Abstract
Chronic paroxysmal intracranial hypertension leading to syncope is a phenomenon not reported previously in patients with refractory cerebral venous sinus thrombosis. We report a case of paroxysmal intracranial hypertension leading to syncopal episodes in a patient with idiopathic autoimmune hemolytic anemia and venous sinus thrombosis. This case demonstrates that intermittent elevations in intracranial pressure can lead to syncope in patients with venous sinus thrombosis and emphasizes the importance of considering this potentially treatable etiology of syncopal episodes.
Keywords: intracranial hypertension, cerebral venous sinus thrombosis, autoimmune hemolytic anemia, Lundberg A wave
Introduction
Transient elevations in intracranial pressure (ICP) are well-studied phenomena in the brain-injured patient,1 but less is known about their presence in healthy patients and also in patients with idiopathic intracranial hypertension (IIH).2,3 We present here the case of a 36-year-old woman with frequent syncopal episodes in the setting of ICP spikes to >60 mm Hg. In the setting of a cerebral venous sinus thrombosis (CVST), the patient developed elevated ICPs with a likely shift of cerebral autoregulation to the passive reactivity range and resultant Lundberg A waves on ICP monitoring. This represents a rare consequence of refractory CVST that is amenable to treatment with ventriculoperitoneal shunt.
Case
A 36-year-old left-handed woman had a history of idiopathic autoimmune hemolytic anemia presenting 6 years prior to her neurological presentation with a hemoglobin of 3.3 g/dL and demand cardiac ischemia. She was treated with a splenectomy in the setting of a poor response to steroids and azathioprine and was then symptom free for several years without immune modulating treatment before reinitiation of azathioprine and rituximab for recurrent anemia 1 month before neurologic symptoms. Her neurologic presentation began with transient episodes of rushing noises in her ears, vertical diplopia on right gaze, and vertigo without headache. Lumbar puncture demonstrated a normal opening pressure, papilledema was noted on ophthalmologic examination, and magnetic resonance imaging (MRI) without dedicated venous imaging was normal at that time. Although she had a normal body mass index (20 kg/m2), the presumed diagnosis was IIH, and she was started on acetazolamide 500 mg twice daily and then a brief course of prednisone (dose unknown).
After 1 year without symptoms, acetazolamide was tapered off. Several months later, she again began having transient episodes of tinnitus, diplopia, and lightheadedness, intermittently accompanied by headache, occurring 5 to 6 times/d lasting 5 to 30 minutes without any clear precipitant or postural predilection. A repeat MRI demonstrated prominent vessels at the skull base, and cerebral angiogram demonstrated thrombosis of the anterior third of the superior sagittal, left transverse, and left sigmoid sinuses. After a lumbar puncture with an opening pressure of 31 mm Hg, acetazolamide was titrated to 1500 mg twice daily, and repeat lumbar punctures every 2 weeks revealed lateral decubitus opening pressures of 16 to 35 mm Hg. Ventriculoperitoneal shunt was considered, but with no abnormalities on visual field testing was not pursued.
Anticoagulation with warfarin was started with enoxaparin bridging once the diagnosis of CVST was made, but episodes persisted and evolved to include confusion and eventually loss of consciousness. One month after initiation of anticoagulation, despite therapeutic INRs, repeat angiogram revealed extension of venous sinus thrombosis to involve the entire superior sagittal sinus. In addition, a dural arteriovenous fistula, supplied by the left middle meningeal artery at the superior sagittal sinus draining into a pial vein, was identified and treated via ethanol embolization. To assure therapeutic anticoagulation, the patient was switched to 1 mg/kg twice daily enoxaparin injections. Given the lack of apparent precipitant, a serologic hypercoagulability workup was initiated, which revealed no abnormalities of factor V Leiden gene, prothrombin 20210 gene, MTHFR gene, janus kinase 2 gene, β2-glycoprotein or cardiolipin antibodies, lupus anticoagulant, Russell viper venom test, paroxysmal nocturnal hemoglobinuria markers, antithrombin III activity, or protein C activity. Her erythrocyte sedimentation rate was 41, and antinuclear antibody was 1:40 speckled, with double-stranded DNA antibody negative. Protein S was mildly depressed at 35% on warfarin with repeat assay off of warfarin normal at 55% (activity was normal at 75%). A direct Coombs test was 3+ for antiglobulin, but Tc99m sulfur colloid nuclear imaging demonstrated no accessory or residual splenic function. No evidence of occult malignancy was found on computed tomography scan of the chest, abdomen, and pelvis.
As her episodes of neurologic symptoms increased in frequency to 1 to 2 times/d with duration up to 10 minutes, they also began occurring only when the patient was sitting or standing and sometimes included total loss of consciousness. As such, several possible etiologies were investigated. Extended video electroencephalographic (EEG) monitoring was done on 3 separate occasions. During the first 2 sessions, no typical events were captured, and no epileptiform activity was seen, but they did demonstrate abnormal intermittent diffuse slow activity, possibly consistent with increased ICP. After her first nonepileptiform EEG, she was started on 50 mg nortriptyline nightly based on a possible diagnosis of complex migraine. Given the repetitive stereotyped nature of her spells, and the known association of CVST with seizure, she was trialed on numerous antiepileptic medications (AEDs), including acetazolamide, valproic acid, levetiracetam, oxcarbazepine, topiramate, zonisamide, and phenytoin. The patient did experience a decrease in episode frequency to once weekly with a combination of acetazolamide 500 mg twice daily and oxcarbazepine 750 mg twice daily. During a third, week long, video EEG monitoring admission, the patient had 4 to 8 syncopal episodes per day, with EEG correlate of diffuse nonrhythmic slowing beginning with excess diffuse theta and progressing over minutes to diffuse 2 to 3 Hz polymorphic delta, correlated with the patient’s symptoms of unresponsiveness. Epochs of slowing lasted 4 to 17 minutes (average 10 minutes). Most episodes were associated with no significant change in heart rate, although occasionally gradual tachycardia occurred several minutes into the episode, consistent with a sympathetic surge in the setting of cerebral hypoxia. Additionally, during 1 observed episode, the patient had downward deviation of her eyes concerning for Parinaud syndrome from dorsal midbrain compression. After this admission, the patient’s AEDs were tapered off.
Complete cardiac workup was pursued, including a 30-day event monitor, showing no arrhythmia or changes in heart rate during 16 typical episodes. During tilt table testing, the patient lost consciousness without changes in systemic blood pressure or heart rate. She was given a brief course of metoprolol without benefit.
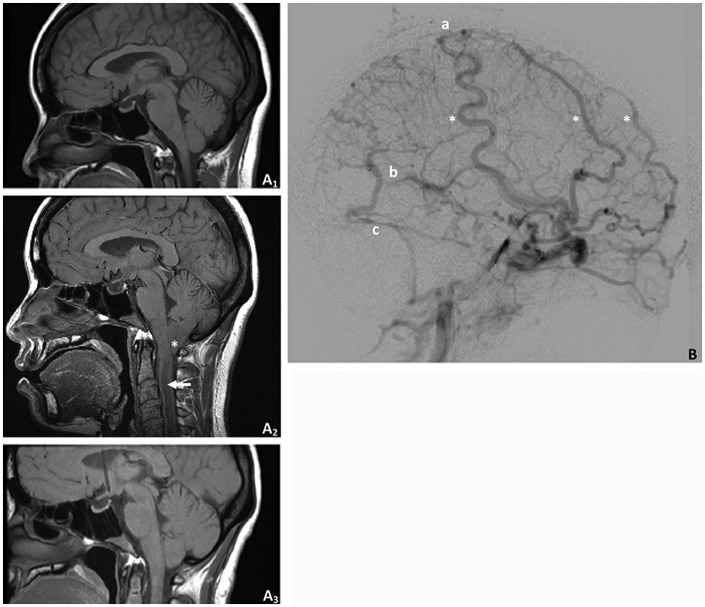
Due to no change in the extent of CVST after 4 months on enoxaparin, anticoagulation was transitioned to fondaparinux. After 3 months on fondaparinux without any improvement in daily episodes of loss of consciousness, a repeat MRI showed 7 mm of cerebellar tonsillar herniation and a cervical cord syrinx (Figure 1), and the patient was admitted to the hospital for further intervention. At that time, repeat angiography showed a new right mid-superior sagittal sinus dural arteriovenous fistula that was embolized. Fondaparinux was held for 4 days, and an external ventricular drain (EVD) was placed with initial ICP of 42 mm Hg and transient plateaus in ICP to greater than 60 mm Hg without loss of consciousness. Ventriculoperitoneal shunting was planned but postponed for 3 days due to concern for infection in the setting of a fever and leukocytosis. During the period that the EVD was in place, she had no syncopal episodes and had greater than 300 cc of cerebrospinal fluid drainage daily to keep ICP less than 20 mm Hg.
Figure 1.
Herniation and venous collaterals. A1, Magnetic resonance imaging (MRI) at initial presentation demonstrated normal posterior fossa alignment. A2, Immediately prior to ventriculoperitoneal shunt placement, there was significant tonsillar herniation (asterisk) and cervical cord syrinx (arrow). A3, Herniation and syrinx resolved and did not recur after shunt placement. B, A conventional angiogram prior to shunt placement demonstrated significant venous collateralization (asterisks) and scant filling of the superior sagittal (a), straight (b), and left transverse (c) sinuses.
One month after placement of ventriculoperitoneal shunt, repeat MRI demonstrated resolution of cervical syrinx though her burden of CVST was unchanged. She had 3 episodes of neurologic symptoms within the first month after shunt placement including 1 spell with loss of consciousness. Since that time, she has remained free of symptoms for 1 year.
Discussion
The interest of the present case is 2-fold: (1) it details the natural history of refractory CVST of unclear precipitant and (2) it demonstrates that chronically increased ICP can present with syncopal episodes, likely mediated by a similar mechanism to that seen with acute causes of elevated ICP.
Although the etiology underlying this patient’s CVST is not clear, it is possible that her persistent thrombocytosis after splenectomy (range 398-836 × 109/L),4 her recent prednisone use, or her autoimmune anemia itself 5,6 precipitated her CVST. Regardless, the persistence of her CVST while therapeutic on warfarin, enoxaparin, or fondaparinux may occur in up to 18% of the cases.7 In this case, we believe that the patient’s chronic CVST likely led to elevated ICP, a situation known to promote abnormal intracranial perfusion dynamics,8 which may result in transient neurological sequelae.9–14 Previous work has demonstrated that among patients with elevated ICP, those with impaired cerebrospinal fluid (CSF) resorption (as this patient had, evidenced by EVD drainage of 300 cc CSF/d), including patients with CVST, are more likely to demonstrate plateau elevations in CSF pressure.15 However, reports demonstrating that these plateau elevations lead to recurrent syncopal events are rare in the literature. It is possible that some cases have been miscategorized secondary to lack of ICP monitoring.16
In 1975, Grubb et al described several cases in which patients experienced syncope that was associated with increased pulsatility of cerebral blood flood (CBF) during tilt table testing as monitored by transcranial Doppler ultrasound (TCD).17 He asserted that this phenomenon was due to aberrant baroreceptor responses leading to deranged cerebral autoregulation and termed the phenomenon cerebral syncope. Unfortunately, in this patient we were not able to monitor CBF to assess whether her transient elevations in ICP led to increased pulsatility of cerebral blood flow as would be expected in cases of cerebral syncope.16 However, the positional nature of her episodes (rarely occurring while lying down and triggered by formal tilt test) do suggest that deranged autoregulation of CBF may have played a role in her syncope.
Another point highlighted by this case is the importance of EEG monitoring in cases of recurrent syncope, given the diagnostic dilemma that this clinical phenotype may present. In this case, the patient’s initial video EEG monitoring did not capture any episodes of syncope. However, her final prolonged EEG recording was sufficiently specific to merit neurosurgical intervention for her elevated ICP in the absence of vision loss (a common criteria for surgical intervention in instances of elevated ICP). Of the previously reported cases of CVST presenting as transient neurological impairment only a few ruled out epilepsy, thus convincingly implicating ICP spikes as the etiology.9,10 Although nonspecific, the predominance of delta waves during episodes of loss of consciousness, along with the absence of epileptiform discharges, is suggestive of hypoxia-mediated syncope.18 Numerous prior series have reported the low yield of EEG in syncope workups,19 but this case emphasizes the utility of EEG in the setting of patients suspected of having elevated ICP. These authors would not recommend invasive ICP monitoring until cardiac telemetry has been proven normal, and findings of either EEG or TCD are suggestive of hypoperfusion during episodic neurological symptoms.
Finally, this case underscores that ICP elevations in the setting of chronic CVST can be of a dynamic nature as opposed to the more commonly considered static elevation leading to papilledema. In these cases, dynamic monitoring of ICP may be useful to make the diagnosis.8
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36(149):1–193 [PubMed] [Google Scholar]
- 2. Warden K, Alizai A, Trobe J, Hoff J. Short-term continuous intraparenchymal intracranial pressure monitoring in presumed idiopathic intracranial hypertension. J Neuroophthalmol. 2011;31(3):202–205 [DOI] [PubMed] [Google Scholar]
- 3. Tokuno T, Yoshida S, Yamamoto T. Benign intracranial hypertension and continuous CSF pressure monitoring: case report. No Shinkei Geka. 1996;24(1):93–98 [PubMed] [Google Scholar]
- 4. Khan PN, Nair RJ, Olivares J, Tingle LE, Li Z. Postsplenectomy reactive thrombocytosis. Proc (Bayl Univ Med Cent). 2009;22(1):9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ataga KI. Hypercoagulability and thrombotic complications in hemolytic anemias. Hematologica. 2009;94(11):1481–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thachil J. Autoimmune haemolytic anaemia—an under-recog-nized risk factor for venous thromboembolism. Transfus Med. 2008;18(6):377–378 [DOI] [PubMed] [Google Scholar]
- 7. Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192 [DOI] [PubMed] [Google Scholar]
- 8. Torbey M, Geocadin R, Razumovsky A, Rigamonti D, Williams M. Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia. 2004;24(6):495–502 [DOI] [PubMed] [Google Scholar]
- 9. Jedynak C, Fournier L, Fischler M, Derome P, Rougerie J, Naquet R. Loss of consciousness and benign intracranial hypertension—correlations between CSF pressure and EEG. Rev Neurol (Paris). 1984;140(3):217–220 [PubMed] [Google Scholar]
- 10. Yildiz O, Cevik S, Cil G, Oztoprak I, Bolayir E, Topaktas S. Cerebral venous sinus thrombosis presenting as transient ischemic attacks in a case with homozygous mutations of MTHFR A1298C and CG677T. J Stroke Cerebrovasc Dis. 2012;21(1):75–77 [DOI] [PubMed] [Google Scholar]
- 11. Manzano Palomo S, Egido Herrero J, Saiz Ayala A, Jorquera Moya M. Transient ischemic attack: the only presenting syndrome of dural sinus thrombosis. Neurologia. 2006;21(3):155–158 [PubMed] [Google Scholar]
- 12. Ferro J, Falcao F, Melo T, Campos J. Dural sinus thrombosis mimicking “capsular warning syndrome”. J Neurol. 2000;247(10):802–803 [DOI] [PubMed] [Google Scholar]
- 13. Bousser M, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis—a review of 38 cases. Stroke. 1985;16(2):199–213 [DOI] [PubMed] [Google Scholar]
- 14. Chang D, Yoon S, Chung K. Superior sagittal sinus thrombosis and transient ischemic attacks: possible mechanism. J Korean Med Sci. 1998;13(5):566–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi M, Kobayashi H, Handa Y, Kawano H, Hirose S, Ishii H. Plateau-wave phenomenon (II) Occurrence of brain herniation in patients with and without plateau waves. Brain. 1991;114(pt 6):2693–2699 [DOI] [PubMed] [Google Scholar]
- 16. Grubb BP, Samoil D, Kosinski D, et al. Cerebral syncope: loss of consciousness associated with cerebral vasoconstriction in the absence of systemic hypotension. Pacing Clin Electrophysiol. 1998;21(4 pt 1):652–658 [DOI] [PubMed] [Google Scholar]
- 17. Grubb R, Raichle M, Phelps M, Racheson R. Effects of increased intracranial pressure on cerebral blood volume, blood flow, and oxygen utilization in monkeys. J Neurosurg. 1975;43(4):385–398 [DOI] [PubMed] [Google Scholar]
- 18. Lescot T, Naccache L, Bonnet MP, Abdennour L, Coriat P, Puybasset L. The relationship of intracranial pressure Lundberg waves to electroencephalograph fluctuations in patients with severe head trauma. Acta Neurochirurgica (Wein). 2005;147(2):125–129 [DOI] [PubMed] [Google Scholar]
- 19. Dantas FG, Cavalcanti AP, Rodrigues Maciel BD, et al. The role of EEG in patients with syncope. J Clin Neurophysiol. 2012;29(1):55–57 [DOI] [PubMed] [Google Scholar]