Abstract
Objectives: To prospectively use a non-invasive algorithm to identify asymptomatic, advanced non-alcoholic fatty liver disease (NAFLD) in a secondary care diabetes clinic and to determine the short-term effect of a multi-disciplinary team (MDT) approach in a liver clinic.
Research design and methods: NAFLD Fibrosis Score (NFS) was calculated in 64 asymptomatic patients with type 2 diabetes. Advanced fibrosis was identified using transient elastography and confirmed with liver biopsy. In a subsequent retrospective study, 95 patients newly referred to the NAFLD MDT clinic were investigated and the impact of the MDT approach assessed.
Results: 25/64 (39.0%) of patients with diabetes had a low NFS (<–1.455). 39/64 (61.0%) patients had a high or indeterminate NFS and were referred for review in the NAFLD MDT clinic, of which 23/39 attended for assessment. 19/23 (82.6%) were diagnosed with NAFLD, of which 6/19 (31.6%) patients had a positive transient elastography (≥8 kPa). Liver biopsy confirmed advanced fibrosis in 5/6 cases, with moderate fibrosis in 1 case.
In the retrospective study, 65/95 (68.4%) new referrals to the NAFLD MDT clinic had a diagnosis of NAFLD. Over a median 98 days (IQR 70–182) follow-up, there was a significant improvement in weight (–0.8 kg; P = 0.024), total cholesterol (–0.2 mmol/L; P = 0.044), ALT (alanine transmaminase, −12.5 IU/L; P < 0.001) and GGT (gammu-glutamyl transferase, −13.0 IU/L; P < 0.0001). 7/28 (25%) of patients with diabetes achieved >5% weight loss.
Conclusions: A significant proportion of asymptomatic patients attending type 2 diabetes clinics have undiagnosed advanced NAFLD fibrosis. An MDT approach to NAFLD results in short-term improvements in metabolic and liver parameters.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is an independent risk factor for mortality, with the leading causes of death being cardiovascular disease, malignancy and liver complications.1,2 Type 2 diabetes and NAFLD are reciprocal risk factors and having one condition doubles an individual’s risk of developing the other.3,4 The presence of NAFLD in patients with type 2 diabetes is associated with poor glycaemic control,5 increased cardiovascular events,5 diabetic nephropathy6 and a >2-fold increase in all-cause mortality.7,8 For the majority with simple hepatic steatosis ongoing diabetes care and cardiovascular risk management is appropriate. However, in those with advanced liver fibrosis, specialist input is recommended to maximize metabolic control and monitoring for hepatocellular carcinoma (HCC) and portal hypertension.
Despite the potential magnitude of the clinical problem9 screening for NAFLD in asymptomatic patients with type 2 diabetes is not currently recommended due to uncertainties in diagnostic tools, disease intervention and cost-effectiveness.10 With prevalence amounting to 43–69% in populations with type 2 diabetes5,11,12 referral of this number of patients with NAFLD (on imaging) to specialist liver clinics would not be feasible and is not necessary.10 Identifying those with advanced liver fibrosis and in greatest need of liver specialist input is a clinical challenge as the vast majority are asymptomatic and have normal liver function tests (LFTs).5 Several non-invasive tools (i.e. NAFLD Fibrosis Score (NFS), transient elastography) have emerged in recent years with the aim to accurately rule-out or identify those with advanced liver fibrosis.13–15
The first aim of our study was to prospectively analyse the utility of the NFS in conjunction with transient elastography to identify patients at risk of advanced NAFLD fibrosis within a routine diabetes clinic. Secondly we aimed to determine the effect on clinical parameters of a multi-disciplinary team (MDT) approach to the management of patients with NAFLD.
Methods
1. Prospective analysis of the presence and severity of NAFLD in a routine diabetes clinic
The study protocol was approved by the North Staffordshire Research Ethics Committee (ref. 09/H1204/97). All participants provided informed written consent.
Study population
All asymptomatic patients with type 2 diabetes who attended the general diabetes clinic at Heartlands Hospital, Birmingham during a 3-month period in 2010, were consecutively approached by the same consultant diabetologist (M.A.K., study investigator) for recruitment to the study. Patients were excluded if they were >80 years old, had previous history/signs/symptoms of liver disease or excess alcohol (males > 30 g/day; females > 20 g/day), acute medical illness (as judged by the investigator) and/or failed to given written consent.
Data recorded included: age, gender, ethnicity, duration of diabetes, blood pressure, body mass index (BMI, kg/m2), past/current medical and medications history. Non-fasting full blood count, HbA1c, blood cholesterol and LFTs were measured. The NFS13 was calculated using the web-based calculator (http://NAFLDscore.com) (Supplementary Box 1). Patients with either a high (>0.676) or indeterminate NFS (–1.455 to +0.676) were offered referral to the NAFLD MDT clinic (UHB).
NAFLD assessment
At the NAFLD MDT clinic, in addition to routine clinical assessment (Supplementary Box 2) and observations, patients underwent a full liver aetiology screen and an abdominal ultrasound scan. The diagnosis of NAFLD was based on the following criteria: (i) ultrasound diagnosis of fatty liver, defined as diffusely increased liver echogenicity (>right renal parenchyma) with vascular blurring; (ii) a negative history of excess alcohol consumption; and (iii) exclusion of types of liver disease (drug induced, autoimmune, viral hepatitis, cholestatic and genetic).
Transient elastography (Fibroscan, Echosens, France) was performed on patients diagnosed with NAFLD. A ‘positive’ result was defined as a valid liver stiffness evaluation (LSE) ≥ 8 kPa. An LSE cut-off ≥ 8 kPa has been used in previous studies to determine the presence of significant fibrosis; values above this indicate that further investigation is appropriate.16,17 In the event of a positive LSE or significant discordance between the NFS and LSE (e.g. high NFS, negative LSE), the patient underwent an ultrasound-guided liver biopsy performed according to standard clinical practice. Liver biopsies were scored based on the Kleiner classification by expert liver histopathologists18 In patients, that declined a liver biopsy, an alternative non-invasive liver fibrosis biomarker was used (Fibrotest, Biopredictive, France), which has been shown to correlate with biopsy fibrosis stage in NAFLD.19
2. MDT management of NAFLD
The study protocol was approved by the clinical research committee at UHB, UK (ref CA5-03331-10).
Study population and data collection
All new patients referred to the NAFLD MDT clinic at UHB were retrospectively identified for a 1-year period between 1 January 2010 and 31 December 2010. All new patients that were subsequently diagnosed with NAFLD were included in the study. Patients found to have other liver disease aetiology including significant alcohol history, positive liver aetiology screen, drug-induced fatty liver and/or decompensated chronic liver disease were excluded from analysis.
Clinical data were collected from the patients’ initial clinic visit (visit 1) and their next follow-up clinic visit (visit 2). A new diagnosis of type 2 diabetes was made with either a 75 g oral glucose tolerance test (OGTT) or with two fasting blood glucose samples ≥7.0 mmol/L, according to established criteria.20 The dietary assessment and advice given is summarized in the Supplementary Box 3.
Data analysis
Continuous clinical and laboratory variables are reported as medians and interquartile ranges (IQRs) as all variables had non-parametric distribution. Categorical variables are reported as number and percentages. Wilcoxon matched-pairs signed rank tests (continuous variables) and Fisher exact/chi-squared tests (categorical variables) were used to assess whether there had been a change in the patients clinical/metabolic parameters between visit 1 and visit 2 (Prism 6.0, Graphpad Software Inc, USA).
Results
1. Prospective analysis of the presence and severity of NAFLD in a routine diabetes clinic
Study population
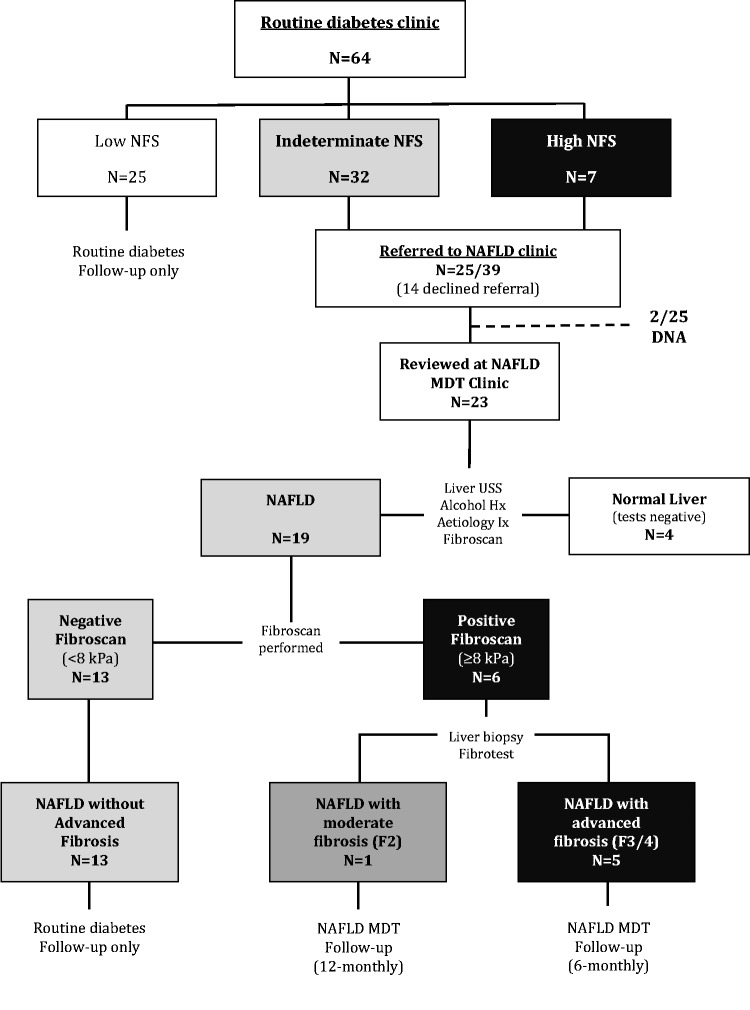
64 patients were recruited into the study, after excluding 16 patients due to age (n = 9), past medical history of viral hepatitis B (n = 1), excess alcohol (n = 5) and a current chest infection (n = 1). The clinical characteristics of the study patients are summarized in Supplementary Table 1. The median duration of diabetes was 8.5 years and HbA1c 7.8% (62 mmol/mol). 21/64 (32.8%) patients had abnormal blood liver enzymes. 25/64 (39.0%) of patients with diabetes had a low NFS (<–1.455), 32/64 (50.0%) had an indeterminate NFS (–1.455 to 0.676) and 7/64 (10.9%) had a high NFS (>0.676) suggestive of advanced liver fibrosis. 25/39 (64.1%) patients with diabetes and a high or indeterminate NFS agreed to a referral to the NAFLD MDT clinic.
Liver assessment at the NAFLD clinic
23/25 (92%) patients referred were reviewed in the NAFLD MDT clinic. Two patients failed to attend after repeated invitations (Figure 1). Of these 23 patients, 4 patients (17.4%) were successfully discharged after one consultation as they had a normal liver ultrasound, a negative liver aetiology screen and a negative LSE (<8 kPa). The remaining 19/23 patients were diagnosed with NAFLD according to the criteria outlined above (Supplementary Table 1). Disease severity in these patients was then assessed with transient elastography (LSE).
Figure 1.
Flow diagram of the step-wise identification of advanced NAFLD fibrosis in a routine secondary diabetes clinic. Data from the prospective study using the NFS (13) and LSE to stratify type 2 diabetes patients at risk of advanced liver fibrosis (Heartlands Hospital Birmingham). Key: F0–F4, Kleiner stages of fibrosis (F3/4 = advanced fibrosis/cirrhosis).
6/19 patients had LSE ≥8 kPa and liver biopsy was recommended in all cases. Five patients underwent liver biopsy (one declined in whom the Fibrotest was performed) and all had significant liver fibrosis (Kleiner stage ≥2). Of the patients with LSE ≥ 8 kPa, 5/6 were diagnosed with advanced fibrosis/cirrhosis (Kleiner stage 3–4). There were no obvious symptoms, signs or observations that would have led to the diagnosis of advanced liver fibrosis in these five patients in the diabetes clinic (median age 61 years; BMI 35.8 kg/m2; HbA1c 7.5% (58 mmol/mol); ALT (alanine transmaminase) 42 IU/L and AST (aspartate transaminase) 39 IU/L). Under surveillance, one patient was diagnosed with portal hypertension, but there were no cases of HCC on imaging. One patient was diagnosed with moderate fibrosis (Kleiner stage 2 on liver biopsy).
In the 1 case out of 19 with discordance between a high NFS and a negative LSE, a liver biopsy was performed and revealed active non-alcoholic steatohepatitis (active hepatocyte inflammation/ballooning), but with no evidence of fibrosis. The remaining 12/19 (63.2%) were diagnosed with non-invasive tools as having NAFLD without advanced fibrosis and were subsequently discharged back to routine diabetes care.
2. MDT management of NAFLD
Study population
Ninety-five new patient referrals were seen between 1 January 2010 and 31 December 2010. The reasons for referral were abnormal LFTs (44.2%; 42/95), fatty liver on ultrasound (12.6%; 12/95), both (34.7; 33/95) or another reason (8.4%; 8/95). Thirty (31.6%) patients were excluded from analysis because they were found to have an alternative liver disease aetiology (alcoholic liver disease, 7/95; viral hepatitis, 6/95; haemochromatosis, 2/95; hepatic congestion secondary to heart failure, 2/95; and other, 4/95), a normal liver (2/95), or decompensated chronic liver disease (ascites +/– encephalopathy, 7/95).
Metabolic characteristics at initial clinic visit (visit 1)
Sixty-five (68.4%) new patient referrals were diagnosed with NAFLD according to the criteria outlined above. Patient characteristics and demographics are summarized in Table 1. In 7/65 patients a new diagnosis of type 2 diabetes was made as a result of the initial MDT clinic visit and one patient was diagnosed with impaired glucose tolerance. 40.6% (13/32) of patients with type 2 diabetes had their medication regimen changed at visit 1. These changes included: introduction of a new or concomitant anti-hyperglycaemic medication (n = 8), a change in dose of anti-hyperglycaemic medication (n = 3) and/or the addition of a lipid-lowering medication (n = 2). 46.9% (15/32) of type 2 diabetes patients had a one-to-one dietary review by a specialist dietician at visit 1. No patients were started on pioglitazone, vitamin E or other potential hepato-protective medications during the study period.
Table 1.
Demographics and characteristics of patients with and without type 2 diabetes referred to the NAFLD MDT clinic (UHB) in 2010
| Total (n = 65) | Type 2 diabetes (n = 32) | Without type 2 diabetes (n = 33) | P-value (T2DM vs. non-T2DM) | |
|---|---|---|---|---|
| Age | 46.0 (37.5–59.0) | 57.5 (42.5–65.5) | 40.0 (36.0–55.0) | 0.004 |
| Male sex, % (n) | 53.9 (35/65) | 46.9 (15/32) | 60.6 (20/33) | 0.324 |
| Ethnicity | ||||
| White | 52.3 (34/65) | 59.4 (19/32) | 45.5 (15/33) | 0.437 |
| Asian | 18.5 (12/65) | 15.6 (5/32) | 21.2 (7/33) | |
| Black | 1.5 (1/65) | 0.0 (0/32) | 3.0 (1/33) | |
| Other/unknown | 27.7 (18/65) | 25.0 (8/32) | 30.3 (10/33) | |
| Source of referral | ||||
| Primary care | 72.3 (47/65) | 62.5 (20/32) | 81.8 (27/33) | 0.102 |
| Secondary care | 27.7 (18/65) | 37.5 (12/32) | 18.2 (6/33) | |
| Metabolic conditions | ||||
| Obesity (BMI > 30), % (n) | 67.7 (44/65) | 78.1 (25/32) | 57.6 (19/33) | 0.118 |
| Hypertension, % (n) | 47.7 (31/65) | 59.4 (19/32) | 36.4 (12/33) | 0.084 |
| Hyperlipidaemia, % (n) | 43.1 (28/65) | 56.3 (18/32) | 30.3 (10/33) | 0.046 |
| Ischaemic heart disease, % (n) | 10.8 (7/65) | 18.8 (6/32) | 3.0 (1/33) | 0.054 |
| Type 2 diabetes, % (n) | 49.2 (32/65) | |||
| Diabetes therapy, % (n) | ||||
| Nonea | 60.0 (39/65) | 21.9 (7/32) | 97.0 (32/33) | <0.0001 |
| Diet | 7.7 (5/65) | 15.6 (5/32) | 0.0 (0/33) | |
| Oral anti-hyperglycaemic onlyb | 15.4 (10/65) | 28.1 (9/32) | 3.0 (1/33) | |
| Insulin | 16.9 (11/65) | 34.4 (11/32) | 0.0 (0/33) | |
| Lipid-lowering therapy | 36.9 (24/65) | 53.1 (17/32) | 21.2 (7/33) | 0.011 |
| Anti-hypertensive therapy | 41.5 (27/65) | 50.0 (16/32) | 33.3 (11/33) | 0.213 |
| Metabolic parameters | ||||
| Weight (kg) | 92.6 (79.0–101) | 94.3 (78.5–103) | 91.0 (80.0–100) | 0.670 |
| Body mass index (kg/m2) | 33.1 (28.6–36.6) | 34.1 (30.2–38.4) | 31.1 (27.8–35.1) | 0.064 |
| Systolic blood pressure (mmHg) | 131 (121–147) | 138 (121–153) | 129 (121–144) | 0.319 |
| Diastolic blood pressure (mmHg) | 83.0 (76.5–87.0) | 83.5 (74.3–87.0) | 82.0 (78.5–87.0) | 0.794 |
| HbA1c (%) [mmol/mol] | 6.4 (5.7–7.1) | 7.1 (6.7–8.5) | 5.8 (5.5–6.1) | <0.0001 |
| 46 [39–54] | 54 [50–69] | 40 [37–43] | ||
| Total cholesterol (mmol/L) | 4.9 (4.2–5.8) | 4.7 (3.7–5.7) | 5.3 (4.8–6.3) | 0.070 |
| Creatinine (µmol/L) | 77.0 (64.0–85.0) | 75.0 (61.0–85.0) | 78.0 (68.5–87.5) | 0.512 |
| Liver function tests | ||||
| ALT (IU/L) | 43.0 (29.0–68.3) | 38.0 (30.3–52.3) | 49.5 (29.0–80.8) | 0.076 |
| AST (IU/L) | 34.0 (23.0–46.0) | 35.0 (23.0–46.0) | 32.0 (23.3–47.3) | 0.723 |
| GGT (IU/L) | 74.5 (33.0–134) | 91.5 (35.0–151) | 70.5 (33.0–106) | 0.372 |
| ALP (IU/L) | 224 (183–276) | 256 (197–337) | 208 (175–248) | 0.005 |
| Bilirubin (µmol/L) | 7.0 (5.0–12.0) | 7.0 (5.0–11.0) | 7.0 (4.0–13.0) | 0.803 |
| Albumin (g/L) | 47.0 (45.0–50.0) | 46.0 (45.0–48.0) | 49.0 (46.5–50.0) | 0.003 |
Values are medians (IQR), unless stated. Only patients that were referred and had confirmed NAFLD on visit 1 are included. Patients that were referred and had another type of liver disease or decompensated liver disease were excluded. Mann–Whitney U-tests or Fisher exact were used to compare patients with vs. without type 2 diabetes.
aPatient with type 2 diabetes on metformin for polycystic ovarian syndrome.
bNew diagnosis of type 2 diabetes confirmed on first clinic visit.
P-values in bold are significant
Effect of MDT management on NAFLD patients
The median time interval between the initial clinic visit (visit 1) and the follow-up visit (visit 2) was 98 days (IQR 70-182). Fifty-five patients (84.6%) attended the follow-up visit 2, whereas 4 (6.2%) did not attend follow-up (reason unknown) and 6 (9.2%) patients were discharged after visit 1 with a diagnosis of simple hepatic steatosis.
Between visits 1 and 2, there was a significant reduction in weight (–0.8 kg, IQR −4.7–1.8; P = 0.024) and BMI (–0.38 kg/m2; P = 0.027) in the NAFLD patients (Table 2). The greatest weight loss was in patients with type 2 diabetes (–1.6 kg; P = 0.037), of whom 25.0% (7/28) achieved >5% weight loss. No significant differences were seen in median blood pressure or HbA1c. However, 40.0% (6/15) of patients with type 2 diabetes with an HbA1c ≥ 7.0% (≥53 mmol/mol) at their initial visit achieved a target HbA1c (<7.0%, <53 mmol/mol) by visit 2. In the whole cohort, cholesterol decreased significantly between visits 1 and 2 (–0.2 mmol/L; P = 0.044), but only 15.4% (4/26) patients with a high total cholesterol at visit 1 improved to a target of <5.0 mmol/L.
Table 2.
Changes in metabolic and liver parameters between the initial visit 1 and follow-up visit 2 in the NAFLD MDT clinic (UHB)
| Total patients (n = 55) | Type 2 diabetes (n = 28) | Without type 2 diabetes (n = 27) | |
|---|---|---|---|
| Clinical differences (visit 2 – visit 1) | |||
| Weight (kg) | |||
| Median difference (IQR) | –0.8 (–4.7,1.8)* | –1.6 (–5.6,1.3)* | –0.3 (–4.2, 1.8) |
| % (n) patient with >5% improvement | 21.8 (12/55) | 25.0 (7/28) | 18.5 (5/27) |
| Body mass index (kg/m2) | |||
| Median difference (IQR) | –0.38 (–1.8, 0.6)* | –0.67 (–2.1, 0.49)* | –0.06 (–1.5, 0.72) |
| % (n) patient with >5% improvement | 23.6 (13/55) | 28.6 (8/28) | 18.5 (5/27) |
| Systolic blood pressure (mmHg) | |||
| Median difference (IQR) | 1.0 (–6.3, 11.3) | 0.0 (–14.5, 9.0) | 3.5 (–4.0, 12.5) |
| % (n) patients improving to target BP < 130 mmHg | 11.8 (4/34) | 22.2 (4/18) | 0.0 (0/16) |
| Diastolic blood pressure (mmHg) | |||
| Median difference (IQR) | –2.0 (–7.0, 2.0) | –2.0 (–7.0, 2.8) | –1.0 (–6.8, 2.0) |
| % (n) patients improving to target BP < 80 mmHg | 30.2 (13/43) | 35.0 (7/20) | 26.1 (6/23) |
| HbA1c % [mmol/mol] | |||
| Median difference (IQR), % | 0.0 (–0.3, 0.2) | –0.1 (–0.5, 0.4) | 0.0 (–0.2, 0.1) |
| Median difference (IQR), mmol/mol | 0.0 (–3.0, 2.0) | –1.0 (–6.0, 4.5) | 0.0 (–2.0, 1.0) |
| % (n) patients improving to target HbA1c < 7.0% [<53 mmol/mol] | 37.5 (6/16) | 40.0 (6/15) | 0.0 (0/1) |
| Total cholesterol (mmol/L) | |||
| Median difference (IQR) | –0.2 (–0.6, 0.1)* | –0.2 (–1.1, 0.2) | –0.2 (–0.4, 0.1) |
| % (n) patients improving to target < 5.0 mmol/L | 15.4 (4/26) | 22.2 (2/9) | 11.8 (2/17) |
| ALT (IU/L) | |||
| Median difference (IQR) | –12.5 (–22, 2.5)*** | –10.0 (–21, 1.5)* | –16.0 (–28, 6.3)* |
| % (n) patients normalizing to ALT ≤ 41 IU/L | 47.1 (14/34) | 66.7 (8/12) | 27.2 (6/22) |
| AST (IU/L) | |||
| Median difference (IQR) | –4.0 (–15, 1.5)* | –4.5 (–17, 2.5)* | –2.0 (–12, 1.0) |
| % (n) patients normalizing to ALT ≤ 43 IU/L | 47.4 (9/19) | 66.7 (6/9) | 30.0 (3/10) |
| GGT (IU/L) | |||
| Median difference (IQR) | –13.0 (–51, 3.0)*** | –14.5 (–53, 17) | –13.0 (–46, 3.0)** |
| % (n) patients normalizing to GGT ≤ 50 IU/L | 18.4 (7/38) | 20.0 (4/20) | 16.7 (3/18) |
Values are median differences (25th centile, 75th centile), unless stated. Newly diagnosed type 2 diabetes are included in the diabetes cohort. Only patients that attended visit 1 and visit 2 were included in the paired analysis of differences in median values (non-parametric Wilcoxon-rank test). Baseline vs. P-wave, *<0.05, **<0.01, ***<0.001.
There was a significant improvement in ALT (–12.5 IU/L; P < 0.001), AST (–4.0 IU/L; P = 0.0067) and GGT (gammu-glutamyl transferase, −13.0 IU/L; P < 0.0001) after the initial visit to the NAFLD MDT clinic (Table 2). 41.2% (14/34) of NAFLD patients normalized their ALT (≤41 IU/L) after the initial NAFLD MDT review. 66.7% (8/12) of the patients with co-existing type 2 diabetes and NAFLD normalized their ALT.
Discussion
NAFLD is a prevalent and under-diagnosed condition that is strongly associated with features of the metabolic syndrome.21 Our pilot analysis has highlighted that application of the NFS in a routine diabetes clinic results in the identification of a significant proportion of patients with advanced NAFLD fibrosis (7.8%), who require specialist liver input. In addition, our retrospective short-term analysis demonstrates the role of the MDT in the management of patients with NAFLD in particular those with co-existing type 2 diabetes, resulting in significant weight loss and reduction in markers of liver injury.
Previous large studies support the notion for a temporal relationship in which type 2 diabetes precedes the insidious onset of chronic liver disease (+/– HCC).3 However, due to a lack of research on diagnostic tools and long-term data on the survival benefits/cost-effectiveness there remain no guidelines for identifying advanced NAFLD fibrosis in patients attending type 2 diabetes clinics [14]. Previous histological studies have highlighted that NASH occurs in 25–30% of patients with type 2 diabetes,21 but there remains a paucity of data on identifying advanced fibrosis in day-to-day diabetes clinic. Our data highlights that without systematic screening in asymptomatic high-risk individuals, advanced liver disease could go undiagnosed. Solely relying on routine LFTs and USS (ultrasound) for this purpose lacks sensitivity and specificity. The recent development of simple, cheap non-invasive scoring systems could provide a solid platform for identifying those in diabetes clinics who require further assessment. NFS is a cheap, user-friendly and non-invasive method of excluding advanced NAFLD fibrosis.13,22 A low NFS provides an excellent negative predictive value for the presence of advanced liver fibrosis,13 meaning we could confidently exclude advanced fibrosis in 39.0% of study participants. Despite the lack of symptoms/signs and LFT abnormality, 7.8% (5/64) of unselected patients with type 2 diabetes had undiagnosed advanced liver fibrosis (F3/F4) necessitating ongoing MDT input.
In adult patients with NAFLD, weight loss, lifestyle modification and diabetes management remain the mainstay of intervention at present. This requires an MDT approach which is rarely available in the majority of liver units or assessed in the literature.23 Our MDT approach resulted in significant weight loss, notably 25% of patients with type 2 diabetes achieved >5% weight loss (amount reported to improve insulin sensitivity and hepatic steatosis on liver biopsy)24 over a median of 3 months. Furthermore, 47% of patients had normalized their ALT levels by the follow-up visit. In the 15 patients with poor glycaemic control at visit 1 (HbA1c > 7.0%, >53 mmol/mol), 40% achieved a HbA1c < 7.0% (<53 mmol/mol) by the follow-up visit. In addition, 7/40 patients (17.5%) had a new diagnosis of type 2 diabetes made in the MDT NAFLD clinic.
To date, no studies have utilized a prospective step-wise strategy (NFS and transient elastography) to identifying advanced NAFLD fibrosis in a routine secondary care diabetes clinic. Liver biopsy (5/6 cases) and Fibrotest (1/6 cases) confirmed the diagnostic accuracy of high LSE readings. A broader interpretation of our data at present is limited as the small cohort size (n = 64), which only represents ∼20% of those attending diabetes clinic during the study period. The low recruitment rate was likely due to the fact that only a single diabetes consultant (M.A.K.) actively recruited patients into the study. Even though we cannot rule-out a degree of selection bias, the patient characteristics of those recruited matched those of the whole clinic population and previous published reports.25 Furthermore, our study potentially underestimates the magnitude of the clinical problem, as MDT referral was recommended in 39/64 cases (based on the NFS), but was only performed in 23/39 patients (59.0%) due to patient refusal and failure to attend. In those patients who actually attended for MDT assessment 21.7% (5/23) had advanced liver fibrosis confirmed on liver biopsy. The refusal rate might reflect a lack of patient understanding of the associated risks of type 2 diabetes and NAFLD or the added inconvenience and anxiety of attending an additional hospital specialist clinic. In 2009, our NAFLD MDT clinic was one of the first and largest to be set up in the UK National Health Service. Even though our pilot study provides a useful insight into the use of an MDT approach to NAFLD in routine clinic practice, it is important to interpret the findings with caution due its retrospective design, short duration of follow-up and lack of a control (non-MDT) group for comparison. Appropriately powered, controlled prospective studies are therefore required before a wider interpretation of such an approach can be made.
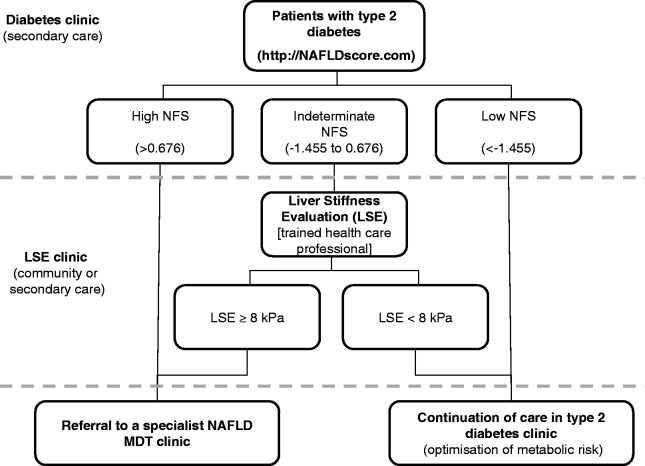
In summary, our study highlights that a significant proportion of asymptomatic patients (unremarkable LFTs) attending routine type 2 diabetes clinics have undiagnosed advanced NAFLD fibrosis. In the absence of validated standards of care, we would advocate the pilot use of the NFS to stratify patients at risk of advanced fibrosis in routine diabetes clinics and a one-off LSE (nurse-led clinic in the community or secondary care)17 when indicated (Figure 2). Implementing an MDT approach to NAFLD is both feasible and results in promising short-term improvements in metabolic and liver parameters. Until prospective study and mortality/morbidity data become available, the long-term efficacy and cost-effectiveness of the MDT approach will remain unknown.
Figure 2.
Proposed strategy for identifying and managing patients with type 2 diabetes at risk of advanced liver fibrosis in secondary care. This guide is based on author opinion only.
Supplementary material
Supplementary material is available at QJMED online.
Acknowledgements
M.J.A. and J.M.H. are the guarantors and take full responsibility for the integrity of the data from inception to the published article. M.J.A., J.M.H., M.A.K., P.N.N. and J.W.T. contributed to the concept and design of the study. M.J.A., J.M.H., R.P., M.A.K., E.K., J.M., S.K., A.P., L.C., J.J., M.R. and G.H. collected the data. M.J.A., J.M.H. and J.W.T. analysed the data and wrote the manuscript. All authors approved the final version of the manuscript.
Funding
M.J.A. is in receipt of a Wellcome Trust Clinical Research Fellowship. R.P. is in receipt of a MRC Clinical Research Fellowship. J.W.T. is in receipt of a MRC Senior Clinical Fellowship (G0802765). The funders did not contribute to the current study.
Conflict of interest: None declared.
References
- 1.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Rhee EJ, Bae JC, Park SE, Park CY, Cho YK, et al. Increased risk of type 2 diabetes in subjects with both elevated liver enzymes and ultrasonographically diagnosed nonalcoholic fatty liver disease: a 4-year longitudinal study. Arch Med Res. 2013;44:115–20. doi: 10.1016/j.arcmed.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–21. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444–50. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–73. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–5. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–40. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–44. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite NC, Salles GF, Araujo ALE, Villela-Nogueira CA, Cardoso CRL. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–9. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 14.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 15.Casey SP, Kemp WW, McLean CA, Topliss DJ, Adams LA, Roberts SK. A prospective evaluation of the role of transient elastography for the detection of hepatic fibrosis in type 2 diabetes without overt liver disease. Scand J Gastroenterol. 2012;47:836–41. doi: 10.3109/00365521.2012.677955. [DOI] [PubMed] [Google Scholar]
- 16.Roulot D, Czernichow S, Le Clésiau H, Costes J-L, Vergnaud A-C, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606–13. doi: 10.1016/j.jhep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 17.McCorry RB, Palaniyappan N, Chivinge A, Kaye P, James MW, Aithal GP. Development and evaluation of a nurse-led transient elastography service for the staging of hepatic fibrosis in patients with suspected chronic liver disease. QJM. 2012;105:749–54. doi: 10.1093/qjmed/hcs043. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a World Health Organisation/Internation Diabetes Federation consultation. Geneva, 2006. [Google Scholar]
- 21.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 22.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Natale S, Manini R, Agostini F. Review article: the treatment of fatty liver disease associated with the metabolic syndrome. Aliment Pharmacol Ther. 2005;22(Suppl. 2):37–9. doi: 10.1111/j.1365-2036.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 24.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–6. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 25.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434–41. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.