heptapeptide repeat domain of Pol II. TFIIS activity counteracts backtracking and arrests (Adelman et al. 2005). The FACT complex aids transcript elongation by remodeling nucleosomes to allow for Pol II transit through the gene (Orphanides et al. 1998; Winkler and Luger 2011). It is the recruitment of these factors by various transcriptional regulators that therefore plays a key role in effecting the gene expression program of cells.
RECRUITMENT OF TRANSCRIPTION ELONGATION CONTROL FACTORS
Transcription elongation is regulated by at least three types of protein complexes: the Mediator complex, a protein associated with both Mediator and acetylated nucleosomes called BRD4, and a variety of DNA-binding transcription factors.
The Mediator coactivator complex functions as a molecular switch capable of regulating both the initiation and elongation stages of transcription. DNA-binding transcription factors bind directly to the Mediator complex, which, in turn, binds to the transcriptional machinery (Malik and Roeder 2010; Meyer et al. 2010; Knuesel and Taatjes 2011). The human Mediator complex is approximately 1.2 mDa and consists of about 30 subunits. Mediator coordinates transcription and higher-ordered chromatin structure through interactions with numerous transcription factors, cofactors, and Pol II. A domain in the MED26 subunit has been identified that can interact with either the general initiation factor TFIID or P-TEFb, and can contribute alternately to initiation and pause release (Takahashi et al. 2011). The MED23 subunit can also contribute to P-TEFb recruitment and pause release (Wang et al. 2013). This is consistent with evidence that Mediator is required for activator-dependent stimulation of RNA Pol II transcription when RNA Pol II is associated with the negative elongation factor Gdown1 in in vitro transcription assays (Hu et al. 2006). Furthermore, Mediator can be copurified with BRD4 and co-occupies promoters genome-wide with BRD4 (Jiang et al. 1998; Loven et al. 2013; Whyte et al. 2013), and BRD4 is involved in P-TEFb recruitment, as described in more detail below. These studies argue that Mediator plays an important role in coordinating initiation and elongation.
The BET bromodomain protein BRD4, which contains two bromodomains that interact with acetylated lysines in the nucleosomal histones of active promoter regions, binds the active form of P-TEFb and thereby stimulates pause release (Jang et al. 2005; Yang et al. 2005; Krueger et al. 2010). BRD4 has been shown to interact with the transcription factors MYC/MAX, c-Jun, AP2, YY1, p53, C/EBPα, and C/EBPβ, suggesting that all these factors may influence elongation (Wu et al. 2013). Interestingly, Brd4 interacts with MYC/MAX heterodimers, but not MAX or MXD/MAX complexes, suggesting that a structural feature present on MYC but not on the structurally similar MAX or Mad accounts for the interaction with BRD4 (Wu et al. 2013). BRD4 thus serves as an adaptor protein to link active P-TEFb complex to transcriptional activators and chromatin to coordinate pause release.
Several families of sequence-specific transcription factors can recruit pause-release factors and may function through postinitiation mechanisms. Basic helix-loop-helix transcription factors including MYC, nuclear hormone receptors such as ERα, and cytokine-responsive factors including NFκB and CIITA have all been shown to recruit P-TEFb to control postinitiation regulation at regulated genes (Peterlin and Price 2006). Aire induces expression of peripheral tissue antigens in thymic epithelial cells via pause release in which Aire deficiency has been shown to have little effect on initiation, but results in a block in elongation (Oven et al. 2007; Giraud et al. 2012). The transcription factor p53 can regulate transcription through postinitiation mechanisms by modulating Mediator structure and function (Donner et al. 2010).
MYC Oncogenic Activity Alters Cellular Gene Expression Programs
MYC is one of the most potent oncogenes and possesses broad oncogenic activity in a wide range of human cancers. MYC’s primary mode of deregulation in cancer is through altered levels of MYC protein, resulting in deregulated MYC activity. A broad spectrum of cellular roles has been attributed to MYC in cancer, including regulation of cell cycle, cell proliferation, response to growth factors, ribosome biogenesis, protein synthesis, cell adhesion and cytoskeleton, angiogenesis, metabolic pathways, apoptosis, DNA replication, mRNA capping, and chromatin structure (Amati et al. 1998, 2001; Facchini and Penn 1998; Nilsson and Cleveland 2003; Hurlin and Dezfouli 2004; Secombe et al. 2004; Gallant 2005; Bernard and Eilers 2006; Dang et al. 2006, 2009; Kuttler and Mai 2006; Meyer et al. 2006; Cowling and Cole 2007, 2010; Lebofsky and Walter 2007; Nieminen et al. 2007; Shchors and Evan 2007; Sutphin et al. 2007; Cole and Cowling 2008; Dai and Lu 2008; Eilers and Eisenman 2008; Hoffman and Liebermann 2008; Meyer and Penn 2008; Prochownik 2008; Herold et al. 2009; Lin et al. 2009; Ruggero 2009; Singh and Dalton 2009; Dang 2010; van Riggelen et al. 2010; Hanahan and Weinberg 2011; Peterson and Ayer 2012; Conacci-Sorrell et al. 2013). How does oncogenic MYC activity produce these broad effects?
Two models have been proposed to explain the impact of oncogenic MYC activity on the cellular gene expression program. Distinct thresholds of MYC expression are required for increasing proliferation and apoptosis in vivo (Murphy et al. 2008). In their preview of this study, Freie and Eisenman (2008) proposed two models to explain how increased MYC levels can account for these cellular effects. In the first model, MYC binds and activates a new set of genes when expressed at increased levels. In the second model, MYC binds more of the genes it occupies when expressed at lower levels, whereby increased binding results in increased expression of the same set of genes.
It has been widely assumed that MYC, when expressed at high levels, binds and activates a new set of genes. Numerous gene expression studies have identified specific sets of genes whose expression levels are altered by changes in MYC levels; these so-called MYC targets might thus explain MYC’s role in cancer (Schuhmacher et al. 2001; Schlosser et al. 2005; Dang et al. 2006; Kim et al. 2006; Ji et al. 2011). However, it is evident that these “MYC signatures” tend to vary greatly across cell types (Chandriani et al. 2009), which has made it difficult to ascribe MYC’s oncogenic properties to a specific set of target genes.
Transcriptional Amplification
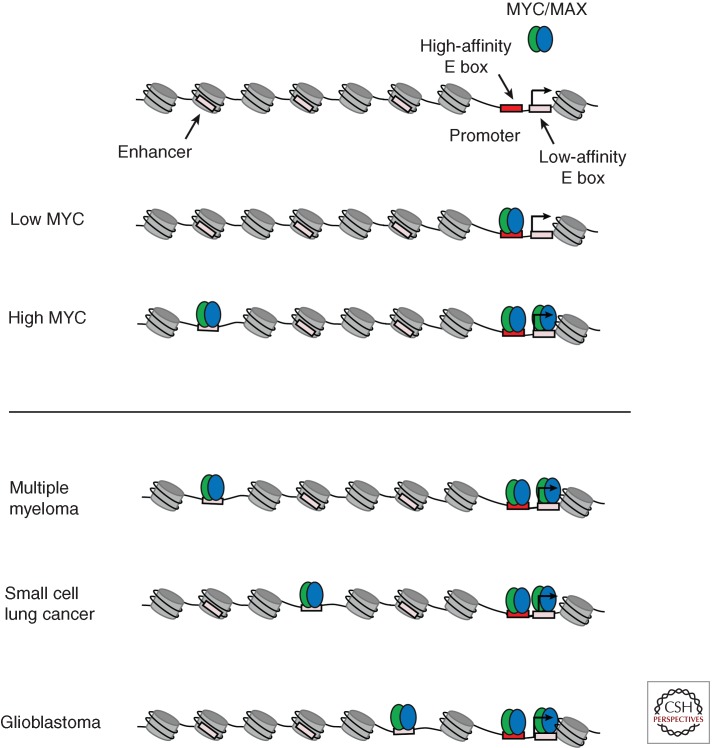
MYC’s dominant transcriptional role in embryonic stem cells is to regulate transcriptional pause release genome-wide (Rahl et al., 2010), but it was not clear from this study how exceptionally high levels (oncogenic levels) of MYC might impact tumor cells. The MYC-inducible P493-6 B cell lymphoma cell line model and various other MYC-dependent human cancer cell lines were recently used to study this issue (Lin et al. 2012). The effect of elevated levels of MYC on its occupancy was analyzed using ChIP-seq analysis. In general, MYC occupied the core promoter of active genes together with RNA Pol II. Increasing MYC protein levels 28-fold in P493-6 cells had little effect on the total number of genes bound by MYC or the number of genes that were actively transcribed. Rather, the predominant effect on MYC occupancy was increased levels of MYC binding at the promoters of the same set of active genes. Increased levels of MYC also caused it to occupy the enhancers of actively transcribed genes. MYC occupied lower affinity E-box sequences at core promoters and enhancers when expressed at high levels (Fig. 2). Similar results were obtained with human cancer cell lines overexpressing MYC. The predominant effect of increased MYC occupancy at genes was increased transcriptional pause release. Increased MYC occupancy led to increased P-TEFb occupancy, increased levels of RNAPII Serine 2 (a modification associated with elongation), increased levels of elongating RNAPII, and increased levels of mRNA for the active gene expression program. Thus, the primary effect of elevated levels of MYC is transcriptional amplification: the production of increased levels of transcripts within the cell’s gene expression program (Fig. 3).
Figure 2.
Elevated levels of MYC leads to altered genome-wide occupancy. (Top) When expressed at low levels, MYC/MAX dimers occupy high-affinity E-box sites in the genome, which are generally located near transcriptional start sites. When expressed at elevated levels, MYC/MAX dimers saturate high-affinity E-box sites and occupy lower affinity binding sites near transcription start sites and at enhancer regions. (Bottom) When overexpressed in different cancer types, MYC/MAX dimers bind low-affinity sites at enhancers. Because many enhancers are used in a tissue-specific manner, open chromatin regions with low-affinity binding sites can vary between cancer types, thus leading to different MYC/MAX binding profiles at enhancers in different cancer types.
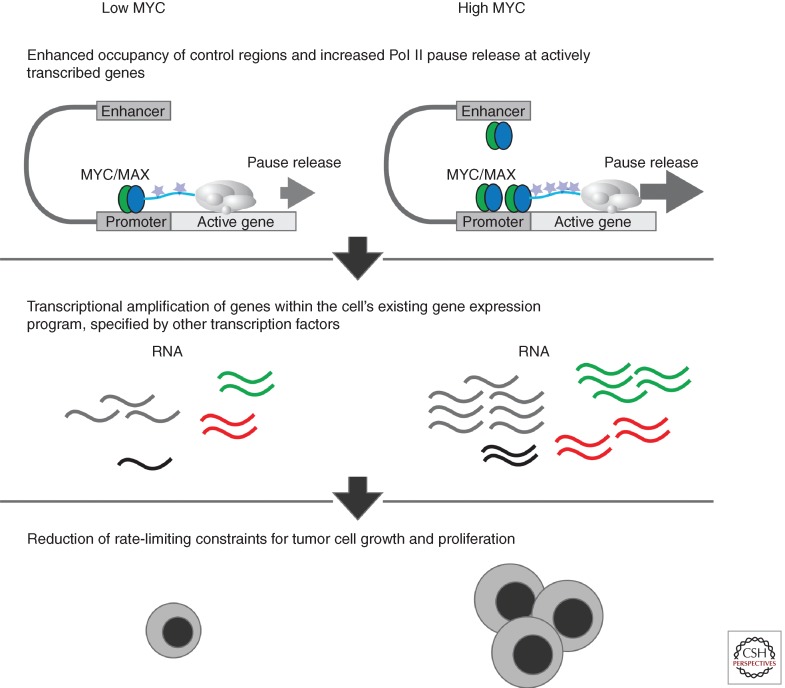
Figure 3.
Elevated levels of MYC leads to transcriptional amplification. (Top) High levels of MYC lead to enhanced occupancy at transcriptional start sites and enhancer elements and increased Pol II pause release. (Middle) High levels of MYC lead to transcriptional amplification of the actively transcribed genes in the cell. The cell’s existing gene expression program is specified by other transcription factors. (Bottom) The amplification of the gene expression program can reduce rate-limiting constraints for cell growth and proliferation. (From Lin et al. 2012; adapted, with permission, from the author.)
MYC acts as a transcriptional amplifier in nonpathological settings as well. By studying MYC activity in murine lymphocyte activation and embryonic stem cells, Levens and colleagues found that MYC does not specifically activate or repress genes, rather it is a nonlinear amplifier of most actively transcribed genes (Nie et al. 2012). For example, RNA Pol II loading at promoters of resting and activated B cells is highly similar. MYC expression in activated B cells simply elevates the expression level of genes already expressed. Therefore, MYC is an amplifier under normal physiologic conditions and cancer exploits this function through deregulating its activity (see Levens 2013).
MYC overexpression consistently results in global transcriptional amplification with widespread increases in transcripts per cell following increases in MYC levels (Loven et al. 2012). Importantly, however, a prolonged increase of MYC activity can lead to repression of certain genes as secondary effects begin to occur. For example, increased expression of repressors, including miRNAs and Polycomb proteins, can lead to repression of some genes (Neri et al. 2012). Thus, the net effect of global transcriptional amplification can ultimately cause repression of certain genes.
Transcriptional amplification of the cell’s gene expression program can account for Myc’s diverse roles in cancer and explain why MYC plays a critical role in tumorigenesis in a wide variety of human tissues. MYC signatures vary greatly across multiple cell types (Chandriani et al. 2009). The transcriptional amplification model provides an explanation for this variation. The set of genes whose expression is altered by MYC should, in fact, be different in different cell types, as oncogenic MYC will amplify each cell’s inherent gene expression program and not an MYC-specific program.
MYC’s broad oncogenic activity suggests that it can reduce different rate-limiting constraints for cellular proliferation in different cells (Vita and Henriksson 2006). In this model of transcription amplification, genes rate limiting for growth should be amplified provided they are transcriptionally active before MYC elevation. For example, MYC-mediated transcriptional amplification of ribosomal subunits could increase translational capacity (Arabi et al. 2005; Grandori et al. 2005; Grewal et al. 2005; Dai and Lu 2008). For cellular functions that are limiting for the growth of tumorigenic cells such as translational capacity and aerobic energy metabolism, an increase in this machinery would provide a mechanism to explain how elevated MYC levels contribute to tumorigenesis (Ruggero et al. 2004; Dang et al. 2009; Feng and Levine 2010; Vander Heiden et al. 2010; Hanahan and Weinberg 2011; Bayley and Devilee 2012). It is also possible that the increase in essentially all components of the gene expression program provides cells with an advantage when adapting to the multiple mutated pathways that characterize most tumor cells.
There has been substantial progress in our understanding of MYC-dependent transcriptional control across the genome and how it influences cell state. N-MYC and L-MYC are also powerful oncogenes that function as transcription factors, although less is known about their transcriptional regulatory circuitry. Future studies of N-MYC and L-MYC function should provide important insight into the similarities or differences among the MYC family regulatory circuitry. MYC appears to be a broader-acting oncogene with tumorigenic activity in diverse tissues, whereas N-MYC and L-MYC are often more restricted in the tissues they transform. Despite this difference, which may be largely due to tissue-specific expression, there are likely to be many mechanistic similarities among the MYC family as they are all powerful oncogenes. Such similarity is suggested by different subclasses of medulloblastoma that appear to activate MYC family transcription factors through any means necessary: MYC amplification, MYCN amplification, MYCL1 amplification, or deregulated upstream signaling pathways including Wnt (Northcott et al. 2012; Roussel and Robinson 2013).
ACKNOWLEDGMENTS
We thank our funding sources (National Institutes of Health grants HG002668 and CA146445) and the editors Robert Eisenman and Chi Dang for their comments on the manuscript.
Footnotes
Editors: Chi V. Dang and Robert N. Eisenman
Additional Perspectives on MYC and the Pathway to Cancer available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Adelman K, Marr MT, Werner J, Saunders A, Ni Z, Andrulis ED, Lis JT 2005. Efficient release from promoter-proximal stall sites requires transcript cleavage factor TFIIS. Mol Cell 17: 103–112 [DOI] [PubMed] [Google Scholar]
- Amati B, Alevizopoulos K, Vlach J 1998. Myc and the cell cycle. Front Biosci 3: d250–d268 [DOI] [PubMed] [Google Scholar]
- Amati B, Frank SR, Donjerkovic D, Taubert S 2001. Function of the c-Myc oncoprotein in chromatin remodeling and transcription. Biochim Biophys Acta 1471: M135–M145 [DOI] [PubMed] [Google Scholar]
- Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, et al. 2005. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol 7: 303–310 [DOI] [PubMed] [Google Scholar]
- Ayer DE, Eisenman RN 1993. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev 7: 2110–2119 [DOI] [PubMed] [Google Scholar]
- Ayer DE, Kretzner L, Eisenman RN 1993. Mad: A heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell 72: 211–222 [DOI] [PubMed] [Google Scholar]
- Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol Cell 8: 327–337 [DOI] [PubMed] [Google Scholar]
- Baudino TA, Cleveland JL 2001. The Max network gone mad. Mol Cell Biol 21: 691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley JP, Devilee P 2012. The Warburg effect in 2012. Curr Opin Oncol 24: 62–67 [DOI] [PubMed] [Google Scholar]
- Bernard S, Eilers M 2006. Control of cell proliferation and growth by Myc proteins. Results Probl Cell Differ 42: 329–342 [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Eisenman RN 1991. Max: A helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251: 1211–1217 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Marquardt J, Bras A, Medema RH, Eilers M 2004. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J 23: 2830–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandriani S, Frengen E, Cowling VH, Pendergrass SA, Perou CM, Whitfield ML, Cole MD 2009. A core MYC gene expression signature is prominent in basal-like breast cancer but only partially overlaps the core serum response. PLoS ONE 4: e6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SH, Price DH 2001. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem 276: 31793–31799 [DOI] [PubMed] [Google Scholar]
- Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, Varzavand K, Cooper JJ, Hu X, Gnatt A, et al. 2012. Functional association of Gdown1 with RNA polymerase II poised on human genes. Mol Cell 45: 38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MD, Cowling VH 2008. Transcription-independent functions of MYC: Regulation of translation and DNA replication. Nat Rev Mol Cell Biol 9: 810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Conacci-Sorrell M, McFerrin L, Eisenman RN 2013. An overview of MYC and its interactome. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW 2013. The Mediator complex and transcription elongation. Biochim Biophys Acta 1829: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT 2008. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322: 1845–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, Cole MD 2007. Turning the tables: Myc activates Wnt in breast cancer. Cell Cycle 6: 2625–2627 [DOI] [PubMed] [Google Scholar]
- Cowling VH, Cole MD 2010. Myc regulation of mRNA cap methylation. Genes Cancer 1: 576–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai MS, Lu H 2008. Crosstalk between c-Myc and ribosome in ribosomal biogenesis and cancer. J Cell Biochem 105: 670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV 2010. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res 70: 859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F 2006. The c-Myc target gene network. Semin Cancer Biol 16: 253–264 [DOI] [PubMed] [Google Scholar]
- Dang CV, Le A, Gao P 2009. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15: 6479–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM 2010. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol 17: 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardy SR, Farnham PJ 2001. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J Biol Chem 276: 48562–48571 [DOI] [PubMed] [Google Scholar]
- Eberhardy SR, Farnham PJ 2002. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J Biol Chem 277: 40156–40162 [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN 2008. Myc’s broad reach. Genes Dev 22: 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini LM, Penn LZ 1998. The molecular role of Myc in growth and transformation: Recent discoveries lead to new insights. FASEB J 12: 633–651 [PubMed] [Google Scholar]
- Feng Z, Levine AJ 2010. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 20: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freie BW, Eisenman RN 2008. Ratcheting Myc. Cancer Cell 14: 425–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuda NJ, Ardehali MB, Lis JT 2009. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461: 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P 2005. Myc, cell competition, and compensatory proliferation. Cancer Res 65: 6485–6487 [DOI] [PubMed] [Google Scholar]
- Gargano B, Amente S, Majello B, Lania L 2007. P-TEFb is a crucial co-factor for Myc transactivation. Cell Cycle 6: 2031–2037 [DOI] [PubMed] [Google Scholar]
- Gilchrist DA, Dos Santos G, Fargo DC, Xie B, Gao Y, Li L, Adelman K 2010. Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell 143: 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, Benoist C 2012. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci 109: 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ 2005. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol 7: 311–318 [DOI] [PubMed] [Google Scholar]
- Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA 2005. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol 7: 295–302 [DOI] [PubMed] [Google Scholar]
- Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall’ Olio V, Zardo G, Nervi C, Bernard L, Amati B 2006. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol 8: 764–770 [DOI] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA 2007. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130: 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA 2011. Hallmarks of cancer: The next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Herold S, Herkert B, Eilers M 2009. Facilitating replication under stress: An oncogenic function of MYC? Nat Rev Cancer 9: 441–444 [DOI] [PubMed] [Google Scholar]
- Hoffman B, Liebermann DA 2008. Apoptotic signaling by c-MYC. Oncogene 27: 6462–6472 [DOI] [PubMed] [Google Scholar]
- Hu X, Malik S, Negroiu CC, Hubbard K, Velalar CN, Hampton B, Grosu D, Catalano J, Roeder RG, Gnatt A 2006. A Mediator-responsive form of metazoan RNA polymerase II. Proc Natl Acad Sci 103: 9506–9511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Dezfouli S 2004. Functions of myc:max in the control of cell proliferation and tumorigenesis. Int Rev Cytol 238: 183–226 [DOI] [PubMed] [Google Scholar]
- Hurlin PJ, Queva C, Eisenman RN 1997. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev 11: 44–58 [DOI] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 19: 523–534 [DOI] [PubMed] [Google Scholar]
- Ji H, Wu G, Zhan X, Nolan A, Koh C, De Marzo A, Doan HM, Fan J, Cheadle C, Fallahi M, et al. 2011. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PLoS ONE 6: e26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, Kornberg RD 1998. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci 95: 8538–8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage M, Malik S, Wagner U, Uberheide B, Ishihama Y, Hu X, Chait BT, Gnatt A, Ren B, Roeder RG 2012. Transcriptional regulation by Pol II(G) involving mediator and competitive interactions of Gdown1 and TFIIF with Pol II. Mol Cell 45: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S, Okamoto T, Peterlin BM 2000. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 12: 61–70 [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM 2003. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene 22: 5707–5711 [DOI] [PubMed] [Google Scholar]
- Kim YH, Girard L, Giacomini CP, Wang P, Hernandez-Boussard T, Tibshirani R, Minna JD, Pollack JR 2006. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene 25: 130–138 [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN 2006. Myc influences global chromatin structure. EMBO J 25: 2723–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel MT, Taatjes DJ 2011. Mediator and post-recruitment regulation of RNA polymerase II. Transcription 2: 28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger BJ, Varzavand K, Cooper JJ, Price DH 2010. The mechanism of release of P-TEFb and HEXIM1 from the 7SK snRNP by viral and cellular activators includes a conformational change in 7SK. PLoS ONE 5: e12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttler F, Mai S 2006. c-Myc, genomic instability and disease. Genome Dyn 1: 171–190 [DOI] [PubMed] [Google Scholar]
- Lebofsky R, Walter JC 2007. New Myc-anisms for DNA replication and tumorigenesis? Cancer Cell 12: 102–103 [DOI] [PubMed] [Google Scholar]
- Lee TI, Young RA 2013. Transcriptional regulation and its misregulation in disease. Cell 152: 1237–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Levens D 2013. Cellular MYCro economics: Balancing MYC function with MYC expression. Cold Spring Harb Perspect Med 5: a014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Malina A, Pelletier J 2009. c-Myc and eIF4F constitute a feedforward loop that regulates cell growth: Implications for anticancer therapy. Cancer Res 69: 7491–7494 [DOI] [PubMed] [Google Scholar]
- Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA 2012. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151: 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Orlando DA, Sigova AA, Lin CY, Rahl PB, Burge CB, Levens DL, Lee TI, Young RA 2012. Revisiting global gene expression analysis. Cell 151: 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA 2013. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153: 320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Roeder RG 2010. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet 11: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, et al. 2012. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149: 590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD 1998. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94: 363–374 [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wood MA, Cole MD 2000. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol Cell Biol 20: 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Penn LZ 2008. Reflecting on 25 years with MYC. Nat Rev Cancer 8: 976–990 [DOI] [PubMed] [Google Scholar]
- Meyer N, Kim SS, Penn LZ 2006. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol 16: 275–287 [DOI] [PubMed] [Google Scholar]
- Meyer KD, Lin SC, Bernecky C, Gao Y, Taatjes DJ 2010. p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol 17: 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI 2008. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell 14: 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, Adelman K 2007. RNA polymerase is poised for activation across the genome. Nat Genet 39: 1507–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechaev S, Adelman K 2011. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochim Biophys Acta 1809: 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechaev S, Fargo DC, dos Santos G, Liu L, Gao Y, Adelman K 2010. Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science 327: 335–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri F, Zippo A, Krepelova A, Cherubini A, Rocchigiani M, Oliviero S 2012. Myc regulates the transcription of the PRC2 gene to control the expression of developmental genes in embryonic stem cells. Mol Cell Biol 32: 840–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. 2012. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151: 68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen AI, Partanen JI, Klefstrom J 2007. c-Myc blazing a trail of death: Coupling of the mitochondrial and death receptor apoptosis pathways by c-Myc. Cell Cycle 6: 2464–2472 [DOI] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL 2003. Myc pathways provoking cell suicide and cancer. Oncogene 22: 9007–9021 [DOI] [PubMed] [Google Scholar]
- Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stutz AM, Korshunov A, Reimand J, Schumacher SE, et al. 2012. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 488: 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D 1998. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92: 105–116 [DOI] [PubMed] [Google Scholar]
- Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM 2007. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol 27: 8815–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kunjibettu S, McMahon SB, Cole MD 2001. The ATM-related domain of TRRAP is required for histone acetyltransferase recruitment and Myc-dependent oncogenesis. Genes Dev 15: 1619–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BM, Price DH 2006. Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23: 297–305 [DOI] [PubMed] [Google Scholar]
- Peterson CW, Ayer DE 2012. An extended Myc network contributes to glucose homeostasis in cancer and diabetes. Front Biosci 17: 2206–2223 [DOI] [PubMed] [Google Scholar]
- Prochownik EV 2008. c-Myc: Linking transformation and genomic instability. Curr Mol Med 8: 446–458 [DOI] [PubMed] [Google Scholar]
- Ptashne M, Gann A 1997. Transcriptional activation by recruitment. Nature 386: 569–577 [DOI] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA 2010. c-Myc regulates transcriptional pause release. Cell 141: 432–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Roussel MF, Robinson GW 2013. Role of MYC in medulloblastoma. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D 2009. The role of Myc-induced protein synthesis in cancer. Cancer Res 69: 8839–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP 2004. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med 10: 484–486 [DOI] [PubMed] [Google Scholar]
- *.Sabò A, Amati B 2013. Genome recognition by Myc. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser I, Holzel M, Hoffmann R, Burtscher H, Kohlhuber F, Schuhmacher M, Chapman R, Weidle UH, Eick D 2005. Dissection of transcriptional programmes in response to serum and c-Myc in a human B-cell line. Oncogene 24: 520–524 [DOI] [PubMed] [Google Scholar]
- Schuhmacher M, Kohlhuber F, Holzel M, Kaiser C, Burtscher H, Jarsch M, Bornkamm GW, Laux G, Polack A, Weidle UH, et al. 2001. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res 29: 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secombe J, Pierce SB, Eisenman RN 2004. Myc: A weapon of mass destruction. Cell 117: 153–156 [DOI] [PubMed] [Google Scholar]
- Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, Young RA, Sharp PA 2008. Divergent transcription from active promoters. Science 322: 1849–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchors K, Evan G 2007. Tumor angiogenesis: Cause or consequence of cancer? Cancer Res 67: 7059–7061 [DOI] [PubMed] [Google Scholar]
- Sigova AA, Mullen AC, Molinie B, Gupta S, Orlando DA, Guenther MG, Almada AE, Lin C, Sharp PA, Giallourakis CC, et al. 2013. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci 110: 2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Dalton S 2009. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell 5: 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS 2012. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell 151: 994–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin PD, Giaccia AJ, Chan DA 2007. Energy regulation: HIF MXIes it up with the C-MYC powerhouse. Dev Cell 12: 845–846 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, et al. 2011. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell 146: 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, et al. 2010. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329: 1492–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riggelen J, Yetil A, Felsher DW 2010. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10: 301–309 [DOI] [PubMed] [Google Scholar]
- Vita M, Henriksson M 2006. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol 16: 318–330 [DOI] [PubMed] [Google Scholar]
- Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog GA, Winston F, et al. 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev 12: 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yao X, Huang Y, Hu X, Liu R, Hou D, Chen R, Wang G 2013. Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb. Transcription 4: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA 2013. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153: 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DD, Luger K 2011. The histone chaperone FACT: Structural insights and mechanisms for nucleosome reorganization. J Biol Chem 286: 18369–18374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM 2013. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell 49: 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H 1999. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97: 41–51 [DOI] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 19: 535–545 [DOI] [PubMed] [Google Scholar]
- Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, Levine M, Young RA 2007. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet 39: 1512–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li T, Price DH 2012. RNA polymerase II elongation control. Annu Rev Biochem 81: 119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]