Abstract
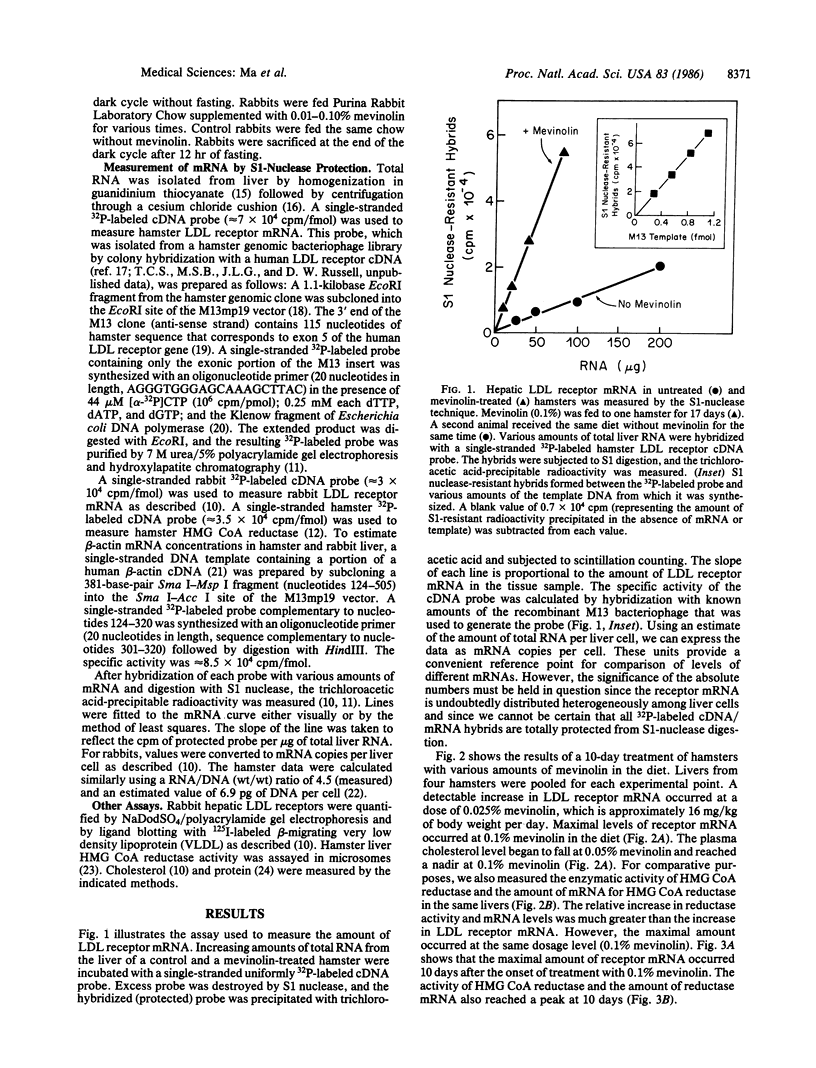
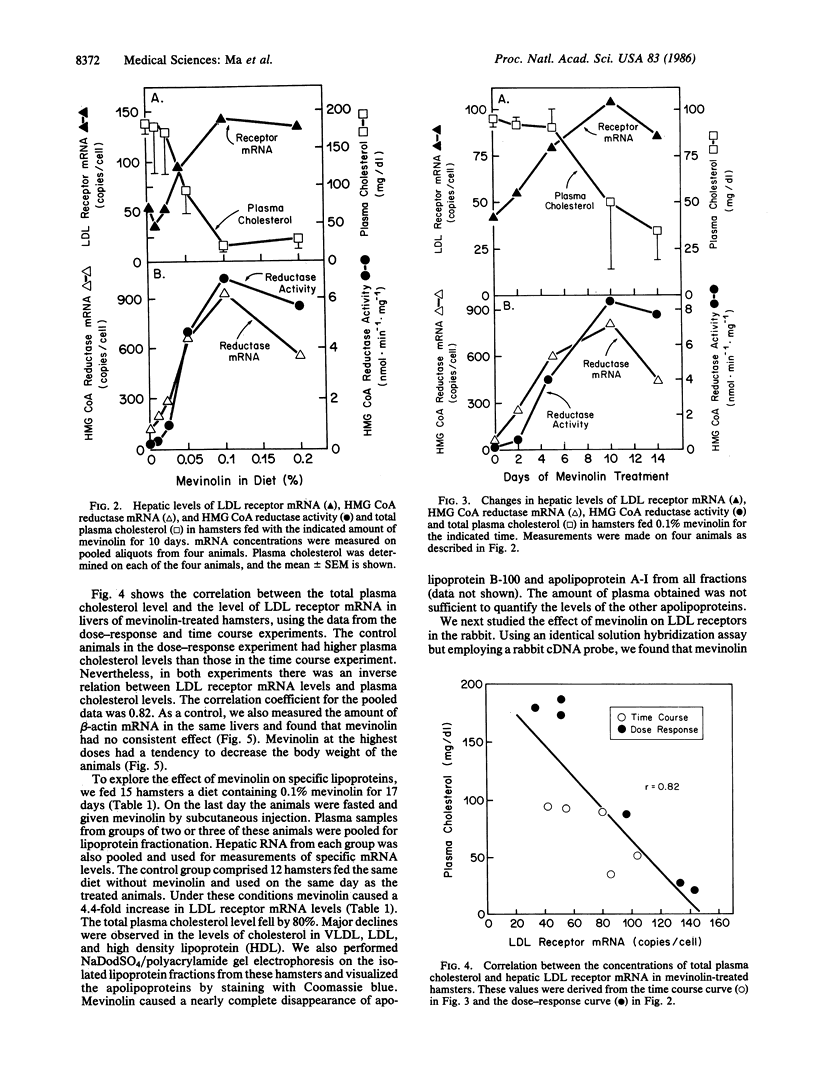
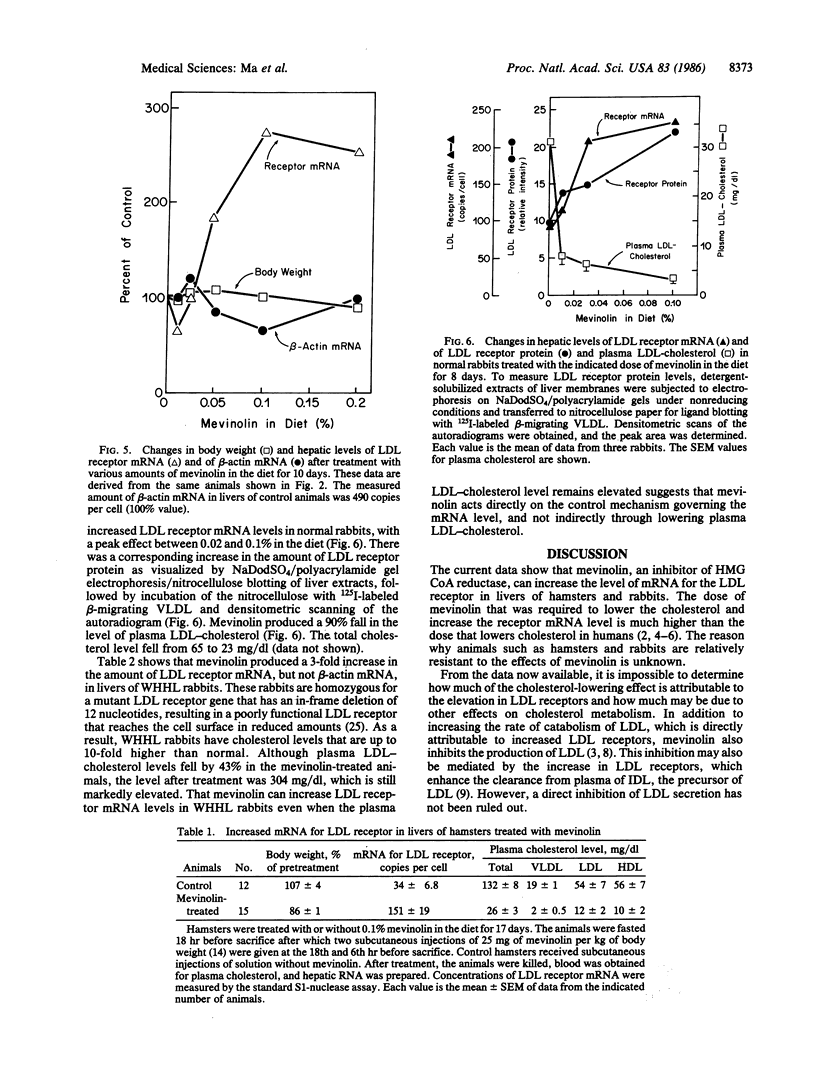
Through the use of a quantitative solution hybridization assay with 32P-labeled cDNA probes, we found that mevinolin, an inhibitor of cholesterol synthesis, elevates the level of mRNA for the low density lipoprotein receptor in livers of hamsters and rabbits. In hamsters the maximal effect (3-fold increase) occurred at 0.1% mevinolin in the diet for 10 days. The same dose produced a maximal induction (10-fold) of mRNA levels for 3-hydroxy-3-methylglutaryl CoA reductase, the rate-limiting enzyme of cholesterol synthesis, and a maximal decrease (80%) in plasma cholesterol. The drug lowered the level of all cholesterol-carrying lipoproteins in plasma. In normal rabbits, mevinolin produced a 90% reduction in plasma low density lipoprotein-cholesterol levels, which was associated with a 2.5-fold increase in low density lipoprotein receptor mRNA levels. A similar induction of receptor mRNA occurred in livers of Watanabe-heritable hyperlipidemic rabbits, although the plasma cholesterol was not reduced to normal, presumably because the receptors produced by the mutant mRNA function poorly. These data are consistent with the hypothesis that mevinolin and other inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase lower plasma cholesterol levels in part by stimulating production of mRNA for the low density lipoprotein receptor in liver.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Grundy S. M., Brown M. S., Goldstein J. L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4124–4128. doi: 10.1073/pnas.80.13.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Chao Y. S., Kroon P. A., Yamin T. T., Thompson G. M., Alberts A. W. Regulation of hepatic receptor-dependent degradation of LDL by mevinolin in rabbits with hypercholesterolemia induced by a wheat starch-casein diet. Biochim Biophys Acta. 1983 Nov 29;754(2):134–141. doi: 10.1016/0005-2760(83)90154-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Endo A. HMG-CoA reductase inhibitors. Adv Exp Med Biol. 1985;183:295–310. doi: 10.1007/978-1-4613-2459-1_23. [DOI] [PubMed] [Google Scholar]
- Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell. 1985 May;41(1):249–258. doi: 10.1016/0092-8674(85)90078-9. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res. 1984 Dec 15;25(13):1450–1461. [PubMed] [Google Scholar]
- Grundy S. M., Vega G. L. Influence of mevinolin on metabolism of low density lipoproteins in primary moderate hypercholesterolemia. J Lipid Res. 1985 Dec;26(12):1464–1475. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeg J. M., Maher M. B., Zech L. A., Bailey K. R., Gregg R. E., Lackner K. J., Fojo S. S., Anchors M. A., Bojanovski M., Sprecher D. L. Effectiveness of mevinolin on plasma lipoprotein concentrations in type II hyperlipoproteinemia. Am J Cardiol. 1986 Apr 15;57(11):933–939. doi: 10.1016/0002-9149(86)90733-2. [DOI] [PubMed] [Google Scholar]
- KIT S., FISCUS J., RAGLAND R. S., GRAHAM O. L., GROSS A. L. Biochemical studies on the Chinese hamster (22 chromosomes) and the Syrian hamster (44 chromosomes). Exp Cell Res. 1959 Feb;16(2):411–417. doi: 10.1016/0014-4827(59)90270-8. [DOI] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Bilheimer D. W., Goldstein J. L. Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5693–5697. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Goldstein J. L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J Clin Invest. 1980 Nov;66(5):1094–1100. doi: 10.1172/JCI109938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liscum L., Luskey K. L., Chin D. J., Ho Y. K., Goldstein J. L., Brown M. S. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and its mRNA in rat liver as studied with a monoclonal antibody and a cDNA probe. J Biol Chem. 1983 Jul 10;258(13):8450–8455. [PubMed] [Google Scholar]
- Ma P. T., Yamamoto T., Goldstein J. L., Brown M. S. Increased mRNA for low density lipoprotein receptor in livers of rabbits treated with 17 alpha-ethinyl estradiol. Proc Natl Acad Sci U S A. 1986 Feb;83(3):792–796. doi: 10.1073/pnas.83.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi H., Haba T., Tatami R., Miyamoto S., Sakai Y., Wakasugi T., Watanabe A., Koizumi J., Takeda R. Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med. 1981 Aug 27;305(9):478–482. doi: 10.1056/NEJM198108273050902. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobert J. A., Bell G. D., Birtwell J., James I., Kukovetz W. R., Pryor J. S., Buntinx A., Holmes I. B., Chao Y. S., Bolognese J. A. Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase, in healthy volunteers. J Clin Invest. 1982 Apr;69(4):913–919. doi: 10.1172/JCI110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Newman T. C., Shelness G. S., Gordon D. A. Measurement of apolipoprotein mRNA by DNA-excess solution hybridization with single-stranded probes. Methods Enzymol. 1986;128:671–689. doi: 10.1016/0076-6879(86)28099-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]



